What Ranges of Probe Pressure Are Applied During Ultrasound Examinations? A Systematic Review
Abstract
1. Introduction
- How does the applied probe pressure differ depending on the type of ultrasound examination (e.g., abdominal and musculoskeletal)?
- Are there significant differences in applied pressure between operators and robotic ultrasound systems (RUSs)?
- Does monitoring the probe pressure during diagnostic examinations affect image quality?
- In which cases is pressure monitoring critical?
- What are the methods for measuring applied pressure?
- What types of force sensors are used?
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
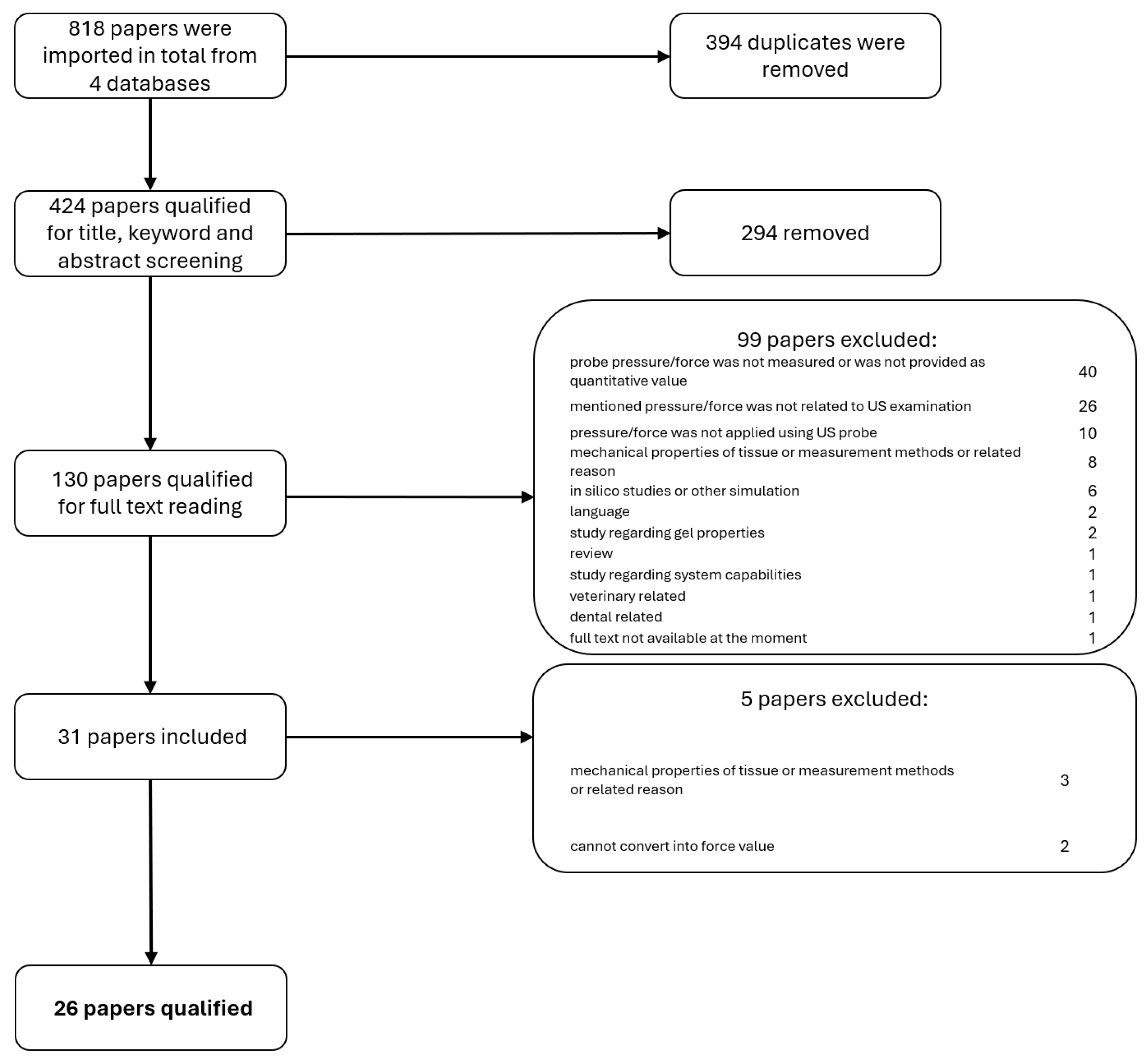
2.3. Selection Process
2.4. Data Collection and Items
2.5. Risk of Bias Assessment
2.6. Synthesis Methods
2.7. Reporting
3. Results
- 13 involved examinations conducted by human operators (e.g., sonographers, physicians, or novices).
- 13 involved examinations incorporating robots or other autonomous devices.
- The anatomical focus included 10 studies on abdominal organs, 6 on the cardiovascular system, and 10 on the musculoskeletal system.
4. Discussion
4.1. Probe Pressure Ranges in Ultrasound Examinations
4.2. Effects of Examination Type on Applied Pressure
4.3. Operator Versus Robotic Ultrasound Systems: Pressure Differences
4.4. Impact of Probe Pressure Monitoring on Image Quality
4.5. Clinical Scenarios Where Pressure Monitoring Is Critical
4.6. Methods for Measuring Applied Probe Pressure and Types of Force Sensors Utilized
4.7. Gaps and Future Research Directions
- The lack of standardized force protocols across exam types and patient groups;
- Few large-scale clinical validations of pressure-sensing systems;
- Limited data on patient comfort thresholds and acceptable pressure ranges across populations (none of the 26 studies report patient discomfort);
- Uncertainty about pressure effects in obese patients.
