Abstract
This article presents an integrated strategy that couples high-efficiency photocatalytic degradation with low-cost, rapid detection to overcome the main drawbacks of conventional TiO2-based photocatalysts, including a weak visible-light response, rapid charge–carrier recombination, and reliance on expensive instrumentation for dye concentration detection. Platinum-decorated TiO2 (Pt/TiO2) was prepared by photoreduction deposition, and systematic characterization confirmed the successful loading of zero-valent Pt nanoparticles onto the TiO2 surface, significantly improving charge separation and extending absorption into the visible region. Methylene blue degradation was quantified under ultraviolet (UV) and simulated sunlight; radical-scavenging tests clarified the reaction pathway. In parallel, smartphone images of the reaction mixture were processed in ImageJto extract red–green–blue (RGB) values, which were related to dye concentration through a partial least-squares (PLS) model validated against reference UV–Vis data. Pt/TiO2 removed 95.0% of methylene blue within 20 min of UV irradiation and 90.2% within 160 min of simulated sunlight—31.8% and 19.1% faster, respectively, than pristine TiO2. The RGB-based PLS model achieved a coefficient of determination (R2) of 0.961 for the prediction set. By integrating photocatalysis with smartphone-based colorimetry, the proposed method enables rapid monitoring of organic dyes concentration, providing an intelligent and economical platform for industrial wastewater treatment.
1. Introduction
Industrial dye effluents represent a growing environmental and public-health concern, because many organic dyes are toxic to aquatic organisms and humans even at trace levels [1,2]. Consequently, the development of efficient, rapid, and accurate methods for both dye degradation and concentration monitoring have become a key research focus in the field of environmental management.
Photocatalytic degradation technology has garnered widespread attention due to its high efficiency, environmental friendliness, and low energy consumption [1,3]. Among various photocatalysts, titanium dioxide (TiO2) is extensively applied in dye wastewater treatment owing to its chemical stability, low toxicity, and cost-effectiveness [4]. However, TiO2 absorbs only ultraviolet (UV) light, and the fast recombination of photogenerated electrons and holes significantly limits its photocatalytic efficiency [5]. Noble-metal deposition—particularly with platinum (Pt) [6], gold (Au) [7], or silver (Ag) [8]—has therefore been used to enhance charge separation and extend the optical response into the visible region. Among these options, Pt/TiO2 exhibits the greatest performance gain, because metallic Pt effectively captures photogenerated electrons, preventing electron–hole recombination and thereby enhancing catalytic efficiency [6,9]. Moreover, metallic Pt nanoparticles can induce localized surface plasmon resonance (LSPR) effects under visible-light irradiation, which significantly enhances visible-light absorption and consequently promotes photocatalytic activity, thereby further broadening the applicability of photocatalytic technology [6].
Although photocatalysis efficiently removes dyes, it does not directly provide real-time monitoring or quantitative analysis of dye concentrations. Conventional analytical techniques such as UV–Vis spectrophotometry or high-performance liquid chromatography (HPLC) are precise, but require costly, laboratory-based instrumentation that is unsuitable for on-site or high-throughput monitoring. Integrating photocatalytic degradation with a simple and low-cost sensing strategy would therefore enable rapid and accurate monitoring of organic dye concentrations, providing timely and reliable data support for industrial process optimization and emission management.
Smartphone colorimetry—based on red, green, and blue (RGB) channel analysis coupled with a partial least squares (PLS) regression model—has recently emerged as a promising tool for quantitative chemical sensing [10,11,12]. By extracting RGB intensities from a single image, dye concentration can be predicted rapidly without specialized equipment, making the technique attractive for field applications.
The present study combined Pt/TiO2 photocatalysis with an RGB-based PLS detection scheme to achieve rapid methylene blue (MB) concentration monitoring. A Pt/TiO2 photocatalyst was synthesized by photoreduction and systematically characterized using X-ray diffraction (XRD), UV–Vis diffuse reflectance spectroscopy (UV–Vis DRS), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). Photocatalytic degradation kinetics were determined under UV and simulated sunlight, while RGB data acquired with a smartphone were correlated with MB concentration through a calibrated PLS model validated against UV–Vis measurements. This innovation—high-efficiency Pt/TiO2 photocatalysis combined with instrument-free RGB colorimetry—provides a rapid, low-cost platform for dye-wastewater treatment and rapid quantitative monitoring.
