Can EMG-Derived Upper Limb Muscle Synergies Serve as Markers for Post-Stroke Motor Assessment and Prediction of Rehabilitation Outcome?
Abstract
1. Introduction
2. Methods
2.1. Subject Recruitment
2.2. Clinical Assessments
2.3. EMG Recording and Extraction of Muscle Synergies
2.4. Rationale and Computation of Muscle Synergy Indices
2.5. Statistical Analysis
3. Results
3.1. All Muscle Synergy Indices (MSIs) Correlated with Motor Impairment Post-Stroke
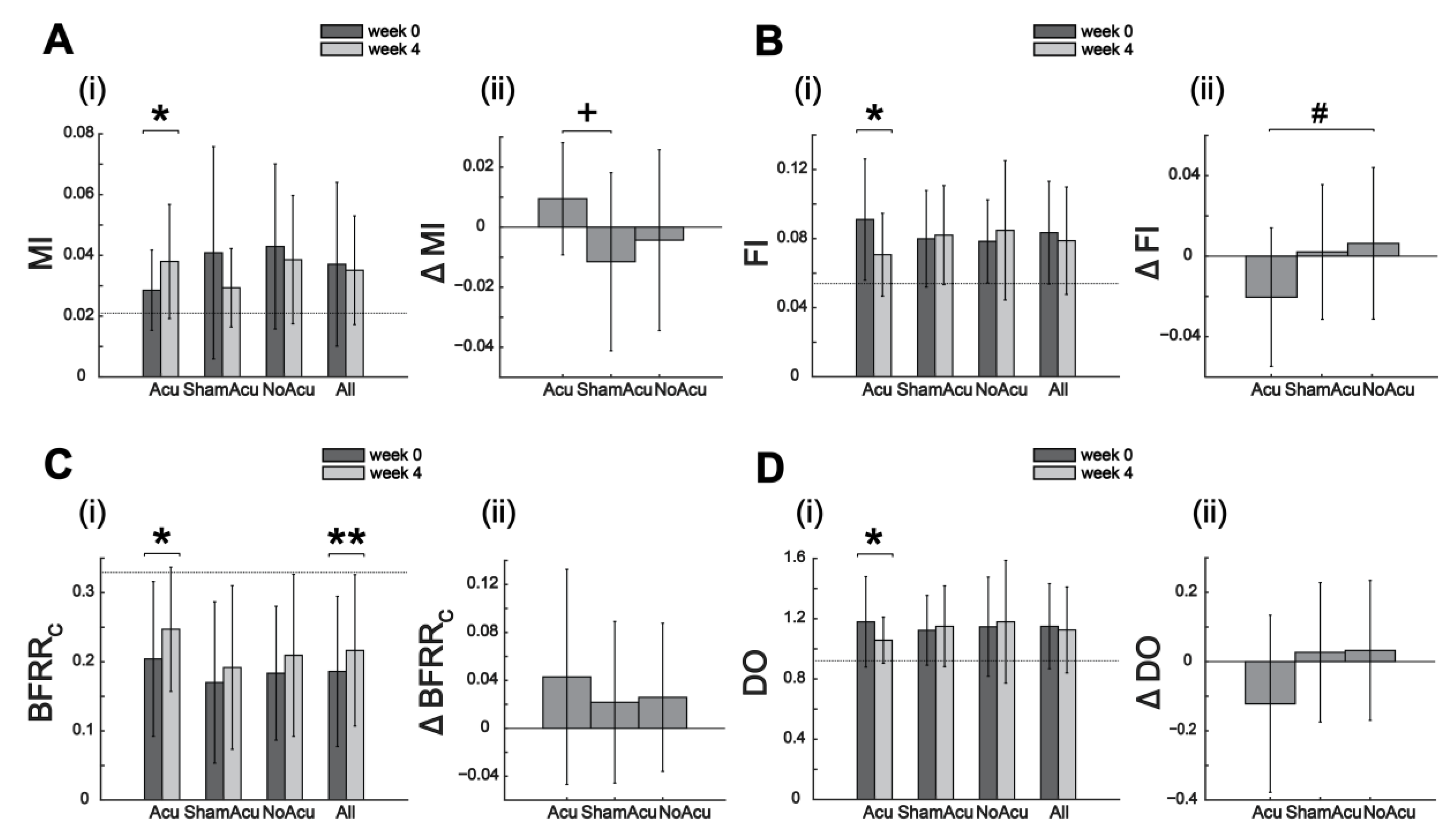
3.2. Effects of Acupuncture on Muscle Synergy Restoration
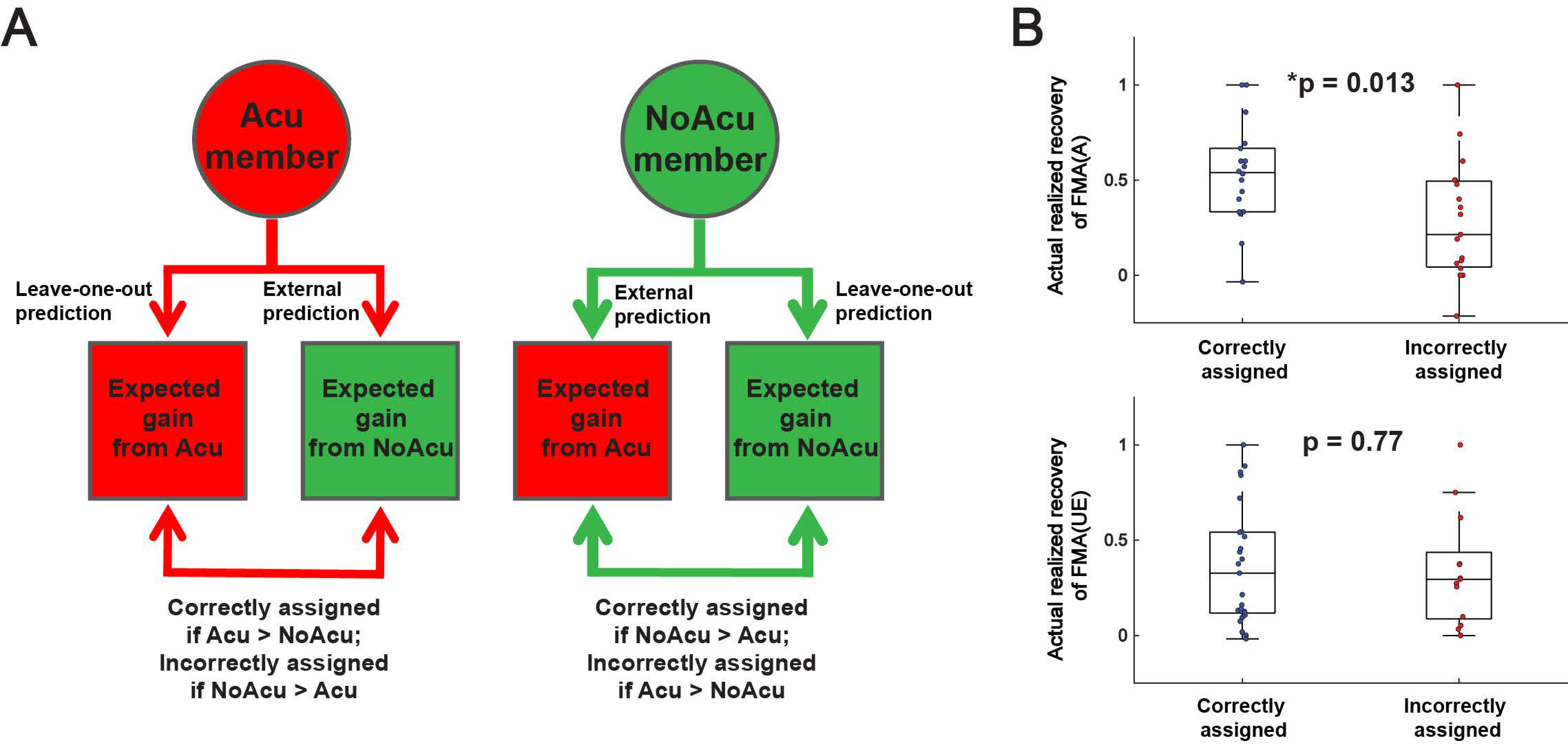
3.3. Subjects Assigned by MSI Predictive Models Had Greater Recovery in Gross Motor Control
4. Discussion
5. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke Care 2: Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Leff, A.P.; Copland, D.A.; Carey, L.M.; et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 480–493. [Google Scholar] [CrossRef]
- Micera, S.; Caleo, M.; Chisari, C.; Hummel, F.C.; Pedrocchi, A. Advanced Neurotechnologies for the Restoration of Motor Function. Neuron 2020, 105, 604–620. [Google Scholar] [CrossRef]
- Piscitelli, D. Motor rehabilitation should be based on knowledge of motor control. Arch. Physiother. 2016, 6, 1–3. [Google Scholar] [CrossRef]
- Maier, M.; Ballester, B.R.; Verschure, P.F.M.J. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front. Syst. Neurosci. 2019, 13, 74. [Google Scholar] [CrossRef]
- Tresch, M.C.; Cheung, V.C.K.; d’Avella, A. Matrix factorization algorithms for the identification of muscle synergies: Evaluation on simulated and experimental data sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef]
- Bizzi, E.; Cheung, V.C.K.; d’Avella, A.; Saltiel, P.; Tresch, M. Combining modules for movement. Brain Res. Rev. 2008, 57, 125–133. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Seki, K. Approaches to revealing the neural basis of muscle synergies: A review and a critique. J. Neurophysiol. 2021, 125, 1580–1597. [Google Scholar] [CrossRef]