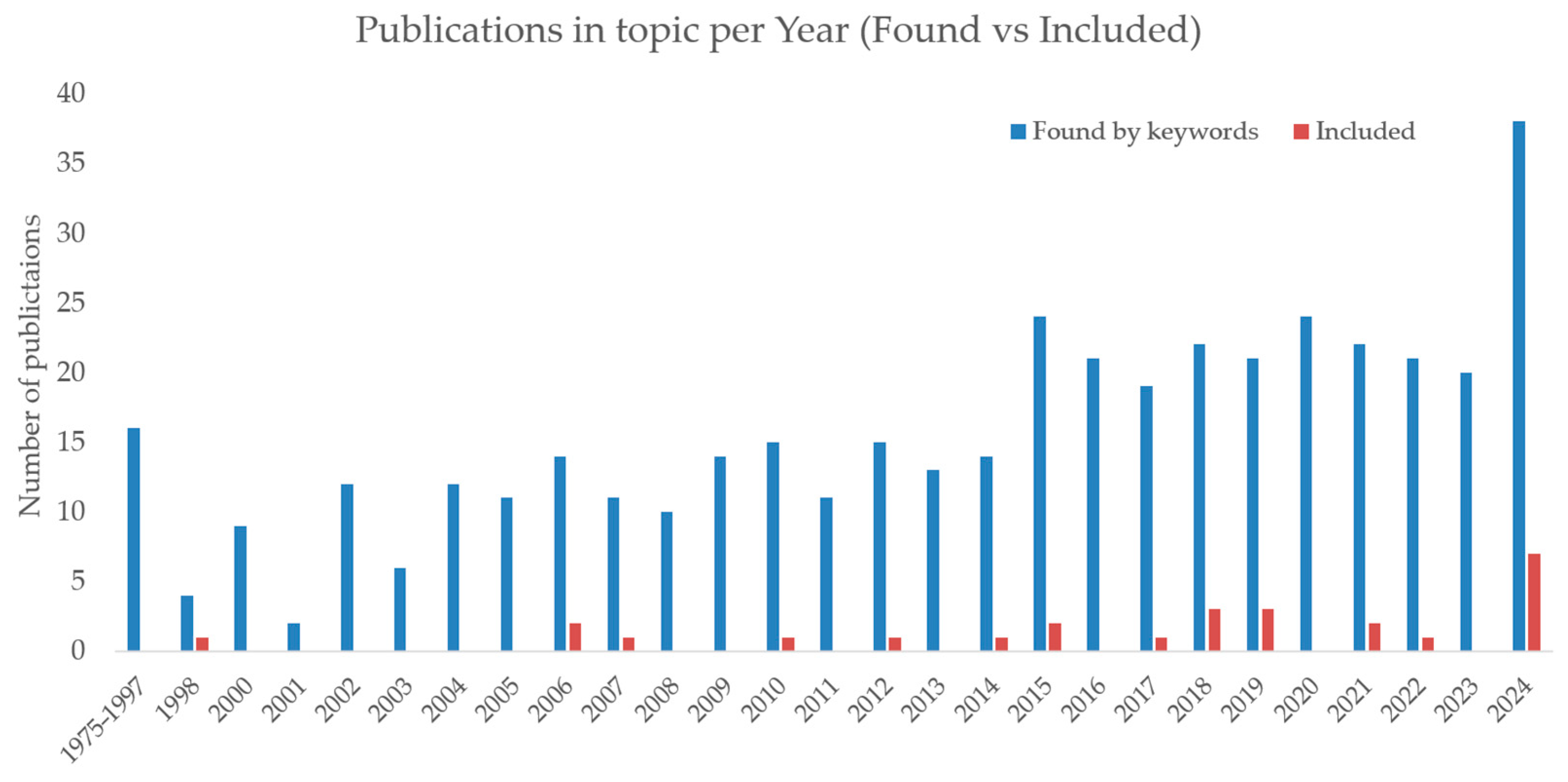
4.8. Trends in Publication Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| US | Ultrasound |
| RUS | Robotic ultrasound system |
References
- Winder, M.; Owczarek, A.J.; Chudek, J.; Pilch-Kowalczyk, J.; Baron, J. Are We Overdoing It? Changes in Diagnostic Imaging Workload during the Years 2010–2020 Including the Impact of the SARS-CoV-2 Pandemic. Healthcare 2021, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Ciekalski, M.; Rosół, I.; Filipek, M.; Gruca, M.; Hankus, M.; Hanslik, K.; Pieniążek, W.; Wężowicz, J.; Miller-Banaś, A.; Guzik-Kopyto, A.; et al. Work-Related Musculoskeletal Disorders in Polish Sonographers—A Questionnaire Study. Curr. Probl. Diagn. Radiol. 2024, 53, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Brunese, M.C.; Setola, S.V.; Ottaiano, A.; Cardone, C.; Avallone, A.; Patrone, R.; Pradella, S.; et al. Radiomics and Machine Learning Analysis by Computed Tomography and Magnetic Resonance Imaging in Colorectal Liver Metastases Prognostic Assessment. Radiol. Med. 2023, 128, 1310–1332. [Google Scholar] [CrossRef]
- Kalańska-Łukasik, B.; Gładyś, A.; Jadczyk, T.; Gruz-Kwapisz, M.; Wojakowski, W.; Kowalska, M. Readiness for Telemedical Services in Patients With Cardiovascular Diseases: Cross-Sectional Study. JMIR Form. Res. 2022, 6, e33769. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, G.; Charisopoulou, D.; Bomba, P.; Stachura, J.; Gasior, P.; Harpula, J.; Zarifis, J.; Marlicz, W.; Hudziak, D.; Jadczyk, T. Robotic-Assisted Solutions for Invasive Cardiology, Cardiac Surgery and Routine On-Ward Tasks: A Narrative Review. J. Cardiovasc. Dev. Dis. 2023, 10, 399. [Google Scholar] [CrossRef]
- Leal Ghezzi, T.; Campos Corleta, O. 30 Years of Robotic Surgery. World J. Surg. 2016, 40, 2550–2557. [Google Scholar] [CrossRef]
- Hidalgo, E.M.; Wright, L.; Isaksson, M.; Lambert, G.; Marwick, T.H. Current Applications of Robot-Assisted Ultrasound Examination. JACC Cardiovasc. Imaging 2023, 16, 239–247. [Google Scholar] [CrossRef]
- Von Haxthausen, F.; Böttger, S.; Wulff, D.; Hagenah, J.; García-Vázquez, V.; Ipsen, S. Medical Robotics for Ultrasound Imaging: Current Systems and Future Trends. Curr. Robot. Rep. 2021, 2, 55–71. [Google Scholar] [CrossRef]
- Rella, R.; Belli, P.; Giuliani, M.; Bufi, E.; Carlino, G.; Rinaldi, P.; Manfredi, R. Automated Breast Ultrasonography (ABUS) in the Screening and Diagnostic Setting. Acad. Radiol. 2018, 25, 1457–1470. [Google Scholar] [CrossRef]
- PRISMA Website 2024. Available online: https://www.prisma-statement.org/ (accessed on 1 April 2025).