2. Materials and Methods
2.1. Reagents
TiO2 nanopowder and MB were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China); chloroplatinic acid, isopropanol, triethanolamine, and anhydrous ethanol were obtained from Guangfu Technology Development Co., Ltd. (Tianjin, China).
2.2. Preparation and Characterization of Pt/TiO2 Photocatalyst
2.2.1. Preparation of Pt/TiO2 Photocatalyst
The Pt/TiO2 photocatalyst was synthesized using the photoreduction deposition method [13]. Considering both cost and environmental factors, the Pt loading was deliberately limited to 1.0 wt%. Briefly, 0.99 g of TiO2 powder and 0.01 g of chloroplatinic acid were dispersed in 100 mL of an ethanol–water solution (volume ratio 1:1). The resulting suspension was then stirred and irradiated under a 300 W mercury lamp for 4 h at room temperature. During irradiation, photogenerated electrons in the conduction band of TiO2 reduced Pt4+ ions to Pt0 nanoparticles, which subsequently deposited uniformly onto the TiO2 surface. After irradiation, the final product was washed thoroughly with deionized water, filtered to remove residual chloride ions (Cl−), and dried at 80 °C for 12 h to obtain the Pt/TiO2 photocatalyst.
2.2.2. Characterization of Pt/TiO2 Photocatalyst
In this study, multiple analytical techniques were employed to comprehensively characterize the Pt/TiO2 photocatalyst. The crystal structure was determined using XRD (D/MAX-2500, Rigaku, Tokyo, Japan), while optical response properties were evaluated via UV–Vis DRS (Lambda 750, PerkinElmer, Waltham, MA, USA). The elemental composition and chemical states were analyzed using XPS (Axis Ultra DLD, Kratos Analytical Ltd., Manchester, UK), with the binding energy of adsorbed carbon at 284.6 eV used as a reference for charge correction. The morphology and elemental distribution were examined using SEM (JSM-7800F, JEOL Ltd., Tokyo, Japan).
Photocatalytic degradation experiments were conducted using a photocatalytic reactor (XPA-7 series, Xujiang Electromechanical Plant, Nanjing, China). Images of MB solutions undergoing photocatalytic degradation were captured using a smartphone, and RGB values were extracted and processed using ImageJ software (1.52a).
2.3. Photocatalytic Performance and Mechanistic Investigation of Pt/TiO2 Photocatalyst
Photocatalytic degradation experiments were conducted using MB as the model pollutant to investigate the photocatalytic performance of Pt/TiO2. Irradiation was performed using either a 300 W mercury lamp (UV light) or a 500 W xenon lamp (simulated sunlight). Pure TiO2 nanopowder was employed as a reference material to evaluate the enhanced photocatalytic activity of the Pt/TiO2 photocatalyst. All photocatalytic experiments were conducted in triplicate to ensure reproducibility.
2.3.1. Photocatalytic Performance Testing
In a typical experiment, 50 mg of either pure TiO2 nanopowder or Pt/TiO2 photocatalyst was dispersed into 60 mL of a MB solution (54 μM). The resulting suspension was magnetically stirred in the dark for 30 min to establish adsorption–desorption equilibrium. After equilibration, 1.0 mL of the solution was collected, centrifuged, and the absorbance of the supernatant was measured spectrophotometrically to determine the initial concentration (C0). Subsequently, the light source (UV or simulated sunlight) was activated, and aliquots were withdrawn at predetermined time intervals. The absorbance of each collected sample was analyzed, and the corresponding MB concentration (C) was determined. The photocatalytic degradation efficiency was evaluated by plotting the relative concentration ratio (C/C0) against irradiation time (t).
The recycling performance of the Pt/TiO2 photocatalyst was investigated through five consecutive recycling tests under identical conditions (UV irradiation), and the photocatalyst was recovered after each cycle, washed, and reused in five consecutive degradation trials.
2.3.2. Mechanistic Investigation Testing
To identify the primary reactive species involved in the photocatalytic degradation process, radical trapping experiments were performed. Triethanolamine, isopropanol, and nitrogen gas (N2) were used as scavengers for photogenerated holes (h+), hydroxyl radicals (·OH), and superoxide radicals (·O2−), respectively. Experimental procedures for radical trapping were identical to those described in Section 2.3.1, with the respective scavengers introduced before initiating irradiation.