- Takei, T.; Confais, J.; Tomatsu, S.; Oya, T.; Seki, K. Neural basis for hand muscle synergies in the primate spinal cord. Proc. Natl. Acad. Sci. USA 2017, 114, 8643–8648. [Google Scholar] [CrossRef] [PubMed]
- Overduin, S.A.; d’Avella, A.; Carmena, J.M.; Bizzi, E. Microstimulation activates a handful of muscle synergies. Neuron 2012, 76, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Yarossi, M.; Brooks, D.H.; Erdoğmuş, D.; Tunik, E. Similarity of hand muscle synergies elicited by transcranial magnetic stimulation and those found during voluntary movement. J. Neurophysiol. 2022, 128, 994–1010. [Google Scholar] [CrossRef]
- Zhao, K.K.; Zhang, Z.S.; Wen, H.Y.; Liu, B.; Li, J.Q.; d’Avella, A.; Scano, A. Muscle synergies for evaluating upper limb in clinical applications: A systematic review. Heliyon 2023, 9, e16202. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef]
- Cheung, V.C.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef]
- Roh, J.; Rymer, W.Z.; Beer, R.F. Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment. Front. Hum. Neurosci. 2015, 9, 6. [Google Scholar] [CrossRef]
- Tropea, P.; Monaco, V.; Coscia, M.; Posteraro, F.; Micera, S. Effects of early and intensive neuro-rehabilitative treatment on muscle synergies in acute post-stroke patients: A pilot study. J. Neuroeng. Rehabil. 2013, 10, 103. [Google Scholar] [CrossRef]
- García-Cossio, E.; Broetz, D.; Birbaumer, N.; Ramos-Murguialday, A. Cortex integrity relevance in muscle synergies in severe chronic stroke. Front. Hum. Neurosci. 2014, 8, 744. [Google Scholar] [CrossRef]
- Li, S.; Zhuang, C.; Niu, C.M.; Bao, Y.; Xie, Q.; Lan, N. Evaluation of Functional Correlation of Task-Specific Muscle Synergies with Motor Performance in Patients Poststroke. Front. Neurol. 2017, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Sun, Y.; Xie, B.; Huang, Z.; Wu, J.; Hou, J.; Liu, Y.; Huang, Z.; Zhang, Z. Alterations of Muscle Synergies During Voluntary Arm Reaching Movement in Subacute Stroke Survivors at Different Levels of Impairment. Front. Comput. Neurosci. 2018, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Huang, Z.; Jin, T.; Wu, J.; Zhang, Z.; Shen, Y. Motor Function Assessment of Upper Limb in Stroke Patients. J. Healthc. Eng. 2021, 2021, 6621950. [Google Scholar] [CrossRef] [PubMed]
