Intelligent Ultrasonic Aspirator Controlled by Fiber-Optic Neoplasm Sensor Detecting 5-Aminolevulinic Acid-Derived Porphyrin Fluorescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Fiber Optic Neoplasm Sensor
2.2. Control System for Intelligent Ultrasonic Aspirator
- Direct contact between the sensor tip and the area of interest, such as a tumor.
- Minimal gap between the sensor and aspirator tips.
- Effective removal of obstructions like blood via saline irrigation and aspiration.

2.3. Comparison of Fiber-Optic Neoplasm Sensor and Human Visual Assessment of PpIX Fluorescence
2.4. Application of the Intelligent Ultrasonic Aspirator on Resected Tumor Masses
- Detection of PpIX fluorescence by the fiber-optic sensor under microscopic illumination.
- Deactivation of the ultrasonic aspirator in normal brain tissue.
- Real-time response and time delay of the ultrasonic aspirator.
2.5. Preliminary Clinical Application in Malignant Glioma Resection
- Whether the system could detect tumors under white light and allow appropriate operation of the ultrasonic aspirator.
- Whether the system could halt operation at the tumor-brain border to protect surrounding normal brain tissue.
3. Results
3.1. Comparison of Sensitivity to PpIX Fluorescence Between the Fiber-Optic Sensor and the Naked Eye
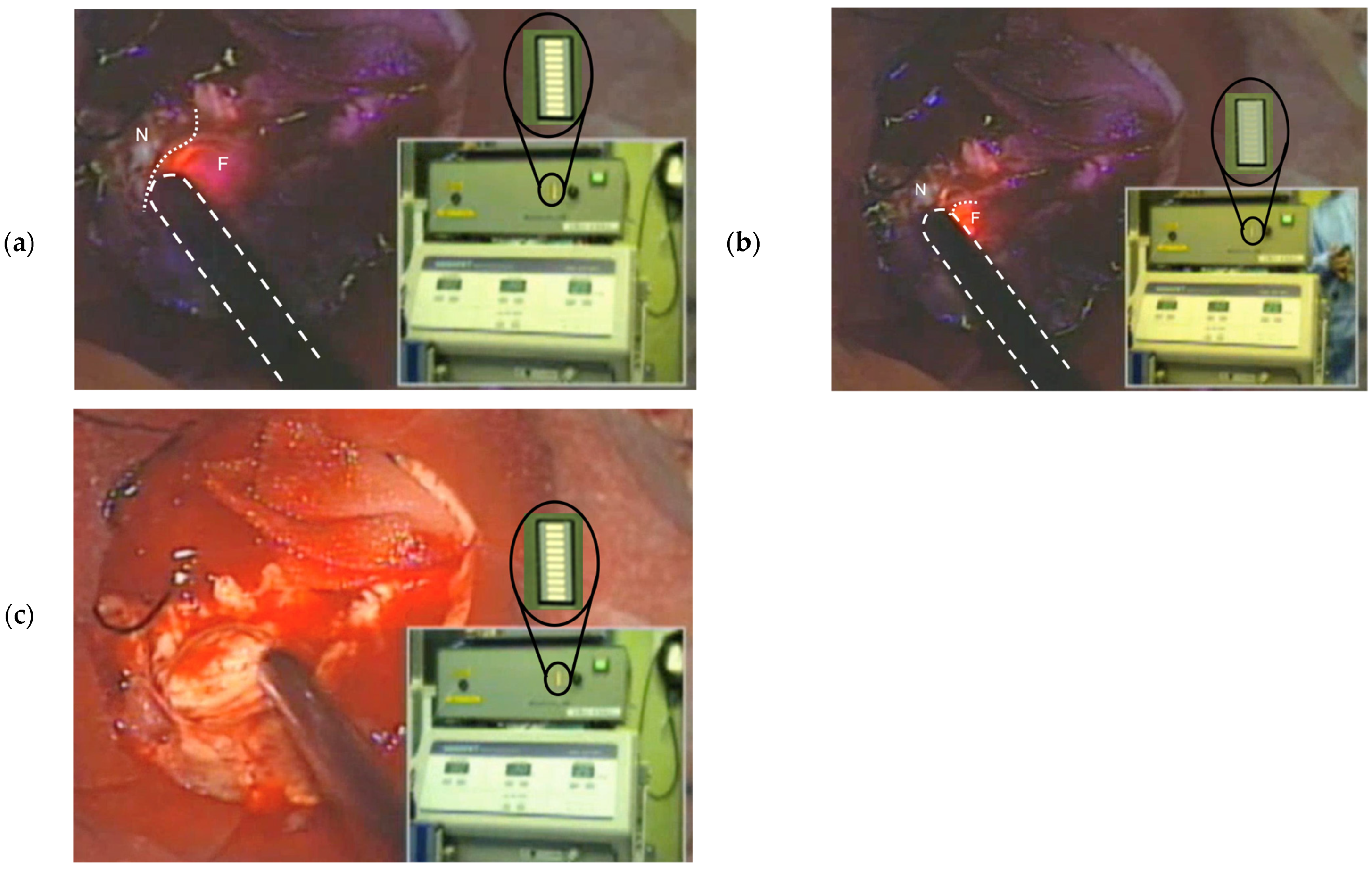
3.2. Experimental Application on Resected Tumor Masses Under an Operating Microscope
3.3. Clinical Application of the Intelligent Ultrasonic Aspirator
4. Discussion
4.1. Intelligent Ultrasonic Aspirator for Microscopic Tumor Resection
4.2. Complementary Role of the Intelligent Ultrasonic Aspirator in Fluorescence-Guided Surgery
4.3. Mitigation of Intensive Microscope Illumination Effects
4.4. Overcoming Light Blockage by Blood at the Sensor Tip
4.5. Time Resolution and System Responsiveness
4.6. Application to Alternative Fluorescent Labeling Agents
4.7. Towards Sensor-Controlled Robotic Surgical Systems
4.8. Standalone Use and Integration with Surgical Navigation Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDD | Photodynamic diagnosis |
| 5-ALA | 5-aminolevulinic acid |
| PpIX | protoporphyrin IX |
| LED | Light Emitting Diode |
References
- Kriegmair, M.; Baumgartner, R.; Knüchel, R.; Stepp, H.; Hofstädter, F.; Hofstetter, A. Detection of early bladder cancer by 5-aminolevulinic acid induced porphyrin fluorescence. J. Urol. 1996, 155, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zaak, D.; Sroka, R.; Khoder, W.; Adam, C.; Tritschler, S.; Karl, A.; Reich, O.; Knuechel, R.; Baumgartner, R.; Tilki, D.; et al. Photodynamic diagnosis of prostate cancer using 5-aminolevulinic acid—First clinical experiences. Urology 2008, 72, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.M.; Gamarra, F.; Hautmann, H.; Häußinger, K.; Wagner, S.; Castro, M.; Baumgartner, R. 5-Aminolaevulinic acid (ALA) for the fluorescence detection of bronchial tumors. Diagn. Ther. Endosc. 1999, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, Y.; Kuroiwa, T.; Miyatake, S.; Ichioka, T.; Miyashita, M.; Tanaka, H.; Tsuji, M. Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence: Case report. J. Neurosurg. 2007, 106, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.; Kuroiwa, T.; Kajimoto, Y.; Miyashita, M.; Tanaka, H.; Tsuji, M. Fluorescence of non-neoplastic, magnetic resonance imaging-enhancing tissue by 5-aminolevulinic acid: Case report. Neurosurgery 2007, 61, E1101–E1104. [Google Scholar] [CrossRef] [PubMed]