2.4. RGB-Based PLS Model for Quantitative Detection of Methylene Blue Concentration
Initially, a series of MB standard solutions with concentrations ranging from 0 to 54 μM (with 6 μM intervals) were prepared. Each standard solution was transferred onto a white ceramic well plate. To minimize variations due to lighting and camera positioning, stringent image acquisition protocols were adopted: the smartphone camera was fixed at a consistent distance and angle, ambient lighting was kept uniform, and a neutral background was used for all photographs.
Subsequently, RGB channel values were extracted from the captured images using ImageJ software. Extracted RGB data and corresponding MB concentrations were imported into the PLS toolbox in MATLAB (R2024a) to establish a quantitative calibration model correlating RGB values with MB concentrations. The predictive performance of the calibration model was assessed by cross-validation, determining key parameters such as the root mean square error of calibration (RMSEC), root mean square error of cross-validation (RMSECV), and correlation coefficient (R2).
Finally, images of MB solutions undergoing photocatalytic degradation were taken under identical imaging conditions, and RGB values were extracted. These RGB data were then input into the previously established PLS calibration model to predict MB concentrations. The concentrations predicted using the RGB-based PLS model were validated by comparing them against results obtained with a standard UV–Vis spectrophotometer.
3. Results and Discussion
3.1. SEM Analysis
Figure 1 displays SEM micrographs of the Pt/TiO2 photocatalyst. Aggregated nanoparticles were observed, and the individual Pt particles (approximately 3–5 nm) were too small to be resolved directly. Nevertheless, the successful loading of Pt nanoparticles onto the TiO2 nanopowder was confirmed through elemental mapping and UV–Vis DRS, and XPS data.
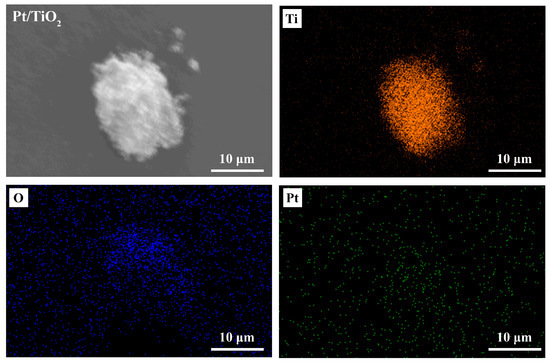
Figure 1.
SEM images and elemental mapping of Pt/TiO2 photocatalyst.
3.2. Structural and Optical Characterization (XRD, UV–Vis DRS, and XPS)
The crystal structure of the Pt/TiO2 photocatalyst was analyzed using XRD. The XRD image (Figure 2A) shows only reflections of anatase TiO2 (JCPDS No. 21-1272), with diffraction peaks at 25.28°, 37.82°, 48.12°, 53.94°, 55.18°, 62.82°, 68.86°, 70.36°, and 75.26°, corresponding to the (101), (004), (200), (105), (211), (204), (116), (220), and (215) crystal planes, respectively. These peaks align well with the standard reference card, indicating the high crystallinity of the synthesized photocatalyst. No additional peaks were detected, indicating that Pt deposition did not alter the host crystal phase and that the photocatalyst was phase-pure and highly crystalline.
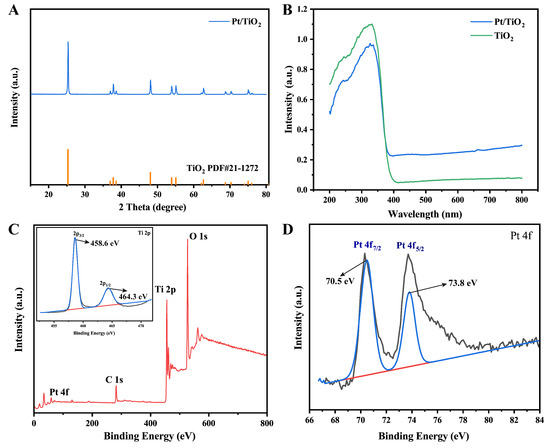
Figure 2.
(A) XRD spectra of Pt/TiO2 photocatalyst; (B) UV–Vis DRS spectra of TiO2 and Pt/TiO2 photocatalysts; (C) XPS wide-spectrum of Pt/TiO2 photocatalyst and corresponding narrow scan spectra of Ti 2p; and (D) corresponding XPS narrow scan spectra of Pt 4f.