- Pierella, C.; Pirondini, E.; Kinany, N.; Coscia, M.; Giang, C.; Miehlbradt, J.; Magnin, C.; Nicolo, P.; Dalise, S.; Sgherri, G.; et al. A multimodal approach to capture post-stroke temporal dynamics of recovery. J. Neural Eng. 2020, 17, 045002. [Google Scholar] [CrossRef] [PubMed]
- Irastorza-Landa, N.; García-Cossio, E.; Sarasola-Sanz, A.; Brötz, D.; Birbaumer, N.; Ramos-Murguialday, A. Functional synergy recruitment index as a reliable biomarker of motor function and recovery in chronic stroke patients. J. Neural Eng. 2021, 18, 046061. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, J.H.; Lee, H.; Roh, J.; Park, H.S. Alterations in intermuscular coordination underlying isokinetic exercise after a stroke and their implications on neurorehabilitation. J. Neuroeng. Rehabil. 2021, 18, 110. [Google Scholar] [CrossRef]
- Maistrello, L.; Rimini, D.; Cheung, V.C.K.; Pregnolato, G.; Turolla, A. Muscle Synergies and Clinical Outcome Measures Describe Different Factors of Upper Limb Motor Function in Stroke Survivors Undergoing Rehabilitation in a Virtual Reality Environment. Sensors 2021, 21, 8002. [Google Scholar] [CrossRef]
- Sheng, Y.; Tan, G.; Liu, J.; Chang, H.; Wang, J.; Xie, Q.; Liu, H. Upper Limb Motor Function Quantification in Post-Stroke Rehabilitation Using Muscle Synergy Space Model. IEEE Trans. Biomed. Eng. 2022, 69, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Kishta, A.; Mugler, E.; Slutzky, M.W.; Roh, J. Myoelectric interface training enables targeted reduction in abnormal muscle co-activation. J. Neuroeng. Rehabil. 2022, 19, 67. [Google Scholar] [CrossRef]
- Funato, T.; Hattori, N.; Yozu, A.; An, Q.; Oya, T.; Shirafuji, S.; Jino, A.; Miura, K.; Martino, G.; Berger, D.; et al. Muscle synergy analysis yields an efficient and physiologically relevant method of assessing stroke. Brain Commun. 2022, 4, fcac200. [Google Scholar] [CrossRef]