- Ødegaard, S.; Kimmey, M.B.; Martin, R.W.; Yee, H.C.; Cheung, A.H.S.; Silverstein, F.E. The Effects of Applied Pressure on the Thickness, Layers, and Echogenicity of Gastrointestinal Wall Ultrasound Images. Gastrointest. Endosc. 1992, 38, 351–356. [Google Scholar] [CrossRef]
- Joo, W.J.; Fukui, M.; Kooguchi, K.; Sakaguchi, M.; Shinzato, T. Transcutaneous Pressure at Which the Internal Jugular Vein Is Collapsed on Ultrasonic Imaging Predicts Easiness of the Venous Puncture. J. Anesth. 2011, 25, 308–311. [Google Scholar] [CrossRef]
- Igarasihi, R.; Koizumi, N.; Nishiyama, Y.; Tomita, K.; Shigenari, Y.; Shoji, S. Sagittal Alignment in an MR-TRUS Fusion Biopsy Using Only the Prostate Contour in the Axial Image. ROBOMECH J. 2020, 7, 4. [Google Scholar] [CrossRef]
- Prager, R.; Gee, A.; Treece, G.; Berman, L. Freehand 3D Ultrasound without Voxels: Volume Measurement and Visualisation Using the Stradx System. Ultrasonics 2002, 40, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, N.; Giraud, R.; Muller, H.; Bendjelid, K. Demonstration of Inferior Vena Cava Compression by Probe Pressure during Subxiphoid Echocardiography. J. Clin. Ultrasound 2012, 40, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lv, G.; Chen, X.; Li, S.; Lin, H. Ultrasound Probe Pressure but Not Maternal Valsalva Maneuver Alters Doppler Parameters during Fetal Middle Cerebral Artery Doppler Ultrasonography. Prenat. Diagn. 2010, 30, 1192–1197. [Google Scholar] [CrossRef]
- Dobler, B.; Mai, S.; Ross, C.; Wolff, D.; Wertz, H.; Lohr, F.; Wenz, F. Evaluation of Possible Prostate Displacement Induced by Pressure Applied during Transabdominal Ultrasound Image Acquisition. Strahlenther. Onkol. 2006, 182, 240–246. [Google Scholar] [CrossRef]
- Li, M.; Hegemann, N.-S.; Manapov, F.; Kolberg, A.; Thum, P.D.; Ganswindt, U.; Belka, C.; Ballhausen, H. Prefraction Displacement and Intrafraction Drift of the Prostate Due to Perineal Ultrasound Probe Pressure. Strahlenther. Onkol. 2017, 193, 459–465. [Google Scholar] [CrossRef]
- Kruse, A.; Stafilidis, S.; Tilp, M. Ultrasound and Magnetic Resonance Imaging Are Not Interchangeable to Assess the Achilles Tendon Cross-Sectional-Area. Eur. J. Appl. Physiol. 2017, 117, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Dall’Alba, D.; Magnabosco, E.; Tenga, C.; Peterlik, I.; Fiorini, P. Position-Based Modeling of Lesion Displacement in Ultrasound-Guided Breast Biopsy. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1329–1339. [Google Scholar] [CrossRef]
- Tagliabue, E.; Dall’Alba, D.; Magnabosco, E.; Peterlik, I.; Fiorini, P. Biomechanical Modelling of Probe to Tissue Interaction during Ultrasound Scanning. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1379–1387. [Google Scholar] [CrossRef]
- Liu, D.; Meyer, T.; Usmani, N.; Kay, I.; Husain, S.; Angyalfi, S.; Sloboda, R. Implanted Brachytherapy Seed Movement Reflecting Transrectal Ultrasound Probe-Induced Prostate Deformation. Brachytherapy 2015, 14, 809–817. [Google Scholar] [CrossRef]
- Golshan, M.; Mahdavi, S.S.; Samei, G.; Lobo, J.; Pickles, T.; James Morris, W.; Keyes, M.; Peacock, M.; Salcudean, S.E.; Spadinger, I. Prostate Brachytherapy Intraoperative Dosimetry Using a Combination of Radiographic Seed Localization with a C-Arm and Deformed Ultrasound Prostate Contours. Brachytherapy 2020, 19, 589–598. [Google Scholar] [CrossRef]
- Boursier, J.; Konaté, A.; Gorea, G.; Reaud, S.; Quemener, E.; Oberti, F.; Hubert–Fouchard, I.; Dib, N.; Calès, P. Reproducibility of Liver Stiffness Measurement by Ultrasonographic Elastometry. Clin. Gastroenterol. Hepatol. 2008, 6, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Fargier-Voiron, M.; Presles, B.; Pommier, P.; Munoz, A.; Rit, S.; Sarrut, D.; Biston, M.-C. Evaluation of a New Transperineal Ultrasound Probe for Inter-Fraction Image-Guidance for Definitive and Post-Operative Prostate Cancer Radiotherapy. Phys. Med. 2016, 32, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Ajossa, S.; Gerada, M.; D’Aquila, M.; Piras, B.; Melis, G.B. “Tenderness-Guided” Transvaginal Ultrasonography: A New Method for the Detection of Deep Endometriosis in Patients with Chronic Pelvic Pain. Fertil. Steril. 2007, 88, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Artignan, X.; Smitsmans, M.H.P.; Lebesque, J.V.; Jaffray, D.A.; Van Her, M.; Bartelink, H. Online Ultrasound Image Guidance for Radiotherapy of Prostate Cancer: Impact of Image Acquisition on Prostate Displacement. Int. J. Radiat. Oncol. 2004, 59, 595–601. [Google Scholar] [CrossRef]
- McGahan, J.P.; Ryu, J.; Fogata, M. Ultrasound Probe Pressure as a Source of Error in Prostate Localization for External Beam Radiotherapy. Int. J. Radiat. Oncol. 2004, 60, 788–793. [Google Scholar] [CrossRef]
- Fung, A.Y.C.; Ayyangar, K.M.; Djajaputra, D.; Nehru, R.M.; Enke, C.A. Ultrasound-Based Guidance of Intensity-Modulated Radiation Therapy. Med. Dosim. 2006, 31, 20–29. [Google Scholar] [CrossRef]
- Dehghan, E.; Lee, J.; Fallavollita, P.; Kuo, N.; Deguet, A.; Le, Y.; Clif Burdette, E.; Song, D.Y.; Prince, J.L.; Fichtinger, G. Ultrasound–Fluoroscopy Registration for Prostate Brachytherapy Dosimetry. Med. Image Anal. 2012, 16, 1347–1358. [Google Scholar] [CrossRef]
- Hu, Y.; Gibson, E.; Ahmed, H.U.; Moore, C.M.; Emberton, M.; Barratt, D.C. Population-Based Prediction of Subject-Specific Prostate Deformation for MR-to-Ultrasound Image Registration. Med. Image Anal. 2015, 26, 332–344. [Google Scholar] [CrossRef]
- Fargier-Voiron, M.; Presles, B.; Pommier, P.; Rit, S.; Munoz, A.; Liebgott, H.; Sarrut, D.; Biston, M.-C. Impact of Probe Pressure Variability on Prostate Localization for Ultrasound-Based Image-Guided Radiotherapy. Radiother. Oncol. 2014, 111, 132–137. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.; Brown, A.; Edwards, C. Impact of Transperineal Ultrasound on Perineal Skin Dose in Prostate Radiation Therapy. Tech. Innov. Patient Support Radiat. Oncol. 2022, 23, 27–32. [Google Scholar] [CrossRef]
- Wang, M.; Rohling, R.; Duzenli, C.; Clark, B.; Archip, N. Evaluation of Targeting Errors in Ultrasound-Assisted Radiotherapy. Ultrasound Med. Biol. 2008, 34, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Köksoy, C.; Kuzu, A.; Kutlay, J.; Erden, Î.; Özcan, H.; Ergîn, K. The Diagnostic Value of Colour Doppler Ultrasound in Central Venous Catheter Related Thrombosis. Clin. Radiol. 1995, 50, 687–689. [Google Scholar] [CrossRef]
- Serago, C.F.; Chungbin, S.J.; Buskirk, S.J.; Ezzell, G.A.; Collie, A.C.; Vora, S.A. Initial Experience with Ultrasound Localization for Positioning Prostate Cancer Patients for External Beam Radiotherapy. Int. J. Radiat. Oncol. 2002, 53, 1130–1138. [Google Scholar] [CrossRef]
- Fontanarosa, D.; Van Der Meer, S.; Bamber, J.; Harris, E.; O’Shea, T.; Verhaegen, F. Review of Ultrasound Image Guidance in External Beam Radiotherapy: I. Treatment Planning and Inter-Fraction Motion Management. Phys. Med. Biol. 2015, 60, R77–R114. [Google Scholar] [CrossRef]
