Development of an E-Nose System for the Early Diagnosis of Sepsis During Mechanical Ventilation: A Porcine Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Population and Treatment
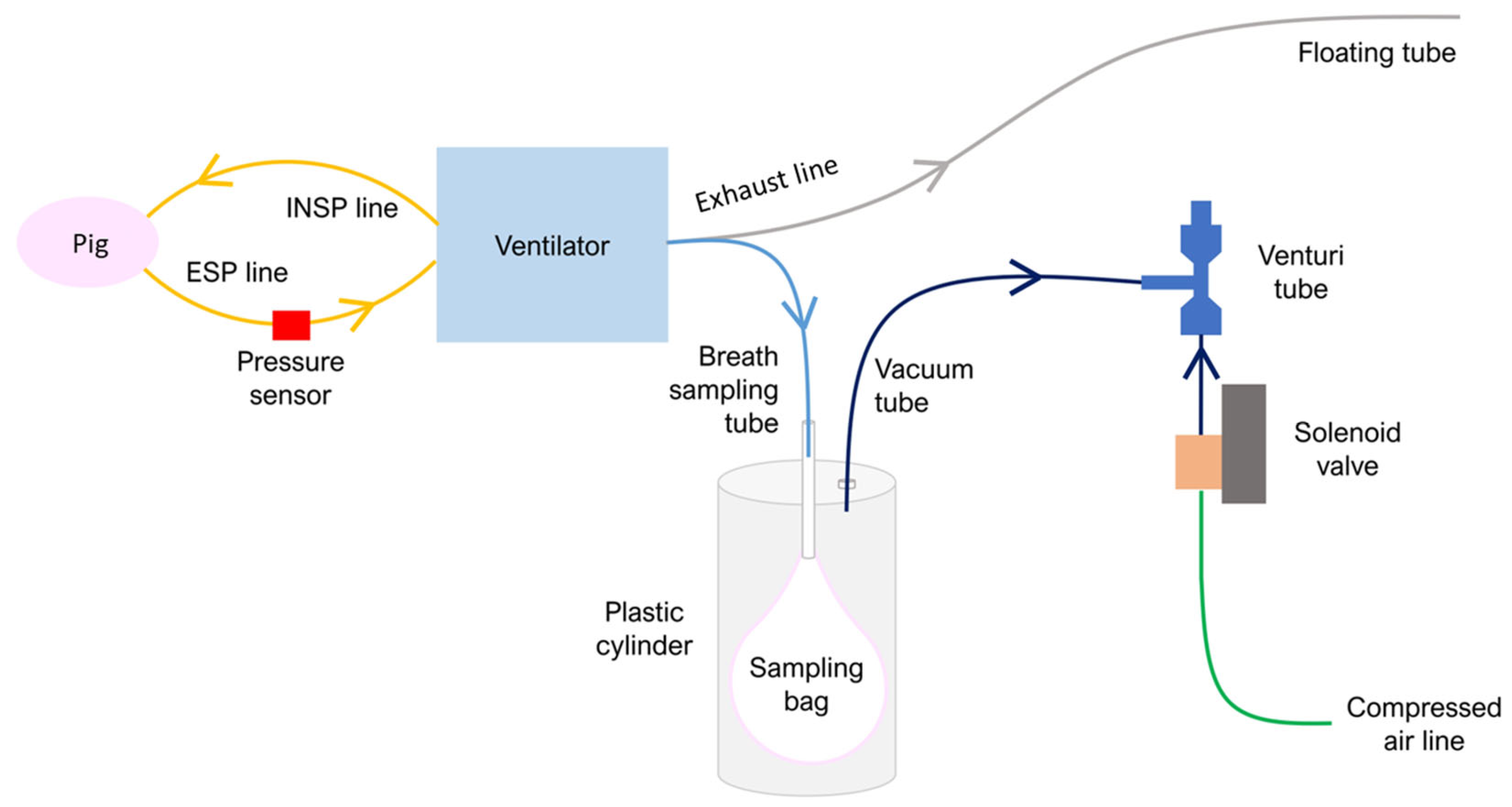
2.2. Exhaled Breath Sampling Protocol
- Circulation: MAP, diastolic blood pressure (DBP), systolic blood pressure (SBP), HR, and T.
- Ventilation: FiO2, PEEP, and peak inspiratory pressure (PIP).