- Chavez, L.M.; Huang, S.S.; MacDonald, I.; Lin, J.G.; Lee, Y.C.; Chen, Y.H. Mechanisms of Acupuncture Therapy in Ischemic Stroke Rehabilitation: A Literature Review of Basic Studies. Int. J. Mol. Sci. 2017, 18, 2270. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Xiong, B.H.; Zhang, P.M.; Wang, Y.Q.; Tang, Y.Y.; Zhan, L.C.; Lu, L.M. Abdominal Acupuncture as an Adjunctive Therapy for the Recovery of Motor Function After Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2021, 12, 705771. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Mills, E.; Moher, D.; Seely, D. Acupuncture in Poststroke Rehabilitation A Systematic Review and Meta-Analysis of Randomized Trials. Stroke 2010, 41, E171–E179. [Google Scholar] [CrossRef]
- Stinear, C.M.; Barber, P.A.; Petoe, M.; Anwar, S.; Byblow, W.D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012, 135, 2527–2535. [Google Scholar] [CrossRef]
- Naeser, M.A.; Alexander, M.P.; Stiassny-Eder, D.; Galler, V.; Hobbs, J.; Bachman, D. Real Versus Sham Acupuncture in the Treatment of Paralysis in Acute Stroke Patients: A CT Scan Lesion Site Study. Neurorehabilit. Neural Repair. 1992, 6, 163–174. [Google Scholar] [CrossRef]
- Banks, C.L.; Pai, M.M.; McGuirk, T.E.; Fregly, B.J.; Patten, C. Methodological Choices in Muscle Synergy Analysis Impact Differentiation of Physiological Characteristics Following Stroke. Front. Comput. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef]
- Bo, Z.Y. On abdominal acupuncture therapy. Chin. Acupunct. Moxibustion 2001, 21, 474–476. [Google Scholar]
- Pan, R.; Ling, S.; Xie, J.; Guo, Y.; Zhong, Y.; Huang, Y.; Zhan, J.; Cheung, V.C.K.; Chen, H. Efficacy and Mechanism of Abdominal Acupuncture as a Poststroke Intervention for Promoting Upper-limb Motor Recovery: A Study Protocol of A Randomized Controlled Trial. Res. Sq. [Prepr. Serv.] 2019. [Google Scholar] [CrossRef]
- Salter, K.; Campbell, N.; Richardson, M.; Mehta, S.; Jutai, J.; Zettler, L.; Moses, M.; McClure, A.; Mays, R.; Foley, N.; et al. 20 Outcome Measures in Stroke Rehabilitation. Evidence-Based Review of Stroke Rehabilitation. Available online: http://www.ebrsr.com/evidence-review/20-outcome-measures-stroke-rehabilitation (accessed on 5 May 2025).