- Van Der Meer, S.; Bloemen-van Gurp, E.; Hermans, J.; Voncken, R.; Heuvelmans, D.; Gubbels, C.; Fontanarosa, D.; Visser, P.; Lutgens, L.; Van Gils, F.; et al. Critical Assessment of Intramodality 3D Ultrasound Imaging for Prostate IGRT Compared to Fiducial Markers. Med. Phys. 2013, 40, 071707. [Google Scholar] [CrossRef]
- Kliper, Y.; Ben-Ami, M.; Perlitz, Y. Effect of Mild Pressure Applied by the Ultrasound Transducer on Fetal Cephalic Measurements at 20–24 Weeks’ Gestation. Fetal Diagn. Ther. 2014, 36, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Killewich, L.A.; Bedford, G.R.; Beach, K.W.; Strandness, D.E. Diagnosis of Deep Venous Thrombosis. A Prospective Study Comparing Duplex Scanning to Contrast Venography. Circulation 1989, 79, 810–814. [Google Scholar] [CrossRef]
- Kumar, S.; Bhavana, K.; Kumar, B.; Sinha, A.K.; Kumar, P. Image Guided Sclerotherapy of Masseteric Venous Malformations. Ann. Otol. Rhinol. Laryngol. 2020, 129, 548–555. [Google Scholar] [CrossRef]
- Bakerp, M.; Juhler-Nøttrup, T.; Behrens, C.F. Impact of Ultrasound Probe Pressure on Uterine Positional Displacement in Gynecologic Cancer Patients. Womens Health 2014, 10, 583–590. [Google Scholar] [CrossRef]
- Baker, M.; Behrens, C.F. Prostate Displacement during Transabdominal Ultrasound Image-Guided Radiotherapy Assessed by Real-Time Four-Dimensional Transperineal Monitoring. Acta Oncol. 2015, 54, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Dixit, R.; Pradhan, G.S. Venous Malformation in the Breast: Imaging Features to Avoid Unnecessary Biopsies or Surgery. J. Radiol. Case Rep. 2023, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, C.; Jereczek-Fossa, B.A.; Zerini, D.; Cambria, R.; Ferrari, A.; Serafini, F.; Cattani, F.; Tagaste, B.; Fodor, C.; Luraschi, R.; et al. Image-Guided Radiotherapy for Prostate Cancer Using 3 Different Techniques: Localization Data of 186 Patients. Tumori J. 2015, 101, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Alfageme Roldän, F.; Solivetti, F.M.; Scotto Di Santolo, M.; Bouer, M.; Wortsman, X. Color Doppler Sonography of Extradigital Glomus Tumors: Color Doppler Sonography of Extradigital Glomus Tumors. J. Ultrasound Med. 2017, 36, 231–238. [Google Scholar] [CrossRef]
- Fattorello Salimbeni, A.; Kulyk, C.; Favruzzo, F.; De Rosa, L.; Viaro, F.; Pieroni, A.; Mozzetta, S.; Vosko, M.R.; Baracchini, C. Robotic Assisted Transcranial Doppler Monitoring in Acute Neurovascular Care: A Feasibility and Safety Study. Neurocrit. Care 2025, 42, 457–464. [Google Scholar] [CrossRef]
- De Silva, T.; Fenster, A.; Cool, D.W.; Gardi, L.; Romagnoli, C.; Samarabandu, J.; Ward, A.D. 2D-3D Rigid Registration to Compensate for Prostate Motion during 3D TRUS-Guided Biopsy: 2D-3D Rigid Registration to Compensate for Prostate Motion. Med. Phys. 2013, 40, 022904. [Google Scholar] [CrossRef]
- Guerrero, J.; Salcudean, S.E.; McEwen, J.A.; Masri, B.A.; Nicolaou, S. Measurement-Based Deep Venous Thrombosis Screening System. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2003; Ellis, R.E., Peters, T.M., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2003; Volume 2878, pp. 214–221. ISBN 978-3-540-20462-6. [Google Scholar]
- Li, Q.; Zhang, F.; Xi, Q.; Jiao, Z.; Ni, X. Nondeformed Ultrasound Image Production Method for Ultrasound-Guided Radiotherapy. Technol. Cancer Res. Treat. 2023, 22, 15330338231194546. [Google Scholar] [CrossRef]
- Şen, H.T.; Cheng, A.; Ding, K.; Boctor, E.; Wong, J.; Iordachita, I.; Kazanzides, P. Cooperative Control with Ultrasound Guidance for Radiation Therapy. Front. Robot. AI 2016, 3, 49. [Google Scholar] [CrossRef]
- Yoshii, Y.; Yuine, H.; Tung, W.; Ishii, T. Quantitative Assessment of Distal Radioulnar Joint Stability with Pressure-Monitor Ultrasonography. J. Orthop. Surg. 2019, 14, 195. [Google Scholar] [CrossRef]
- Lediju Bell, M.A.; Sen, H.T.; Iordachita, I.I.; Kazanzides, P.; Wong, J. In Vivo Reproducibility of Robotic Probe Placement for a Novel Ultrasound-Guided Radiation Therapy System. J. Med. Imaging 2014, 1, 1. [Google Scholar] [CrossRef]
- Byenfeldt, M.; Kihlberg, J.; Nasr, P.; Grönlund, C.; Lindam, A.; Bartholomä, W.C.; Lundberg, P.; Ekstedt, M. Altered Probe Pressure and Body Position Increase Diagnostic Accuracy for Men and Women in Detecting Hepatic Steatosis Using Quantitative Ultrasound. Eur. Radiol. 2024, 34, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, A.M.; Huang, A.Y.; Zubajlo, R.; Anthony, B.W. Real-Time Blood Pressure Estimation From Force-Measured Ultrasound. IEEE Trans. Biomed. Eng. 2018, 65, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Dall’Alba, D.; Busellato, L.; Savarimuthu, T.R.; Cheng, Z.; Iturrate, I. Imitation Learning of Compression Pattern in Robotic-Assisted Ultrasound Examination Using Kernelized Movement Primitives. IEEE Trans. Med. Robot. Bionics 2024, 6, 1567–1580. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Li, Z.M.; Choi, A.P.C.; Lu, M.H.; Chen, X.; Huang, Q.H. Ultrasound Palpation Sensor for Tissue Thickness and Elasticity Measurement—Assessment of Transverse Carpal Ligament. Ultrasonics 2006, 44, e313–e317. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Wang, Y.; Jiang, W.; Yang, K.; Jin, H.; Zhu, Y. Self-Adaptive Ultrasound Scanning System for Imaging Human Spine. IEEE Trans. Ind. Electron. 2022, 69, 570–581. [Google Scholar] [CrossRef]
- Fang, T.-Y.; Zhang, H.K.; Finocchi, R.; Taylor, R.H.; Boctor, E.M. Force-Assisted Ultrasound Imaging System through Dual Force Sensing and Admittance Robot Control. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 983–991. [Google Scholar] [CrossRef]
- Virga, S.; Göbl, R.; Baust, M.; Navab, N.; Hennersperger, C. Use the Force: Deformation Correction in Robotic 3D Ultrasound. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Salisbury, K.; Hristov, D. Online Image-Based Monitoring of Soft-Tissue Displacements for Radiation Therapy of the Prostate. Int. J. Radiat. Oncol. 2012, 83, 1633–1640. [Google Scholar] [CrossRef]
- Schimmoeller, T.; Colbrunn, R.; Nagle, T.; Lobosky, M.; Neumann, E.E.; Owings, T.M.; Landis, B.; Jelovsek, J.E.; Erdemir, A. Instrumentation of Off-the-Shelf Ultrasound System for Measurement of Probe Forces during Freehand Imaging. J. Biomech. 2019, 83, 117–124. [Google Scholar] [CrossRef]
- Mestre, S.; Triboulet, J.; Demattei, C.; Veye, F.; Nou, M.; Pérez-Martin, A.; Dauzat, M.; Quéré, I. Noninvasive Measurement of Venous Wall Deformation Induced by Changes in Transmural Pressure Shows Altered Viscoelasticity in Patients with Chronic Venous Disease. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 987–997.e2. [Google Scholar] [CrossRef]
- Schaer, G. Examination of Voiding in Seated Women Using a Remote-Controlled Ultrasound Probe. Obstet. Gynecol. 1998, 91, 297–301. [Google Scholar] [CrossRef]