2.3. Exhaled Breath Collection System
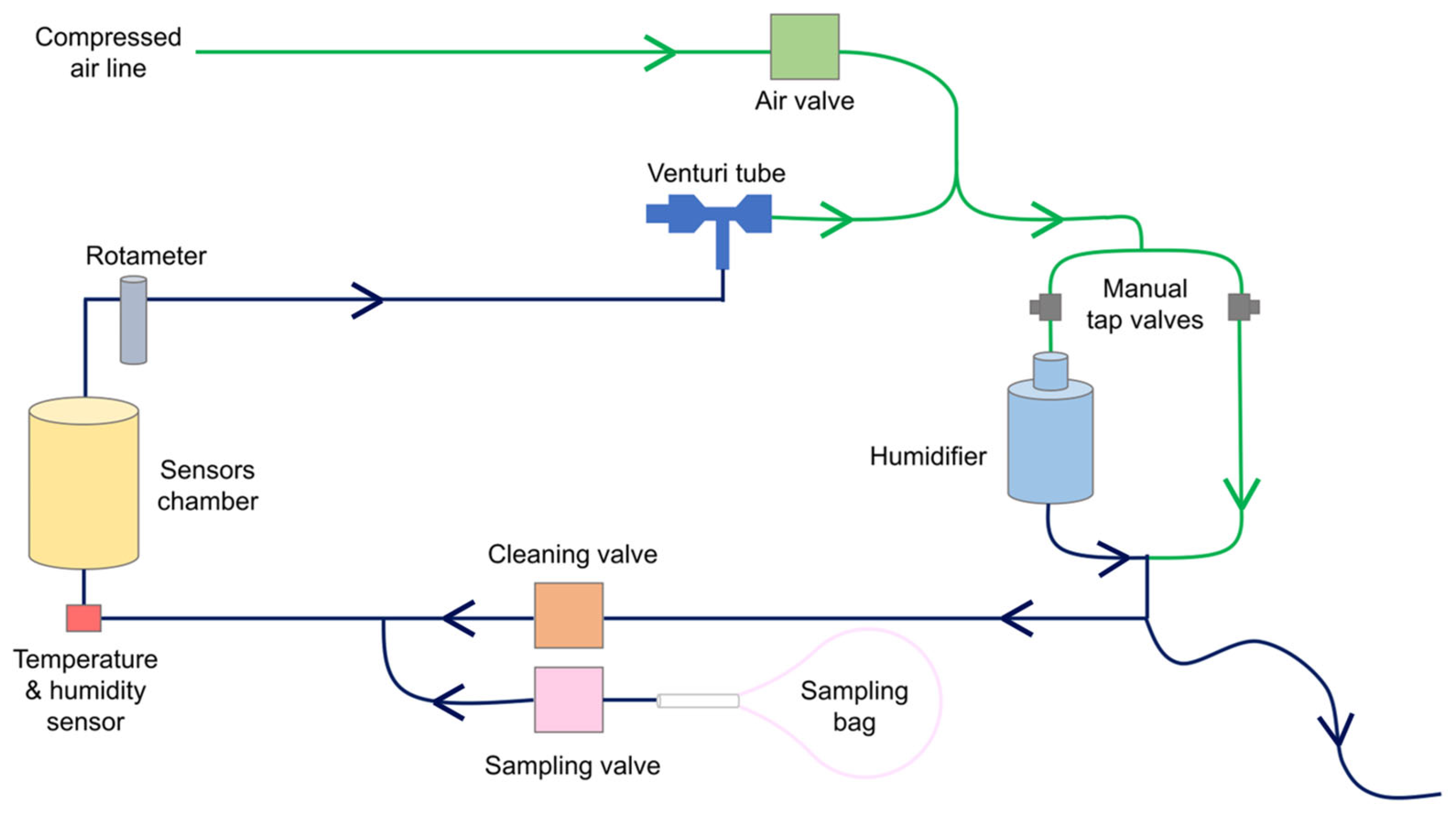
2.4. E-Nose Prototype for Exhaled Breath Analysis
- A temperature and humidity sensor (SHT40, Sensirion AG, Stäfa, Switzerland) positioned at the inlet of the sensors chamber;
- A rotameter (MR3A14BVBN, Key Instruments, Croydon, PA, USA) placed after the sensors chamber to adjust the sampling flow rate in the sensors chamber;
- A Venturi tube (VN-10-H-T3-PQ2-VQ2-RO1 vacuum generator, Festo, Esslingen am Neckar, Germany) connected to the rotameter, used as a negative pressure source to drive the sample flow into the system;
- An electro valve (2V025-08, Heschen, Foshan, China) to control compressed air entering the system;
- Two electro valves (2V025-08, Heschen, Foshan, China), used as “cleaning valve” and “sampling valve”, respectively. The cleaning valve is open before and after sample analysis to have clean air from the compressed air line flowing into the sensors’ chamber. The sampling valve opens during the sample analysis phase (called the “during” phase) to let the exhaled breath flow into the sensors’ chamber;
- A humidification circuit for humidifying the reference air and reducing the humidity difference between the sample and reference gases as the air coming from the compressed air line is dry. Water is a strong competitor of VCs in MOS surface reactions, and maintaining identical humidity levels in all experiments increases measurement reproducibility [32]. The humidification system comprises two manually operable tap valves and a water tank filled with up to 300 mL of distilled water. The compressed air was divided into two branches by adjusting the valves. The air flowing into the water tank branch was humidified and then mixed with the air coming from the second branch. The humidity of the mixed air was, therefore, set by adjusting the opening ratio of the two tap valves. A long tube open to the atmosphere was connected to the exit of the humidification circuit. The tube was used for three reasons: (1) to allow air to exit if it entered the humidification circuit with a flow higher than 1 L/min, (2) to let air flow into the humidification circuit when the cleaning valve was closed, avoiding a non-expected increase in humidity, and (3) to prevent the use of ambient air as a reference during the cleaning phase. The performance of the humidification system was evaluated by computing the humidity difference between reference air and the sample.