Furthermore, XPS analysis was conducted to investigate the chemical state of Pt in the Pt/TiO2 photocatalyst. As shown in Figure 2C, wide-scan XPS spectra confirm the successful incorporation of Pt on the TiO2 surface, and no discernible shifts are observed in the Ti 2p region, suggesting that Ti4+ cations remained stable during photoreduction [14]. High-resolution Pt 4f spectra (Figure 2D) reveal Pt 4f7/2 (~70.5 eV) and Pt 4f5/2 (~73.8 eV) peaks characteristic of metallic Pt0. The narrow width of these peaks, combined with their alignment with reported reference values for metallic platinum, further verify the complete photoreduction of Pt4+ to Pt0 and the uniform dispersion on the support [15,16]. UV–Vis DRS (Figure 2B) further reveals that, compared to pure TiO2 (absorption edge at 400 nm, bandgap of 3.2 eV), Pt/TiO2 exhibited a significant redshift of the absorption edge and broad-spectrum absorption enhancement in the 500–800 nm visible light region. This broadening is attributed to the LSPR effect of Pt0 nanoparticles. Additionally, the XPS-confirmed metallic Pt0 chemical state provides direct electronic structural evidence supporting the LSPR-induced visible-light response mechanism.
Collectively, these characterization results indicate that Pt0 was homogeneously distributed on anatase TiO2, simultaneously improving charge–carrier separation and extending optical absorption via the LSPR effect. These features provide a solid basis for high-efficiency visible-light-driven photocatalysis.
3.3. Photocatalytic Performance and Mechanistic Investigation of Pt/TiO2
The photocatalytic performance of the Pt/TiO2 photocatalyst was evaluated using an MB solution as a target pollutant. Figure 3A shows the degradation profiles of the MB solution under UV irradiation in the presence of Pt/TiO2 and TiO2. The inset photograph clearly illustrates the progressive color change of the MB solution from dark blue to nearly colorless, visually confirming efficient dye removal. Specifically, Pt/TiO2 achieved a degradation efficiency of 95.0% within 20 min, markedly superior to the 63.2% observed for pure TiO2. Figure 3B shows the corresponding first-order kinetic plots (ln(C0/C) vs. time), confirming that MB degradation follows a first-order kinetic [17] as described by the Langmuir–Hinshelwood model:
where k represents the degradation rate constant. The calculated rate constants for Pt/TiO2 and TiO2 were 0.0655 min−1 and 0.0584 min−1, respectively, demonstrating that Pt/TiO2 significantly enhances photogenerated charge–carrier separation efficiency compared to unmodified TiO2.
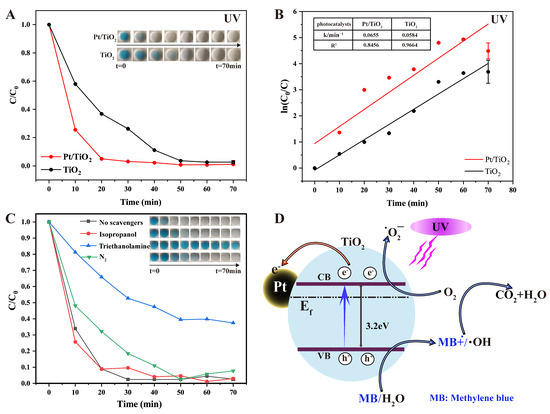
Figure 3.
(A) Photocatalytic degradation of MB by TiO2 and Pt/TiO2 under UV irradiation (inset images present photocatalytic degradation of MB solution at different times); (B) first-order kinetics of MB degradation under UV irradiation in TiO2 and Pt/TiO2 systems at different times (inset tables present rate constants and R2 values of all photocatalysts); (C) photocatalytic degradation of MB upon addition of various scavengers under UV irradiation in Pt/TiO2 system; and (D) proposed photocatalytic mechanism of Pt/TiO2 system under UV irradiation.
Radical trapping experiments were conducted to identify the primary reactive species involved in MB degradation, and results are presented in Figure 3C. The addition of triethanolamine (a scavenger of photogenerated holes, h+) significantly suppressed degradation efficiency, indicating that h+ was the predominant reactive species. Introducing nitrogen gas (an ·O2− scavenger) partially inhibited degradation, suggesting superoxide radicals (·O2−) served an auxiliary role. Conversely, adding isopropanol (a hydroxyl radical, ·OH, scavenger) had minimal effect, implying a minor role for ·OH radicals.