- Alpert, Y.; Ben-Moshe, B.; Shvalb, N.; Piura, E.; Tepper, R. The Effect of the Pressure Exerted on the Maternal Abdominal Wall by the US Probe on Fetal MCA Peak Systolic Velocity. Ultraschall Med.-Eur. J. Ultrasound 2015, 38, 44–50. [Google Scholar] [CrossRef]
- Duan, A.; Yang, C.; Zhao, J.; Huo, S.; Zhou, P.; Ma, W.; Zheng, Y.; Navarro-Alarcon, D. Safe Learning by Constraint-Aware Policy Optimization for Robotic Ultrasound Imaging. IEEE Trans. Autom. Sci. Eng. 2024, 22, 2349–2360. [Google Scholar] [CrossRef]
- Guerrero, J.; Salcudean, E.; McEwen, A.; Masri, B.A.; Nicolaou, S. System for Deep Venous Thrombosis Detection Using Objective Compression Measures. IEEE Trans. Biomed. Eng. 2006, 53, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.P.; Nayak, R.; Wang, Y.; Gendin, D.; Larson, N.B.; Fazzio, R.T.; Oberai, A.A.; Hall, T.J.; Barbone, P.E.; Alizad, A.; et al. A Force-Matched Approach to Large-Strain Nonlinearity in Elasticity Imaging for Breast Lesion Characterization. IEEE Trans. Biomed. Eng. 2024, 71, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, A.S.; Handsfield, G.G.; Gizzi, L.; Gerlach, J.; Verl, A.; Besier, T.F.; Röhrle, O. A System for Reproducible 3D Ultrasound Measurements of Skeletal Muscles. IEEE Trans. Biomed. Eng. 2024, 71, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Liu, T.; Yang, K.; Jin, H. A Flexible Ultrasound Scanning System for Minimally Invasive Spinal Surgery Navigation. IEEE Trans. Med. Robot. Bionics 2021, 3, 426–435. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Li, B.; Leng, Y.; Peng, J.; Wu, J.; Luo, B.; Chen, X.; Rong, Y.; Fu, C. Automatic Generation of Autonomous Ultrasound Scanning Trajectory Based on 3-D Point Cloud. IEEE Trans. Med. Robot. Bionics 2022, 4, 976–990. [Google Scholar] [CrossRef]
- Ning, G.; Wang, J.; Liao, H. Cable-Driven Light-Weighting and Portable System for Robotic Medical Ultrasound Imaging. IEEE Trans. Med. Robot. Bionics 2024, 6, 1220–1231. [Google Scholar] [CrossRef]
- Sridhar, M.; Insana, M.F. Ultrasonic Measurements of Breast Viscoelasticity. Med. Phys. 2007, 34, 4757–4767. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Salisbury, K.; Hristov, D. Telerobotic System Concept for Real-time Soft-tissue Imaging during Radiotherapy Beam Delivery. Med. Phys. 2010, 37, 6357–6367. [Google Scholar] [CrossRef] [PubMed]
- Flavell, C.A.; Marshman, L.G.; Gordon, S.J. Measurement of Transversus Abdominis Activation in Chronic Low Back Pain Patients Using a Novel Standardized Real-Time Ultrasound Imaging Method. Ultrasound 2019, 27, 31–37. [Google Scholar] [CrossRef]
- Kennedy, V.L.; Flavell, C.A.; Doma, K. Intra-Rater Reliability of Transversus Abdominis Measurement by a Novice Examiner: Comparison of “Freehand” to “Probe Force Device” Method of Real-Time Ultrasound Imaging. Ultrasound 2019, 27, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-L.; Ha, S.-M.; Jeon, I.-C.; Hong, K.-H. Reliability of Ultrasonography Measurement for the Longus Colli According to Inward Probe Pressure. J. Phys. Ther. Sci. 2015, 27, 3579–3581. [Google Scholar] [CrossRef]
- Măluțan, A.M.; Clinciu, D.; Mirescu, Ș.C.; Ciortea, R.; Dudea-Simon, M.; Mihu, D. Ultrasound Probe Pressure on the Maternal Abdominal Wall and the Effect on Fetal Middle Cerebral Artery Doppler Indices. Medicina 2019, 55, 410. [Google Scholar] [CrossRef]

| Human Operator | RUS | |
|---|---|---|
| Abdomen organs | 30 N [54] | 34.5 N [53] |
| Cardiovascular system | 11 N [55] | 14 N [56] |
| Musculoskeletal system | 20 N [57] | 16 N [58] |
| Reference | Short Study Characterisation | Ultrasound Examination Type | Specific Organs | Operator (Radiologist, Sonographer, Technician, RUS, etc.) | Range of Applied Force or Pressure | (Force/Pressure) Measurement Device | Device/Sensor Placement | Positive Impact on Examination Quality | Effect on Patient Comfort | Key Findings Relevant to Applied Force/Pressure |
|---|---|---|---|---|---|---|---|---|---|---|
| Byenfeldt et al., 2024 [54] | Prospective diagnostic and experimental study; 60 participants (30 men, 30 women); metabolic-dysfunction-associated steatotic liver disease (MASLD) cohort; control group with no MASLD (BMI < 25) | Quantitative ultrasound-guided attenuation parameter (UGAP) | Liver (convex probe) | Trained operator performing UGAP measurements | Normal force: 4 N; increased force: 30 N | Custom cyclic compression apparatus | On the liver parenchyma; real-time quality map-guided region of interest | Yes | Not specified or not explicitly discussed | Optimal diagnostic performance achieved with 30 N probe force |
| Fang et al., 2017 [59] | Experimental validation study with human participants and phantom setup; 6 participants (2 expert sonographers, 4 non-experts); experts and non-experts using phantom models for scanning | Robotic-assisted linear ultrasound | Uterus phantom (linear probe) | Expert sonographers and lay participants | Reduced from 20 N to 5.48 N (73%) without constraint; 13.62 N (32%) with force constraint | Handheld US device with dual force sensors: a 6-DOF sensor and a 1-DOF load cell | One sensor on the robotic arm and another on the handheld probe | Yes | Not specified or not explicitly discussed: focused on sonographer (comfort improved) | Co-robotic system reduced human force effort significantly while improving contact force stability and ultrasound image quality |
| Virga et al., 2018 [60] | Experimental study with robotic platform: 30 volumetric acquisitions on 5 human volunteers; healthy adults aged 26 ± 30 (4 males, 1 female) | Robotic-assisted 3D ultrasound | Soft tissues of the thigh (linear probe) | Robotic system with force sensing; no direct operator involvement | 2 N–15 N | Robotic arm (KUKA LBR iiwa R800) with torque sensors and Ultrasonix RP ultrasound machine | Torque sensors integrated into the robotic arm’s joints | Yes | Not specified or not explicitly discussed | Deformation correction improved 3D image quality by compensating for force-induced tissue displacement, enabling more accurate anatomical reconstructions |
| Schlosser et al., 2012 [61] | Experimental validation study with phantom and in vivo imaging; 5 healthy volunteers and a multimodality pelvic phantom; healthy adults for in vivo imaging; phantom modeling for testing | 2D telerobotic ultrasound for prostate monitoring | Prostate (convex probe but not clearly specified) | Robotically assisted imaging; no direct human operator | 1 N–12 N | 2D Interson 3.5 MHz abdominal ultrasound probe mounted on a robotic manipulator | Infrared tracking for robotic probe localization in six degrees of freedom | Yes | Not specified or not explicitly discussed | Proposed method (with force control) reliably detected prostate displacements |