- “before” (1 min): the baseline response of the MOS gas sensors is recorded while flushing clean, humidified air into the sensors’ chamber.
- “during” (5 min): after opening of the sampling valve, exhaled breath is drawn from the sampling bag into the sensors’ chamber, leading to the adsorption of VCs on the MOS surfaces and to a consequent change in their resistance.
- “after” (10 min): clean air is circulated again inside the sensors’ chamber to recover to baseline conditions.
2.5. Classification Models
2.5.1. Physiological Data Classification Model
2.5.2. E-Nose Data Classification Model
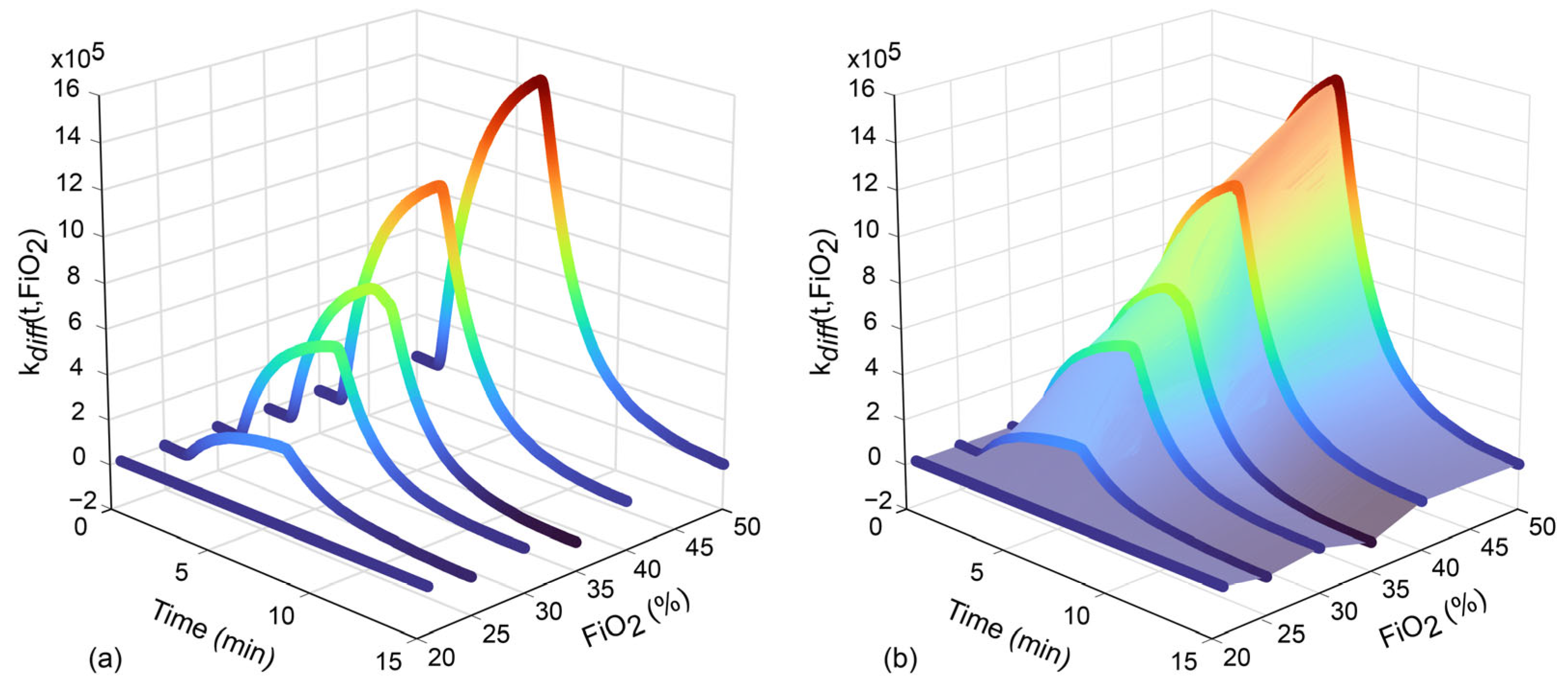
FiO2 Correction
Classification
3. Results
3.1. Exhaled Breath Collection System
3.2. E-Nose Prototype for Exhaled Breath Analysis
3.3. Animal Experiments
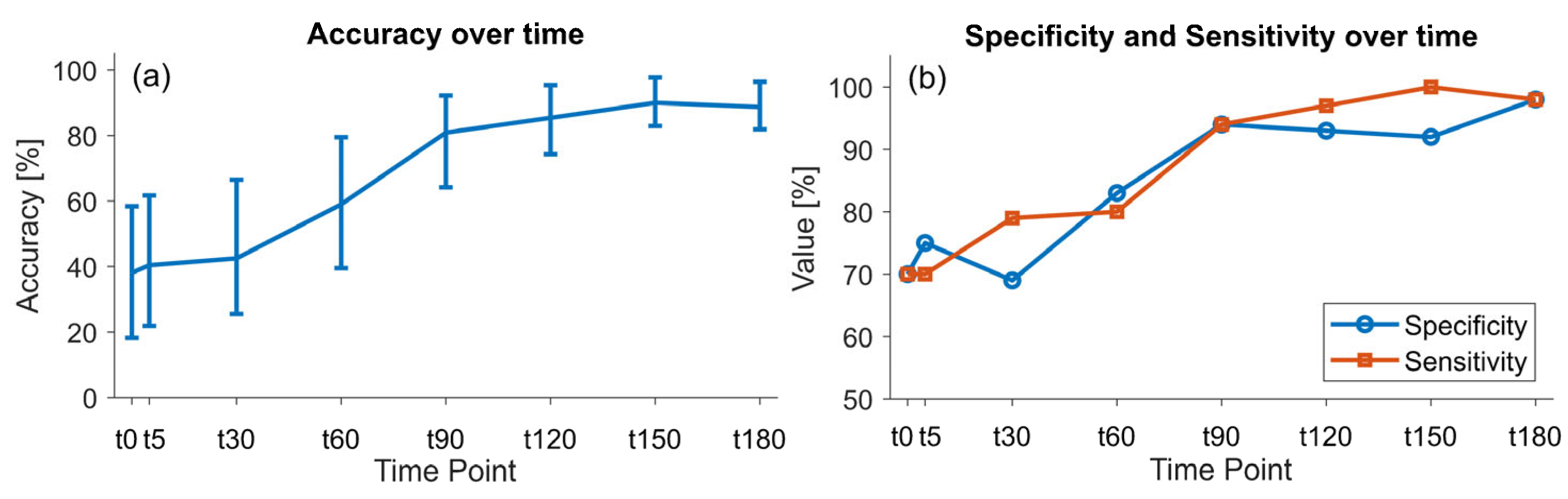
3.4. Physiological Data Classification Model
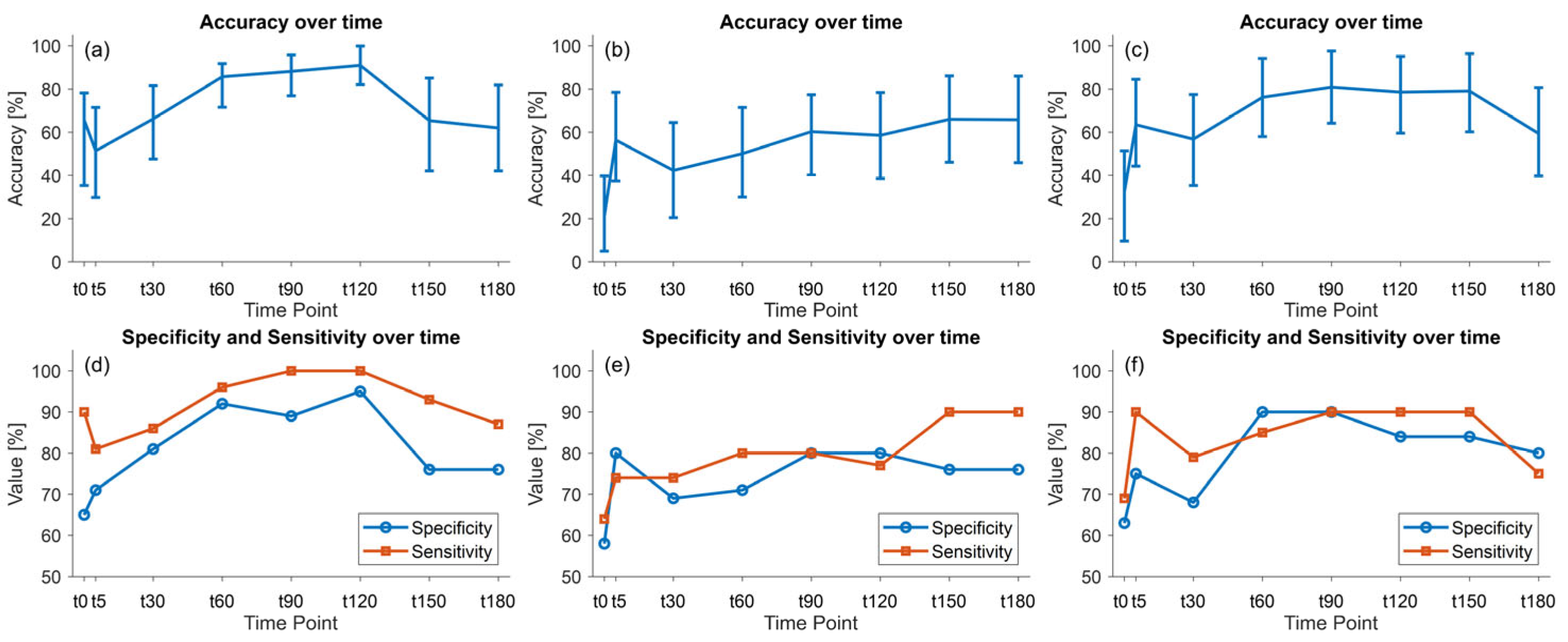
3.5. E-Nose Data Classification Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Bennet, S.R. Sepsis in the Intensive Care Unit. Surgery 2015, 33, 565–571. [Google Scholar]
- Fleischmann-Struzek, C.; Rudd, K. Challenges of Assessing the Burden of Sepsis. Med. Klin. Intensivmed. Notfmed. 2023, 118, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kataria, Y.; Remick, D. Sepsis Biomarkers. In Sepsis—Methods in Molecular Biology; Humana: New York, NY, USA, 2021; pp. 177–189. [Google Scholar]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.-L. Biomarkers of Sepsis: Time for a Reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Faix, J.D. Biomarkers of Sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef]
- Covington, J.A.; Marco, S.; Persaud, K.C.; Schiffman, S.S.; Nagle, H.T. Artificial Olfaction in the 21stCentury. IEEE Sens. J. 2021, 21, 12969–12990. [Google Scholar] [CrossRef]
- Farraia, M.V.; Cavaleiro Rufo, J.; Paciência, I.; Mendes, F.; Delgado, L.; Moreira, A. The Electronic Nose Technology in Clinical Diagnosis: A Systematic Review. Porto Biomed. J. 2019, 4, e42. [Google Scholar] [CrossRef]