Based on these observations, a plausible photocatalytic mechanism is proposed in Figure 3D. Under UV irradiation, electrons from the valence band (VB) of TiO2 migrate to the conduction band (CB), generating electron–hole pairs. Photogenerated electrons subsequently react with adsorbed molecular oxygen to form reactive oxygen species, such as superoxide radicals (·O2−) and hydrogen peroxide (H2O2), facilitating MB degradation. Due to the high Schottky barrier at the Pt–TiO2 interface, electrons transfer efficiently to Pt nanoparticles, significantly suppressing electron–hole recombination and enhancing overall catalytic activity [18]. Simultaneously, photogenerated holes (h+) directly oxidize MB molecules, ultimately decomposing them into non-toxic products such as CO2 and H2O.
Reusability is crucial for practical wastewater treatment applications. Thus, the recycling performance of the Pt/TiO2 photocatalyst was examined over five consecutive degradation cycles under identical UV irradiation (Figure 4A,B). After each cycle, the catalyst was recovered, washed, and reused. Degradation efficiencies remained above 85% even after the fifth reuse, indicating excellent reusability of the Pt/TiO2 photocatalyst.
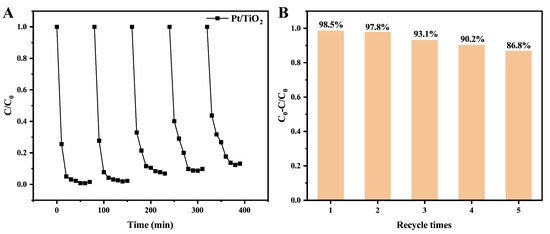
Figure 4.
Recyclability of Pt/TiO2 toward the degradation of MB under UV irradiation. (A) the variation of the ratio C/C0 with time during the UV-induced degradation of MB over Pt/TiO2; (B) the degradation efficiency of Pt/TiO2 for MB under UV irradiation after different recycle times.
Photocatalytic performance was further assessed under simulated sunlight conditions (Figure 5A). Pt/TiO2 achieved a degradation efficiency of 90.2% after 160 min, significantly higher than the 71.1% observed for pure TiO2. Additionally, kinetic analysis confirmed that the rate constant for Pt/TiO2 was approximately 1.9 times higher than that of TiO2, with excellent fitting quality (R2 = 0.9703 for Pt/TiO2 and R2 = 0.9775 for TiO2; Figure 5B). The enhanced photocatalytic efficiency under visible-light conditions is attributed primarily to the LSPR effect of metallic Pt nanoparticles, facilitating improved absorption and utilization of visible-light energy [19,20]. While pure TiO2 was the primary control in this study, Pt was specifically selected due to its established capacity to enhance electron–hole separation and broaden visible-light absorption through the LSPR effect. Although Au- and Ag-modified TiO2 also exhibit enhanced photocatalytic performance according to previous reports, the choice of Pt was guided by its superior catalytic stability and extensively documented efficiency in similar applications [21,22].
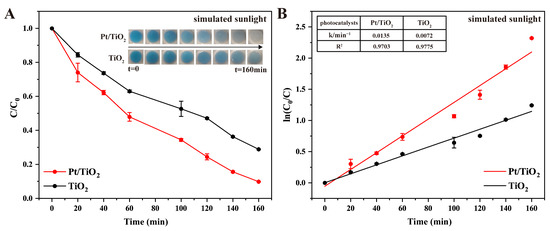
Figure 5.
(A) Photocatalytic degradation of MB by TiO2 and Pt/TiO2 under simulated sunlight irradiation (inset images present photocatalytic degradation of MB at different times), and (B) first-order kinetics of MB degradation under simulated sunlight irradiation in TiO2 and Pt/TiO2 systems at different times (inset table presents rate constants and R2 values of all photocatalysts).
3.4. Rapid Detection of Methylene Blue Concentration Using RGB-Based Sensing Model
Figure 6A presents the absorbance–concentration calibration curve obtained for MB solutions. Within the investigated concentration range, the absorbance of MB demonstrates an excellent linear correlation with its concentration. To enable rapid quantitative detection, an RGB-based PLS model was established using RGB channel values extracted from smartphone images of standard MB solutions. The optimal number of latent variables in the PLS model was determined by performing leave-one-out cross-validation and selecting the number corresponding to the minimum root mean square error of cross-validation (RMSECV).