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Smith, M.C.; Borges, V.M.; Barber, P.A. Proportional Motor Recovery After Stroke: Implications for Trial Design. Stroke 2017, 48, 795–798. [Google Scholar] [CrossRef]
- Lin, D.J.; Cloutier, A.M.; Erler, K.S.; Cassidy, J.M.; Snider, S.B.; Ranford, J.; Parlman, K.; Giatsidis, F.; Burke, J.F.; Schwamm, L.H.; et al. Corticospinal Tract Injury Estimated From Acute Stroke Imaging Predicts Upper Extremity Motor Recovery After Stroke. Stroke 2019, 50, 3569–3577. [Google Scholar] [CrossRef]
- Merletti, R.; Hermens, H. Introduction to the special issue on the SENIAM European Concerted Action. J. Electromyogr. Kinesiol. 2000, 10, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.K.; Cheung, B.F.; Zhang, J.H.; Chan, Z.Y.S.; Ha, S.C.W.; Chen, C.Y.; Cheung, R.T.H. Plasticity of muscle synergies through fractionation and merging during development and training of human runners. Nat. Commun. 2020, 11, 4356. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef]
- Lee, S.W.; Triandafilou, K.; Lock, B.A.; Kamper, D.G. Impairment in task-specific modulation of muscle coordination correlates with the severity of hand impairment following stroke. PLoS ONE 2013, 8, e68745. [Google Scholar] [CrossRef]
- Bizzi, E.; Cheung, V.C.K. The neural origin of muscle synergies. Front. Comput. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Niu, C.X.M.; Li, S.; Xie, Q.; Lan, N. A Novel FES Strategy for Poststroke Rehabilitation Based on the Natural Organization of Neuromuscular Control. Ieee Rev. Biomed. Eng. 2019, 12, 154–167. [Google Scholar] [CrossRef]
- Ferrante, S.; Chia Bejarano, N.; Ambrosini, E.; Nardone, A.; Turcato, A.M.; Monticone, M.; Ferrigno, G.; Pedrocchi, A. A Personalized Multi-Channel FES Controller Based on Muscle Synergies to Support Gait Rehabilitation after Stroke. Front. Neurosci. 2016, 10, 425. [Google Scholar] [CrossRef]
- Niu, C.M.; Chou, C.H.; Bao, Y.; Wang, T.; Gu, L.; Zhang, X.; Cui, L.; Xuan, Z.; Zhuang, C.; Li, S.; et al. A pilot study of synergy-based FES for upper-extremity poststroke rehabilitation. Neurosci. Lett. 2022, 780, 136621. [Google Scholar] [CrossRef]
- Levin, M.F.; Kleim, J.A.; Wolf, S.L. What Do Motor “Recovery” and “Compensation” Mean in Patients Following Stroke? Neurorehabilit. Neural Repair. 2008, 23, 313–319. [Google Scholar] [CrossRef]