- Vadala, R.; Pattnaik, B.; Bangaru, S.; Rai, D.; Tak, J.; Kashyap, S.; Verma, U.; Yadav, G.; Dhaliwal, R.; Mittal, S.; et al. A Review on Electronic Nose for Diagnosis and Monitoring Treatment Response in Lung Cancer. J. Breath Res. 2023, 17, 024002. [Google Scholar] [CrossRef]
- Turner, A.P.F.; Magan, N. Electronic Noses and Disease Diagnostics. Nat. Rev. Microbiol. 2004, 2, 161–166. [Google Scholar] [CrossRef]
- van der Sar, I.G.; Wijbenga, N.; Nakshbandi, G.; Aerts, J.G.J.V.; Manintveld, O.C.; Wijsenbeek, M.S.; Hellemons, M.E.; Moor, C.C. The Smell of Lung Disease: A Review of the Current Status of Electronic Nose Technology. Respir. Res. 2021, 22, 246. [Google Scholar] [CrossRef]
- Swanson, B.; Fogg, L.; Julion, W.; Arrieta, M.T. Electronic Nose Analysis of Exhaled Breath Volatiles to Identify Lung Cancer Cases: A Systematic Review. J. Assoc. Nurses AIDS Care 2020, 31, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Mores, N.; Trové, A.; Mondino, C.; Barnes, P.J. The Electronic Nose in Respiratory Medicine. Respiration 2012, 85, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Wolf, A.; Maurer, F.; Albrecht, F.W.; Heim, N.; Wolf, B.; Hauschild, A.C.; Bödeker, B.; Baumbach, J.I.; Volk, T.; et al. Volatile Organic Compounds during Inflammation and Sepsis in Rats. Anesthesiology 2015, 122, 117–126. [Google Scholar] [CrossRef] [PubMed]
- de Kroon, R.R.; Frerichs, N.M.; Struys, E.A.; de Boer, N.K.; de Meij, T.G.J.; Niemarkt, H.J. The Potential of Fecal Volatile Organic Compound Analysis for the Early Diagnosis of Late-Onset Sepsis in Preterm Infants: A Narrative Review. Sensors 2024, 24, 3162. [Google Scholar] [CrossRef]
- Othman Kombo, K.; Nur Hidayat, S.; Puspita, M.; Kusumaatmaja, A.; Roto, R.; Nirwati, H.; Susilowati, R.; Lutfia Haksari, E.; Wibowo, T.; Wandita, S.; et al. A Machine Learning-Based Electronic Nose for Detecting Neonatal Sepsis: Analysis of Volatile Organic Compound Biomarkers in Fecal Samples. Clin. Chim. Acta 2025, 565, 119974. [Google Scholar] [CrossRef]