| Schimmoeller et al., 2019 [62] | Experimental study on instrumentation design and validation; one subject for in vivo study; phantom and cadaver specimens for testing; in vivo subject: 24-year-old male | Freehand ultrasound imaging | Soft tissue of the arm and leg (linear probe) | Operator using instrumented freehand probe | Minimal force imaging: <1 N; indentation: up to 10.74 N | ACUSON S3000 ultrasound system with Nano25 6-axis load cell and IMU | Probe with integrated load and motion sensors for force and orientation tracking | Yes | Not specified or not explicitly discussed | Instrumented system allowed precise measurement of probe force and orientation, improving reproducibility and enabling minimal force imaging |
| Mestre et al., 2021 [63] | Deformation correction improved 3D image quality by compensating for force-induced tissue displacement, enabling more accurate anatomical reconstructions | Compression ultrasound using a linear probe | Small saphenous vein (SSV) and deep calf vein (DCV) (linear probe) | Measurements performed by trained clinical researchers | Probe force until vein collapse, median force: 1.03 N (controls)–1.22 N (CVD patients) | Logiq-e system with a 12L-RS linear probe equipped with a force sensor | Probe placed over the small saphenous and deep calf veins at mid-calf height | Not specified | Not specified or not explicitly discussed | Force measurement allows one to determine the hysteresis loop, which reflects the viscoelastic properties of the vessel walls |
| Zheng et al., 2006 [57] | Experimental in vivo study; 5 male subjects, mean age 29.8 ± 5.1 years; healthy adult males with no neuro-musculoskeletal disorders in their upper limbs | Ultrasound palpation using TUPS system | Transverse carpal ligament (TCL) (hand-held indentation probe) | Trained operators performed the measurements | 5 N–20 N | Tissue Ultrasound Palpation System (TUPS) with a 5 MHz transducer and load cell | Probe applied to the palm skin overlying the TCL with positioning based on anatomical landmarks | Not specified | Not specified or not explicitly discussed | Skin-TCL layer was softer than TCL-carpal bone layer: stiffness differences reduced at higher forces |
| Schaer, 1998 [64] | Experimental clinical study; 30 women (20 with urinary incontinence, 10 healthy controls); women aged 47.6 ± 13.1 years with and without urinary incontinence | Perineal ultrasound with a 5 MHz curved linear array transducer | Bladder base, bladder neck, mid-urethra, and distal urethra (convex probe but not clearly specified) | Clinical researchers using a remote-controlled probe | Probe force adjustable up to 10 N, maintained gentle perineal contact | Custom-designed micturition chair with a pressure-controlled steering arm | Probe positioned against the perineum for sagittal plane imaging | Yes | None of the patients reported discomfort | Bladder neck opening and descent were observable during voiding; 11 women voided without bladder neck descent |
| Alpert et al., 2015 [65] | Prospective observational study; 29 gravid women (singleton pregnancies); pregnant women in the 2nd to 3rd trimester, median gestational age: 28.5 weeks (range: 22 + 2 to 35 + 6 weeks) | Color and pulsed Doppler ultrasound of fetal middle cerebral artery (MCA) peak systolic velocity (PSV) | Fetal middle cerebral artery (MCA) (convex probe) | Single experienced operator to reduce inter-observer variability | Pressure 1–940 units (~11 N) | GE Voluson E8 ultrasound system with a convex transducer (3.5–5 MHz) equipped with a force sensor | Electronic pressure sensor between the probe and operator’s hand; pressure measured during maternal abdominal scans | Yes | Not specified or not explicitly discussed | Higher applied pressure significantly increased MCA-PSV; excessive pressure could lead to false-positive results in fetal anemia diagnosis |
| Duan et al., 2024 [66] | Experimental validation of a reinforcement learning approach for robotic ultrasound imaging; tissue phantom experiments for spinal diagnosis using a robotic manipulator; phantom model with embedded spine; no human participants | Ultrasound imaging for spinal diagnosis using a robotic system | Spine (phantom) (linear probe but not clearly specified) | Robotic system autonomously handling ultrasound probe via reinforcement learning | 0.5 N–10 N | USB ultrasound probe (Sonoptek) attached to a 6-DoF robotic manipulator with a force/torque sensor (Robotiq FT300) | Probe mounted at the end-effector of the robotic arm, interacting with the phantom’s back | Yes | Phantom-based study | Constraint-aware policy optimization maintained force limits while achieving comparable image quality to unconstrained methods, avoiding excessive force application |
| Guerrero et al., 2006 [67] | Experimental system evaluation on phantoms and healthy human subjects; phantom experiments and healthy volunteers; phantom models with PVA cryogel vessels mimicking venous structures and healthy human volunteers | Compression ultrasound (CUS) with sensorized probe for deep venous thrombosis (DVT) screening | Deep veins of the lower extremities (e.g., femoral vein, popliteal vein) (linear probe) | Operators using sensorized handheld ultrasound probes | 2 N–10 N | Sensorized handheld probe with a 6-DOF force/torque sensor and electromagnetic location sensor | Force/torque sensor integrated into the probe housing; position tracked using an electromagnetic sensor | Not specified | Not specified or not explicitly discussed | The developed method provided an objective measure of venous compressibility, reducing operator dependency and increasing the diagnostic accuracy for DVT screening |
| Zakrzewski et al., 2018 [55] | Experimental study with real-time algorithm validation; 21 healthy volunteers for single-visit measurements; healthy adults aged 18+ | Real-time ultrasound imaging for arterial stiffness and blood pressure estimation | Carotid artery (blood pressure and tissue stiffness assessment) (linear probe) | Operators supervised real-time measurements; volunteers performed self-scans | 1 to 11 N over 10 s during compression sweeps | GE Logiq E9 ultrasound system with integrated FUTEK load cell for force measurement | Force sensor mounted on the ultrasound probe’s faceplate, applied to the carotid artery | Yes | Not specified or not explicitly discussed: system aimed at user-guided ease of use | Demonstrated feasibility of non-invasive and calibration-free blood pressure estimation with high reproducibility |