- Pichlmeier, U.; Bink, A.; Schackert, G.; Stummer, W. Resection and survival in glioblastoma multiforme: An RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008, 10, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid–induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomized controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Stocker, S.; Wagner, S.; Stepp, H.; Fritsch, C.; Goetz, C.; Goetz, A.E.; Kiefmann, R.; Reulen, H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, Y.; Miyatake, S.; Kuroiwa, T. Fiber-optic spectroscopic detection of neoplasm by intraoperative fluorescence labeling. Int. Congr. Ser. 2004, 1259, 33–38. [Google Scholar] [CrossRef]
- Brebner, J.T.; Welford, A.T. Introduction: An historical background sketch. In Reaction Times; Welford, A.T., Ed.; Academic Press: New York, NY, USA, 1980; pp. 1–23. [Google Scholar]
- Jung, M.; Morel, P.; Buehler, L.; Buchs, N.C.; Hagen, M.E. Robotic general surgery: Current practice, evidence, and perspective. Langenbeck’s Arch. Surg. 2015, 400, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.S.; Armijo, P.R.; Krause, C.; Choudhury, S.A.; Oleynikov, D. Review of emerging surgical robotic technology. Surg. Endosc. 2018, 32, 1636–1655. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajimoto, Y.; Ota, H.; Kameda, M.; Nonoguchi, N.; Furuse, M.; Kawabata, S.; Kuroiwa, T.; Takami, T.; Wanibuchi, M. Intelligent Ultrasonic Aspirator Controlled by Fiber-Optic Neoplasm Sensor Detecting 5-Aminolevulinic Acid-Derived Porphyrin Fluorescence. Sensors 2025, 25, 3412. https://doi.org/10.3390/s25113412
Kajimoto Y, Ota H, Kameda M, Nonoguchi N, Furuse M, Kawabata S, Kuroiwa T, Takami T, Wanibuchi M. Intelligent Ultrasonic Aspirator Controlled by Fiber-Optic Neoplasm Sensor Detecting 5-Aminolevulinic Acid-Derived Porphyrin Fluorescence. Sensors. 2025; 25(11):3412. https://doi.org/10.3390/s25113412
Chicago/Turabian StyleKajimoto, Yoshinaga, Hidefumi Ota, Masahiro Kameda, Naosuke Nonoguchi, Motomasa Furuse, Shinji Kawabata, Toshihiko Kuroiwa, Toshihiro Takami, and Masahiko Wanibuchi. 2025. "Intelligent Ultrasonic Aspirator Controlled by Fiber-Optic Neoplasm Sensor Detecting 5-Aminolevulinic Acid-Derived Porphyrin Fluorescence" Sensors 25, no. 11: 3412. https://doi.org/10.3390/s25113412
APA StyleKajimoto, Y., Ota, H., Kameda, M., Nonoguchi, N., Furuse, M., Kawabata, S., Kuroiwa, T., Takami, T., & Wanibuchi, M. (2025). Intelligent Ultrasonic Aspirator Controlled by Fiber-Optic Neoplasm Sensor Detecting 5-Aminolevulinic Acid-Derived Porphyrin Fluorescence. Sensors, 25(11), 3412. https://doi.org/10.3390/s25113412