- Berkhout, D.J.C.; Niemarkt, H.J.; Buijck, M.; van Weissenbruch, M.M.; Brinkman, P.; Benninga, M.A.; van Kaam, A.H.; Kramer, B.W.; Andriessen, P.; de Boer, N.K.H.; et al. Detection of Sepsis in Preterm Infants by Fecal Volatile Organic Compounds Analysis. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e47–e52. [Google Scholar] [CrossRef]
- Rogosch, T.; Herrmann, N.; Maier, R.F.; Domann, E.; Hattesohl, A.; Koczulla, A.R.; Zemlin, M. Detection of Bloodstream Infections and Prediction of Bronchopulmonary Dysplasia in Preterm Neonates with an Electronic Nose. J. Pediatr. 2014, 165, 622–624. [Google Scholar] [CrossRef]
- van der Aart, T.J.; Visser, M.; van Londen, M.; van de Wetering, K.M.H.; Ter Maaten, J.C.; Bouma, H.R. The Smell of Sepsis: Electronic Nose Measurements Improve Early Recognition of Sepsis in the ED. Am. J. Emerg. Med. 2025, 88, 126–133. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S. Electronic-Nose: A Non-Invasive Technology for Breath Analysis of Diabetes and Lung Cancer Patients. J. Breath Res. 2018, 13, 024001. [Google Scholar] [CrossRef]
- Bikov, A.; Pako, J.; Kovacs, D.; Tamasi, L.; Lazar, Z.; Rigo, J.; Losonczy, G.; Horvath, I. Exhaled Breath Volatile Alterations in Pregnancy Assessed with Electronic Nose. Biomarkers 2011, 16, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Lázár, Z.; Horvath, I. Established Methodological Issues in Electronic Nose Research: How Far Are We from Using These Instruments in Clinical Settings of Breath Analysis? J. Breath Res. 2015, 9, 34001. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.; Ahmed, W.M.; Nijsen, T.M.E.; Goodacre, R.; Fowler, S.J. Exhaled Breath Analysis: A Review of ‘Breath-Taking’ Methods for off-Line Analysis. Metabolomics 2017, 13, 110. [Google Scholar] [CrossRef]
- van Oort, P.M.P.; White, I.R.; Ahmed, W.; Johnson, C.; Bannard-Smith, J.; Felton, T.; Bos, L.D.; Goodacre, R.; Dark, P.; Fowler, S.J. Detection and Quantification of Exhaled Volatile Organic Compounds in Mechanically Ventilated Patients—Comparison of Two Sampling Methods. Analyst 2021, 146, 222–231. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, W.-C.; Yang, H.-Y. Diagnosis of Ventilator-Associated Pneumonia Using Electronic Nose Sensor Array Signals: Solutions to Improve the Application of Machine Learning in Respiratory Research. Respir. Res. 2020, 21, 45. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Shih, C.-H.; Abbod, M.F.; Shieh, J.-S.; Hsiao, Y.-J. Development of an E-Nose System Using Machine Learning Methods to Predict Ventilator-Associated Pneumonia. Microsyst. Technol. 2022, 28, 341–351. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.-N.; Vincent, J.-L. Sepsis in Intensive Care Unit Patients: Worldwide Data from the Intensive Care over Nations Audit. Open Forum Infect Dis 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Dhital, R.; Basnet, S.; Poudel, D.R. Predictors and Outcome of Invasive Mechanical Ventilation in Hospitalized Patients with Sepsis: Data from National Inpatient Sample. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 49–52. [Google Scholar] [CrossRef]
- te Nijenhuis, L.H.; Gangaram-Panday, N.H.; Specht, P.A.C.; Fine, I.; Elstein, N.; Mik, E.G.; Harms, F.A.; Reiss, I.K.M.; van Weteringen, W. Detection of Hemodynamic Changes in a Porcine Lipopolysaccharide Model of Systemic Inflammation Using Dynamic Light Scattering Measurements of the Microcirculation. Front. Med. 2025, 12. [Google Scholar] [CrossRef]
- Bax, C.; Robbiani, S.; Zannin, E.; Capelli, L.; Ratti, C.; Bonetti, S.; Novelli, L.; Raimondi, F.; Di Marco, F.; Dellacà, R.L. An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients. Diagnostics 2022, 12, 776. [Google Scholar] [CrossRef]
- Robbiani, S.; Lotesoriere, B.J.; Dellacà, R.L.; Capelli, L. Physical Confounding Factors Affecting Gas Sensors Response: A Review on Effects and Compensation Strategies for Electronic Nose Applications. Chemosensors 2023, 11, 514. [Google Scholar] [CrossRef]
- Robbiani, S.; Bax, C.; Albertazzi, J.; Pappolla, M.; Busini, V.; Apelli, L.; Dellaca, R. A Bench Test System for Developing E-Nose Diagnostic Tools with Exhaled Breath Sampling. In Proceedings of the ISOCS/IEEE International Symposium on Olfaction and Electronic Nose (ISOEN 2022), Aveiro, Portugal, 29 May–1 June 2022; Proceedings. IEEE: Piscataway, NJ, USA, 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Patro, S.G.K.; Sahu, K.K. Leave-One-Out Cross-Validation. In Encyclopedia of Machine Learning; Springer: Boston, MA, USA, 2011; pp. 600–601. [Google Scholar]