| Rosen et al., 2024 [68] | Experimental clinical study with force-controlled compression device; 54 female patients with suspicious breast lesions recommended for biopsy; adult women presenting with suspicious breast lesions; 28 lesions malignant, 26 benign | Elasticity imaging with controlled compression during ultrasound imaging | Breast tissue (malignant and benign lesions) (linear probe) | Experienced sonographers performed lesion segmentation and imaging | 1 N–6 N | Custom force-instrumented uniaxial compression system integrated with verasonics ultrasound system | Force sensors mounted on a compression plate assembly attached to the ultrasound probe | Yes | Not specified or not explicitly discussed | The study demonstrated that nonlinear elasticity metrics, obtained under controlled forces, can effectively distinguish malignant from benign breast lesions, with significant differences in strain observed |
| Sahrmann et al., 2024 [69] | Experimental validation of an automated 3D ultrasound system with force control; 8 trials on phantoms with 3 force settings; 10 trials on human tibialis anterior muscle with 2 force settings; custom-designed phantoms; one healthy female subject for tibialis anterior trials | Automated 3D freehand ultrasound (a3DUS) using Aixplorer MACH30 system | Tibialis anterior muscle; muscle-like and cylindrical phantoms (linear probe) | Operators performed freehand trials; automated system for controlled trials | 4.1 N–6.5 N | Custom motorized ultrasound probe holder with integrated force control and encoders | Probe mounted on a vertical axis actuated by a direct linear motor; phantom placed on a stable platform | Yes | Not specified or not explicitly discussed | Automated 3D ultrasound system demonstrated higher reproducibility and accuracy than freehand methods, with reduced operator dependency |
| Zhang et al., 2022 [58] | Experimental validation on phantoms and human trials; phantom experiments: 3 setups; human experiments: 4 healthy volunteers; phantom models and healthy volunteers aged 20–35 | Automatic ultrasound scanning system using a robotic mechanism (SAUSS) | Spine (thoracic, lumbar, and thoracolumbar regions) (linear probe) | Robotic system autonomously controlled with minimal operator involvement | Probe contact force: 6–10 N; total resultant force: 14–16 N | Ultrasound probe with integrated force sensor and robotic arm with force control | Probe mounted on a self-adaptive attitude-adjusting mechanism for dorsal scanning | Yes | Visual Analogue Scale (VAS) confirmed no pain reported by volunteers during the scanning process | The SAUSS system maintained stable forces, providing reliable and repeatable images for spinal navigation in minimally invasive surgeries, reducing operator dependency |
| Zhang et al., 2021 [70] | Experimental validation of a robotic ultrasound system with flexible fixtures; phantom experiments; phantom models of human dorsal anatomy | Robotic ultrasound scanning for minimally invasive spinal surgery navigation | Spine (phantom models for navigation) (linear probe) | Autonomous robotic system with minimal operator involvement | Probe contact force: 1–10 N for safe operation; maximum allowed impact force: 15 N | Robotic arm (UR5) with a flexible fixture and integrated six-axis force/torque sensor | Probe mounted on a flexible fixture equipped with a torsion spring and RGB-D camera | Yes | Phantom-based study | Flexible system improved image consistency and reduced operator dependency; repeatable images showed relative errors of only 4.49% across scans |
| Tan et al., 2022 [71] | Development and experimental validation of an autonomous ultrasound scanning system; phantom models and validation on bilateral breast regions; no specific human sample size provided; tissue-mimicking phantoms; human anatomical models for breast region testing | Robotic autonomous ultrasound scanning for breast imaging | Breast tissue (phantoms and anatomical models) | System operates autonomously; limited operator involvement | Contact force controlled dynamically within 4.84 N to 5.34 N | RGB-D camera integrated robotic arm equipped with a six-axis force/torque sensor | Ultrasound probe attached to a robotic arm with real-time force and position control | Yes | Phantom-based study | Fully autonomous system reduced scanning variability, enhanced image quality and repeatability, and provided global trajectory coverage within 4 s |
| Ning et al., 2024 [72] | Development and experimental validation of a lightweight cable-driven robotic ultrasound system; phantom-based testing; no human or live animal samples were involved; tissue-mimicking phantoms; designed for application on human anatomy in future trials, though author presented some results on humans | Cable-driven robotic ultrasound system for multi-region imaging | Designed for imaging multiple organs, including spine, kidney, and breast (linear probe) | System intended for autonomous operation; limited operator intervention during experiments | Force application range: 3–14.5 N, calibrated for safety. The max. comp. force measured for the probe: 14.5 N | Ultrasound probe with cable-driven actuators integrated into the system for precise control | Probe connected to the robotic actuator via a cable-sheath mechanism; body-mounted during testing | Yes | Phantom-based study | Lightweight and portable system demonstrated feasibility for multi-organ imaging with high stability, safety, and adaptability in dynamic environments |
| Dall’Alba et al., 2024 [56] | Experimental validation of Kernelized Movement Primitives (KMP) for robotic ultrasound; demonstrations using synthetic vascular phantoms and healthy volunteers; synthetic vascular phantoms; healthy volunteers for initial validation | Robotic ultrasound imaging system with interaction force control | Deep veins in the limbs (phantoms) for DVT simulation (linear probe) | Human demonstrators (expert sonographers) provided training data for RUS | Variable force profiles designed for DVT scanning; forces ranged from gentle coupling to compression 3–14 N | Custom recording device integrating a Clarius HD3 Linear probe with force/torque sensors | Force sensor integrated into a 3D-printed adapter mounted on the ultrasound probe | Yes | Not explicitly discussed; focus was on operator performance | KMP-based RUS successfully replicated expert compression patterns, enabling automated DVT assessment |
| Lediju Bell et al., 2014 [53] | Experimental in vivo study using robotic assistance for ultrasound probe placement; canine model: prostate, liver, pancreas implanted with 3 markers each; canine organs as in vivo models for prostate, liver, and pancreas studies | B-mode ultrasound integrated with robotic positioning for radiation therapy guidance | Prostate, liver, and pancreas (linear probe) | Robotic system autonomously managed probe positioning and tissue deformation reproducibility | Forces ranged from 1.3 N (prostate) to 34.5 N (liver and pancreas) depending on organ and setup | Custom robotic arm equipped with a six-axis force/torque sensor | Probe mounted with a geometric reference for position reproducibility; soft tissue deformations measured | Yes | Not directly applicable; animal model used for evaluation | Position control was more accurate than force control for tissue deformation reproducibility; errors were within acceptable treatment margins for radiation therapy applications |