- Kitago, T.; Liang, J.; Huang, V.S.; Hayes, S.; Simon, P.; Tenteromano, L.; Lazar, R.M.; Marshall, R.S.; Mazzoni, P.; Lennihan, L.; et al. Improvement After Constraint-Induced Movement Therapy: Recovery of Normal Motor Control or Task-Specific Compensation? Neurorehabilit. Neural Repair. 2013, 27, 99–109. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Feldner, H.A.; Howell, D.; Kelly, V.E.; McCoy, S.W.; Steele, K.M. “Look, Your Muscles Are Firing!”: A Qualitative Study of Clinician Perspectives on the Use of Surface Electromyography in Neurorehabilitation. Arch. Phys. Med. Rehab. 2019, 100, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Pedraza, S.; Blasco, G.; Daunis-i-Estadella, J.; Prados, F.; Remollo, S.; Prats-Galino, A.; Soria, G.; Boada, I.; Castellanos, M.; et al. Acute Damage to the Posterior Limb of the Internal Capsule on Diffusion Tensor Tractography as an Early Imaging Predictor of Motor Outcome after Stroke. Am. J. Neuroradiol. 2011, 32, 857–863. [Google Scholar] [CrossRef]
- Brown, A.W.; Therneau, T.M.; Schultz, B.A.; Niewczyk, P.M.; Granger, C.V. Measure of functional independence dominates discharge outcome prediction after inpatient rehabilitation for stroke. Stroke 2015, 46, 1038–1044. [Google Scholar] [CrossRef]
- Koh, C.L.; Pan, S.L.; Jeng, J.S.; Chen, B.B.; Wang, Y.H.; Hsueh, I.P.; Hsieh, C.L. Predicting Recovery of Voluntary Upper Extremity Movement in Subacute Stroke Patients with Severe Upper Extremity Paresis. PLoS ONE 2015, 10, e0126857. [Google Scholar] [CrossRef]
- Feng, W.W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.A.; Kautz, S.A.; Schlaug, G. Corticospinal Tract Lesion Load: An Imaging Biomarker for Stroke Motor Outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef]
- Gandolfi, M.; Boscolo Galazzo, I.; Gasparin Pavan, R.; Cruciani, F.; Vale, N.; Picelli, A.; Storti, S.F.; Smania, N.; Menegaz, G. eXplainable AI Allows Predicting Upper Limb Rehabilitation Outcomes in Sub-Acute Stroke Patients. IEEE J. Biomed. Health Inform. 2023, 27, 263–273. [Google Scholar] [CrossRef]
- Quinlan, E.B.; Dodakian, L.; See, J.; McKenzie, A.; Le, V.; Wojnowicz, M.; Shahbaba, B.; Cramer, S.C. Neural Function, Injury, and Stroke Subtype Predict Treatment Gains after Stroke. Ann. Neurol. 2015, 77, 132–145. [Google Scholar] [CrossRef]