- Patro, S.G.K.; Sahu, K.K. Normalization: A Preprocessing Stage. arXiv 2015, arXiv:1503.06462. [Google Scholar] [CrossRef]
- Sun, M. Support Vector Machine Models for Classification. In Encyclopedia of Business Analytics and Optimization; IGI Global: Hershey, PA, USA, 2014; pp. 2395–2409. [Google Scholar]
- Staerz, A.; Weimar, U.; Barsan, N. Current State of Knowledge on the Metal Oxide Based Gas Sensing Mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Abdullah, A.N.; Kamarudin, K.; Kamarudin, L.M.; Adom, A.H.; Mamduh, S.M.; Juffry, Z.H.M.; Bennetts, V.H. Correction Model for Metal Oxide Sensor Drift Caused by Ambient Temperature and Humidity. Sensors 2022, 22, 3301. [Google Scholar] [CrossRef]
- Ionescu, R.; Vancu, A.; Tomescu, A. Time-Dependent Humidity Calibration for Drift Corrections in Electronic Noses Equipped with SnO2 Gas Sensors. Sens. Actuators B Chem. 2000, 69, 283–286. [Google Scholar] [CrossRef]
- Marco, S.; Gutierrez-Galvez, A. Signal and Data Processing for Machine Olfaction and Chemical Sensing: A Review. IEEE Sens. J. 2012, 12, 3189–3214. [Google Scholar] [CrossRef]
- Du, C.-J.; Sun, D.-W. Object Classification Methods. In Computer Vision Technology for Food Quality Evaluation; Elsevier: Amsterdam, The Netherlands, 2008; pp. 81–107. [Google Scholar]
- Koenig, S.M.; Truwit, J.D. Ventilator-Associated Pneumonia: Diagnosis, Treatment, and Prevention. Clin. Microbiol. Rev. 2006, 19, 637–657. [Google Scholar] [CrossRef]
- Fink, M.P.; Heard, S.O. Laboratory Models of Sepsis and Septic Shock. J. Surg. Res. 1990, 49, 186–196. [Google Scholar] [CrossRef]
- Poli-de-Figueiredo, L.F.; Garrido, A.G.; Nakagawa, N.; Sannomiya, P. Experimental models of sepsis and their clinical relevance. Shock 2008, 30, 53–59. [Google Scholar] [CrossRef]
- Akca, O.; Ball, L.; Belda, F.J.; Biro, P.; Cortegiani, A.; Eden, A.; Ferrando, C.; Gattinoni, L.; Goldik, Z.; Gregoretti, C.; et al. WHO Needs High FIO2? Turk. J. Anesth. Reanim. 2017, 45, 181–192. [Google Scholar] [CrossRef]