| Sridhar and Insana, 2007 [73] | Experimental in vivo study using ultrasonic elasticity imaging; 3 female volunteers (ages 23–28) and 1 patient (age 53) with a fibroadenoma; healthy female volunteers with no history of breast disease and 1 patient with biopsy-confirmed fibroadenoma | B-mode ultrasound using Siemens Sonoline Antares system with VF10-5 linear array transducer | Breast tissue (glandular and fatty regions) (linear probe) | Sonographers manually applied compression with force feedback | 1–20 N applied with a strain rate; linear response observed between 2 and 5 N | Ultrasound system equipped with ATI Industrial Automation force sensor for real-time feedback | Force sensor mounted on transducer with a widened contact surface (4 × 8 cmÂ2). | Not specified | No discomfort reported | Linear viscoelasticity observed between 2 and 5 N; glandular tissues showed higher viscoelastic response compared to fatty tissues; higher applied forces (>5 N) induced nonlinear and rheodictic behavior |
| Schlosser et al., 2010 [74] | Experimental study on a telerobotic ultrasound system integrated with a radiotherapy setup; three in vivo volunteer imaging sessions; phantom experiments for tracking; healthy volunteers; phantom models used for testing beam interaction | 2D transabdominal ultrasound integrated with radiotherapy linear accelerator | Prostate (primary target for imaging) (convex probe) | Robotic system autonomously controlled with remote haptic operator adjustments as needed | 3.15 N–6.01 N | Interson 3.5 MHz abdominal transducer; robotic manipulator with force sensor and haptic control | Transducer mounted on a robotic manipulator designed to minimize collisions with the radiotherapy gantry | Yes | No discomfort reported | The robotic system demonstrated high precision and repeatability, offering significant improvements in volumetric imaging of pelvic organs |
| Flavell et al., 2019 [75] | Blinded pilot intra-observer reliability study using a novel standardized method for ultrasound imaging; 17 patients with chronic low back pain (CLBP); patients with CLBP aged 18+ | Real-time ultrasound imaging with 3.1 MHz curved array probe for TrA thickness measurements | Transversus abdominis muscle (convex probe) | Physiotherapist with 5 years of musculoskeletal ultrasound experience conducted imaging | 0.88 N–5.26 N | Probe force device (PFD) attached to a 3.1 MHz ultrasound probe; data recorded at 60 Hz via LabVIEW | Probe positioned lateral to the umbilicus, midway between the iliac crest and lower ribs | Yes | No significant discomfort reported | Standardized ultrasound imaging method demonstrated superior intra-observer reliability compared to freehand methods |
| Kennedy et al., 2019 [76] | Single-group repeated-measures reliability study comparing freehand and probe force device (PFD) methods; 33 healthy participants (9 males, 24 females); adults aged 18–60 years; excluded if they had low back pain (LBP) in the past year, pregnancy, or other contraindications | Real-time ultrasound imaging with 3.1 MHz curved array probe | Transversus abdominis muscle (convex probe) | Novice examiner with 2 h of prior training conducted imaging and measurements | Force range varied during freehand (uncontrolled) and was standardized in PFD (~4 N) | Probe force device (PFD) integrated with LabVIEW for real-time force and angle monitoring | Probe placed lateral to umbilicus, between the iliac crest and lower ribs, following standardized protocols | Yes | No discomfort reported | PFD method yielded better reliability and lower error in novice examiner’s measurements, especially for contraction phases |
| Jeong et al., 2015 [77] | Experimental study evaluating intra- and inter-rater reliability of ultrasonography for longus colli muscle under varying probe pressures; 13 participants (11 males, 2 females); university students aged 23.1 ± 2.9 years; healthy adults with no history of neck pain, neuromuscular, or musculoskeletal disorders | Real-time ultrasound with 5–12 MHz linear probe in M-mode | Longus colli muscle (cervical region) (linear probe) | Two experienced examiners with 5 and 2 years of orthopedic physical therapy experience | Inward pressures of 0.5 kg (~4.9 N) and 1.0 kg (~9.8 N) applied using a calibrated spring gauge | Ultrasound system integrated with a hands-free probe holder and spring gauge for consistent pressure application | Probe placed perpendicular to the anterior neck for cross-sectional area and parallel for thickness measurements | Yes | No discomfort reported | Consistent inward pressure significantly improved measurement reliability; differences in CSA and MT were minimal between 0.5 kg and 1.0 kg pressures |
| Măluțan et al., 2019 [78] | Prospective study assessing the impact of ultrasound probe pressure on fetal Doppler indices; 40 pregnant women with singleton pregnancies, gestational ages between 24 + 0 and 41 + 3 weeks; pregnant women with no pathologies affecting fetal Doppler parameters (e.g., preeclampsia, fetal distress) | US system with 2–9 MHz convex abdominal transducer | Fetal middle cerebral artery (MCA) and surrounding abdominal wall (convex probe) | Examinations conducted by a single experienced obstetrics and gynecology specialist | 3 levels of pressure (mean values): 106 gf (~1 N), 206 gf (~2 N), 300 gf (~2.9 N) | Convex transducer integrated with electronic pressure sensor | Probe positioned on the maternal abdomen to assess MCA Doppler indices | No | No discomfort reported; (care taken to avoid prolonged high-pressure applications) | The study highlighted the influence of abdominal pressure on fetal Doppler indices, recommending the use of pressure sensors to standardize measurements and improve diagnostic accuracy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchoń, S.; Burkacki, M.; Chrzan, M.; Winder, M. What Ranges of Probe Pressure Are Applied During Ultrasound Examinations? A Systematic Review. Sensors 2025, 25, 3415. https://doi.org/10.3390/s25113415
Suchoń S, Burkacki M, Chrzan M, Winder M. What Ranges of Probe Pressure Are Applied During Ultrasound Examinations? A Systematic Review. Sensors. 2025; 25(11):3415. https://doi.org/10.3390/s25113415
Chicago/Turabian StyleSuchoń, Sławomir, Michał Burkacki, Miłosz Chrzan, and Mateusz Winder. 2025. "What Ranges of Probe Pressure Are Applied During Ultrasound Examinations? A Systematic Review" Sensors 25, no. 11: 3415. https://doi.org/10.3390/s25113415
APA StyleSuchoń, S., Burkacki, M., Chrzan, M., & Winder, M. (2025). What Ranges of Probe Pressure Are Applied During Ultrasound Examinations? A Systematic Review. Sensors, 25(11), 3415. https://doi.org/10.3390/s25113415