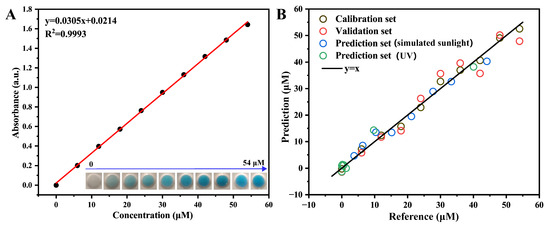
Figure 6.
(A) Absorbance–concentration standard curve of MB (inset photos present standard solutions of MB with concentrations ranging from 0 to 54 μM); and (B) RGB-based PLS model for rapid monitoring of MB concentrations.
The final PLS calibration model for predicting MB concentrations utilized three latent variables, yielding a root mean square error of calibration (RMSEC) of 2.513 and a correlation coefficient (Rc2) of 0.979. Cross-validation results provided an RMSECV of 3.860 and a correlation coefficient (Rcv2) of 0.951. Additionally, the residual predictive deviation (RPD) value of the model was 4.7 (RPD > 3), indicating excellent predictive capability.
To further verify the model’s predictive accuracy, an independent prediction dataset was employed and the model’s accuracy was evaluated using the root mean square error of prediction (RMSEP) and the prediction correlation coefficient (Rp2). As shown in Figure 6B, the model produced a root mean square error of prediction (RMSEP) of 3.736 and a correlation coefficient (Rp2) of 0.961, demonstrating robust reliability and strong linear correlation predictive performance.
Multiple measurements performed under uniform lighting conditions on MB solutions of identical concentrations produced minimal variation in RGB values, confirming excellent reproducibility of the image acquisition protocol and analytical procedure. As this sensing approach involves non-destructive imaging with only a smartphone camera and standard image-processing software (e.g., ImageJ), the RGB-based method can be reused indefinitely without substantial additional costs. Additionally, repeated analyses can be readily conducted by simply replacing the solution in a standard well plate, demonstrating practical suitability for continuous or repeated monitoring.
Compared to traditional UV–Vis spectrophotometric methods, the RGB-based PLS model significantly simplifies sample handling and reduces both material and labor costs. This methodology offers a convenient, low-cost, and instrument-free alternative for the rapid quantitative detection of organic dye concentrations. If further developed into a dedicated mobile application, this approach would allow users to capture a single smartphone image of dye-containing wastewater, enabling immediate concentration determination without complex analytical procedures. Such a capability holds strong practical promises for routine monitoring and management of organic dyes in industrial wastewater.
4. Conclusions
This study successfully demonstrated a rapid detection method for detecting MB concentrations using a smartphone-based RGB sensing approach combined with a PLS model. The developed sensing method was effectively applied to monitor the degradation of MB using a Pt/TiO2 photocatalyst. Characterization results confirmed the uniform dispersion of zero-valent Pt nanoparticles on the anatase TiO2 surface, significantly enhancing the separation efficiency of photogenerated charge carriers and expanding the photocatalyst’s absorption into the visible region through LSPR. The proposed RGB-based PLS sensing approach eliminates reliance on costly analytical instruments, providing an efficient, low-cost, and easily implementable solution for rapid on-site monitoring of organic dyes in industrial wastewater. Furthermore, integrating this method into a dedicated mobile application in the future could substantially advance the synergistic combination of photocatalytic wastewater treatment and intelligent detection technologies.