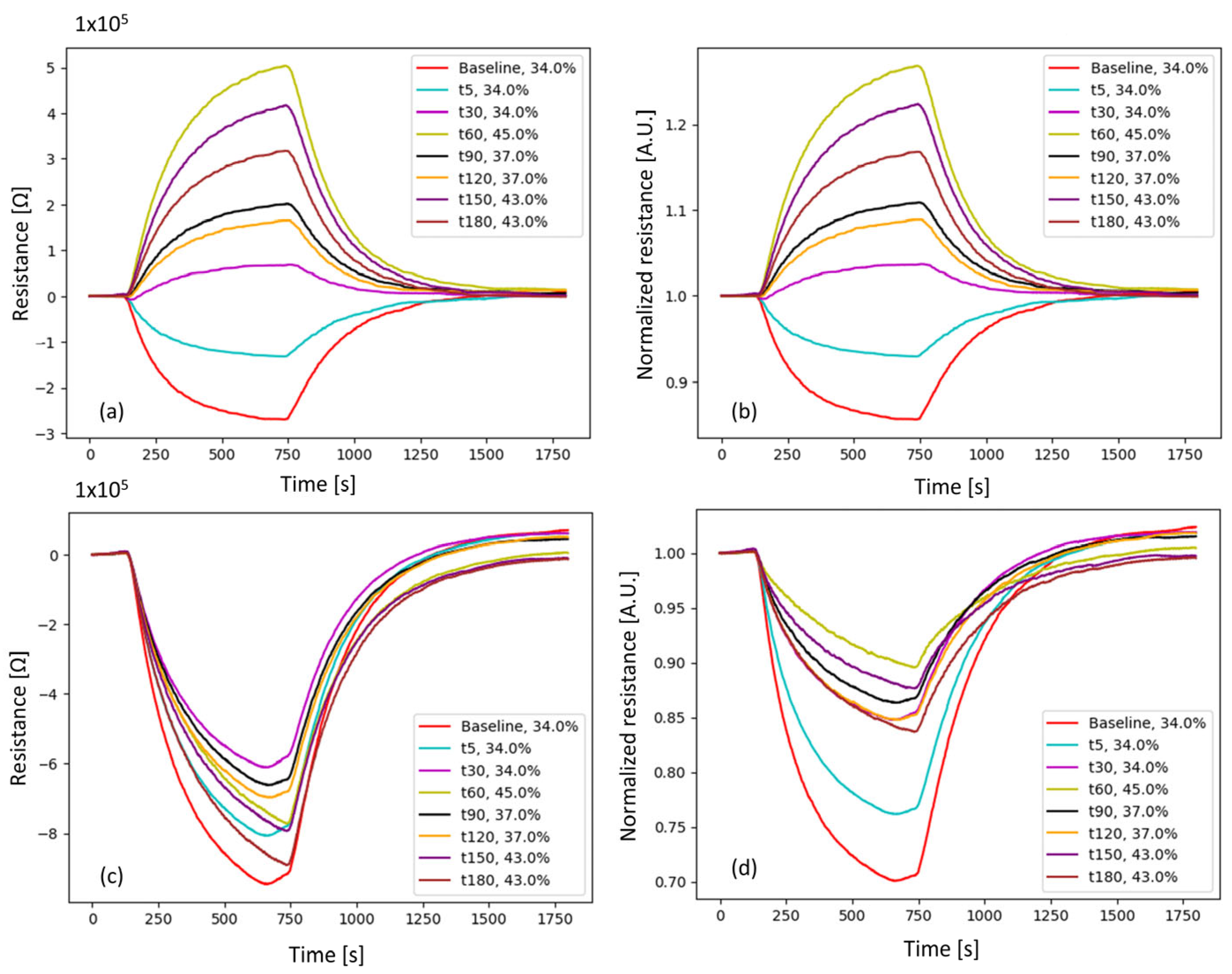
| Strategy 1: kdiff(t,FiO2) Correction | Strategy 2: kratio(t,FiO2) Correction | |
|---|---|---|
| Mean MOS sensor response curve at specific FiO2 series | ||
| Bivariate correction function | ||
| Samples’ curves after baseline removal | ||
| FiO2-corrected samples’ curves |
| Accuracy (%) | 95% Confidence Interval | |
|---|---|---|
| Physiological data | 59.0 | [39.5, 79.5] |
| Raw e-Nose data | 85.7 | [71.6, 91.8] |
| e-Nose data with kdiff(t,FiO2) correction | 48.1 | [27.0, 69.2] |
| e-Nose data with kratio(t,FiO2) correction | 76.2 | [58.0, 94.2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robbiani, S.; te Nijenhuis, L.H.; Specht, P.A.C.; Zanni, E.; Bax, C.; Mik, E.G.; Harms, F.A.; Weteringen, W.v.; Capelli, L.; Dellacà, R.L. Development of an E-Nose System for the Early Diagnosis of Sepsis During Mechanical Ventilation: A Porcine Feasibility Study. Sensors 2025, 25, 3343. https://doi.org/10.3390/s25113343
Robbiani S, te Nijenhuis LH, Specht PAC, Zanni E, Bax C, Mik EG, Harms FA, Weteringen Wv, Capelli L, Dellacà RL. Development of an E-Nose System for the Early Diagnosis of Sepsis During Mechanical Ventilation: A Porcine Feasibility Study. Sensors. 2025; 25(11):3343. https://doi.org/10.3390/s25113343
Chicago/Turabian StyleRobbiani, Stefano, Louwrina H. te Nijenhuis, Patricia A. C. Specht, Emanuele Zanni, Carmen Bax, Egbert G. Mik, Floor A. Harms, Willem van Weteringen, Laura Capelli, and Raffaele L. Dellacà. 2025. "Development of an E-Nose System for the Early Diagnosis of Sepsis During Mechanical Ventilation: A Porcine Feasibility Study" Sensors 25, no. 11: 3343. https://doi.org/10.3390/s25113343
APA StyleRobbiani, S., te Nijenhuis, L. H., Specht, P. A. C., Zanni, E., Bax, C., Mik, E. G., Harms, F. A., Weteringen, W. v., Capelli, L., & Dellacà, R. L. (2025). Development of an E-Nose System for the Early Diagnosis of Sepsis During Mechanical Ventilation: A Porcine Feasibility Study. Sensors, 25(11), 3343. https://doi.org/10.3390/s25113343