Author Contributions
Conceptualization, C.H. and C.G.; methodology, C.H.; software, C.G.; validation, Z.W., J.C. and B.Z.; formal analysis, B.Z.; investigation, S.L.; resources, B.Z.; data curation, L.Y.; writing—original draft preparation, C.H.; writing—review and editing, C.G.; visualization, Y.F.; supervision, C.G.; project administration, C.G.; funding acquisition, C.H. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yancheng Health Commission 2024 Medical Research Project, grant number YK2024187; and the Scientific Research Startup Fund of Jiangsu Medical College, grants number 20216112 and 20246105.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are openly available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TiO2 | Titanium dioxide |
| HPLC | High-performance liquid chromatography |
| PLS | Partial least squares |
References
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, A.; Salimi, M.; Jafari, A.J.; Sobhi, H.R.; Gholami, M.; Kalantary, R.R. Pt-based TiO2 photocatalytic systems: A systematic review. J. Mol. Liq. 2022, 352, 118685. [Google Scholar] [CrossRef]
- Serra-Pérez, E.; Dražić, G.; Takashima, M.; Ohtani, B.; Kovačič, S.; Žerjav, G.; Tušar, N.N. Influence of the surface structure of the TiO2 support on the properties of the Au/TiO2 photocatalyst for water treatment under visible light. Catal. Today 2024, 437, 114764. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Kutiang, F.D.A.; Lim, Y.C.; Goh, P.S. Recent progress of Ag/TiO2 photocatalyst for wastewater treatment: Doping, co-doping, and green materials functionalization. Appl. Mater. Today 2022, 27, 101500. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z. The enhancement of photodegradation efficiency using Pt–TiO2 catalyst. Chemosphere 2002, 48, 1103–1111. [Google Scholar] [CrossRef]
- Botelho, B.G.; Dantas, K.C.F.; Sena, M.M. Determination of allura red dye in hard candies by using digital images obtained with a mobile phone and N-PLS. Chemom. Intell. Lab. Syst. 2017, 167, 44–49. [Google Scholar] [CrossRef]
- Fay, C.D.; Wu, L. Critical importance of RGB color space specificity for colorimetric bio/chemical sensing: A comprehensive study. Talanta 2024, 266, 124957. [Google Scholar] [CrossRef]
- Singh, G.; Singh, H.; Kaur, N.; Singh, N. Azodye-based colorimetric sensor array for identification of biogenic amines: Food forensics by portable RGB-based signal readout. Sens. Actuators B Chem. 2023, 387, 133794. [Google Scholar] [CrossRef]
- Li, C.-H.; Hsieh, Y.-H.; Chiu, W.-T.; Liu, C.-C.; Kao, C.-L. Study on preparation and photocatalytic performance of Ag/TiO2 and Pt/TiO2 photocatalysts. Sep. Purif. Technol. 2007, 58, 148–151. [Google Scholar] [CrossRef]
- He, Z.; Que, W.; Chen, J.; He, Y.; Wang, G. Surface chemical analysis on the carbon-doped mesoporous TiO2 photocatalysts after post-thermal treatment: XPS and FTIR characterization. J. Phys. Chem. Solids 2013, 74, 924–928. [Google Scholar] [CrossRef]
- Kim, K.; Winograd, N.; Davis, R. Electron spectroscopy of platinum-oxygen surfaces and application to electrochemical studies. J. Am. Chem. Soc. 1971, 93, 6296–6297. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chao, J.-H.; Liu, C.-H.; Chang, J.-C.; Wang, F.-C. Effect of calcination temperature on the structure of a Pt/TiO2 nanofiber and its photocatalytic activity in generating H2. Langmuir 2008, 24, 9907–9915. [Google Scholar] [CrossRef]
- Das, A.; Adak, M.K.; Mahata, N.; Biswas, B. Wastewater treatment with the advent of TiO2 endowed photocatalysts and their reaction kinetics with scavenger effect. J. Mol. Liq. 2021, 338, 116479. [Google Scholar] [CrossRef]
- Hufschmidt, D.; Bahnemann, D.; Testa, J.J.; Emilio, C.A.; Litter, M.I. Enhancement of the photocatalytic activity of various TiO2 materials by platinisation. J. Photochem. Photobiol. A Chem. 2002, 148, 223–231. [Google Scholar] [CrossRef]
- Qin, L.; Wang, G.; Tan, Y. Plasmonic Pt nanoparticles—TiO2 hierarchical nano-architecture as a visible light photocatalyst for water splitting. Sci. Rep. 2018, 8, 16198. [Google Scholar] [CrossRef]
- Lv, J.; Gao, H.; Wang, H.; Lu, X.; Xu, G.; Wang, D.; Chen, Z.; Zhang, X.; Zheng, Z.; Wu, Y. Controlled deposition and enhanced visible light photocatalytic performance of Pt-modified TiO2 nanotube arrays. Appl. Surf. Sci. 2015, 351, 225–231. [Google Scholar] [CrossRef]
- Moslah, C.; Kandyla, M.; Mousdis, G.A.; Petropoulou, G.; Ksibi, M. Photocatalytic properties of titanium dioxide thin films doped with noble metals (Ag, Au, Pd, and Pt). Phys. Status Solidi 2018, 215, 1800023. [Google Scholar] [CrossRef]
- Du, Y.; Ma, D.; Li, J.; Huang, Q.; He, Q.; Ji, J.; Ji, H.; Ma, W.; Zhao, J. Visible light-sensitized CO2 methanation along a relaxed heat available route. Chemistry 2024, 30, e202402102. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).