- Vergara-Diaz, G.P.; Sapienza, S.; Daneault, J.F.; Fabara, E.; Adans-Dester, C.; Severini, G.; Cheung, V.C.K.; de Vargas, C.E.R.; Nimec, D.; Bonato, P. Can muscle synergies shed light on the mechanisms underlying motor gains in response to robot-assisted gait training in children with cerebral palsy? J. Neuroeng. Rehabil. 2025, 22, 23. [Google Scholar] [CrossRef]
- Lin, P.J.; Zhai, X.; Li, W.; Li, T.; Cheng, D.; Li, C.; Pan, Y.; Ji, L. A Transferable Deep Learning Prognosis Model for Predicting Stroke Patients’ Recovery in Different Rehabilitation Trainings. IEEE J. Biomed. Health Inform. 2022, 26, 6003–6011. [Google Scholar] [CrossRef]
- Dussault-Picard, C.; Havashinezhadian, S.; Turpin, N.A.; Moissenet, F.; Turcot, K.; Cherni, Y. Age-related modifications of muscle synergies during daily-living tasks: A scoping review. Clin. Biomech. 2024, 113, 106207. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, C.A.; Rao, G.; Berton, E.; Retornaz, F.; Temprado, J.J. The Effect of Aging on Muscular Dynamics Underlying Movement Patterns Changes. Front. Aging Neurosci. 2016, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.; d’Avella, A.; Tresch, M.C.; Bizzi, E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J. Neurosci. 2005, 25, 6419–6434. [Google Scholar] [CrossRef]
- Berger, D.J.; Masciullo, M.; Molinari, M.; Lacquaniti, F.; d’Avella, A. Does the cerebellum shape the spatiotemporal organization of muscle patterns? Insights from subjects with cerebellar ataxias. J. Neurophysiol. 2020, 123, 1691–1710. [Google Scholar] [CrossRef]

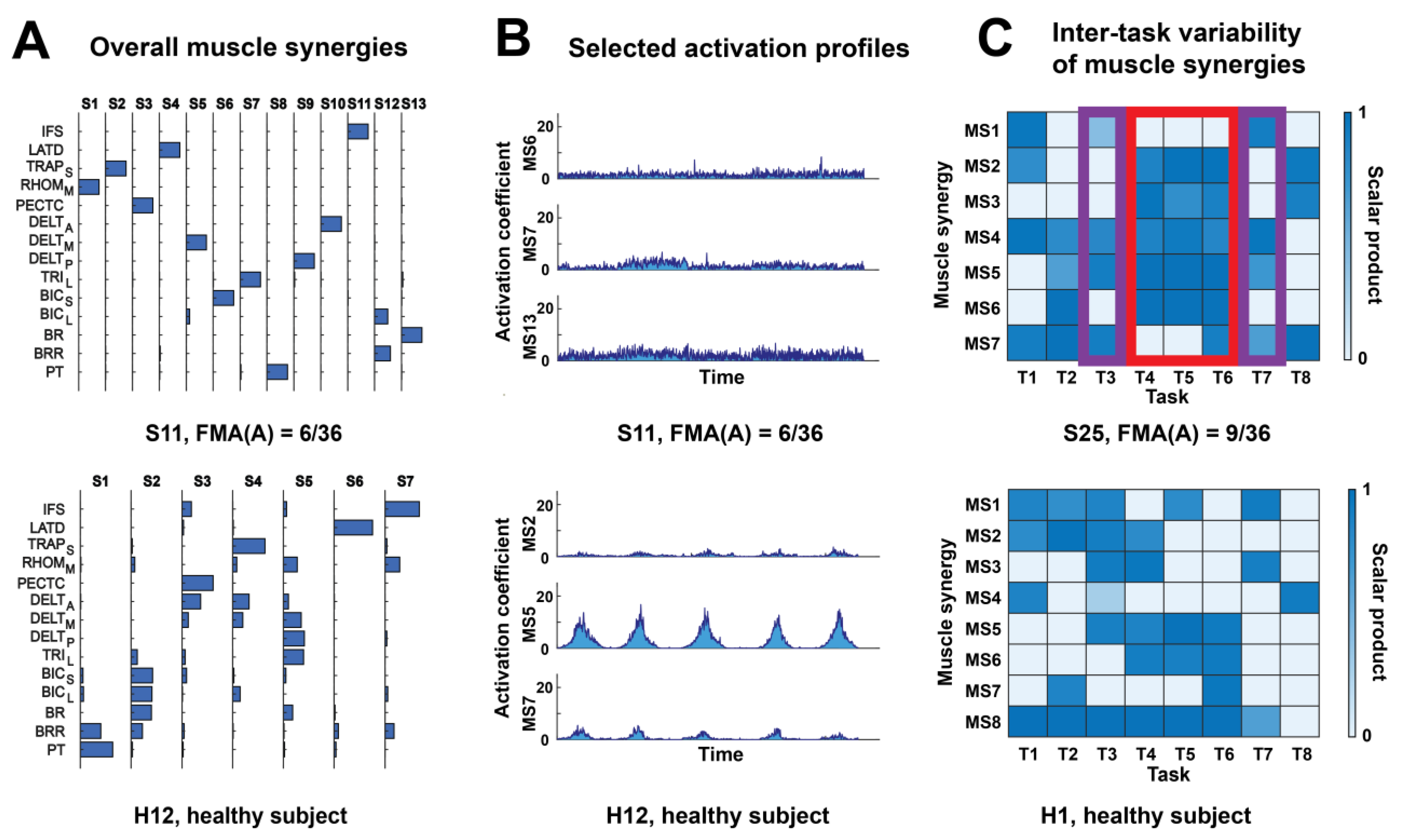




| Index | Description |
|---|---|
| (1) Dimensionality of the stroke-affected limb | |
| DevDO | Deviation in the Dimensionality from normal (Original value) |
| DevDA | Deviation in the Dimensionality from normal (Absolute value) |
| (2) Similarity of the W matrix between the stroke-affected limb and the reference limb | |
| BFRRW | Bidirectional Fitting R2 Ratio (BFRR) of the W matrix , |
| (3) Other features of the W matrix in the stroke-affected limb | |
| MI | Merging index |
| FI | Fractionation index |
| (4) Similarity of the C matrix between the stroke-affected limb and the reference limb | |
| BFRRC | Bidirectional Fitting R2 Ratio (BFRR) of the C matrix , |
| BFRRC(mod) | Bidirectional Fitting R2 Ratio (BFRR) of the modified C matrix , |
| (5) Other features of the C matrix in the stroke-affected limb | |
| DO | Degree of Oscillation of the C matrix |
| MEA | Magnitude of Effective Activation |
| (6) Inter-task variability in the W or C matrix in the stroke-affected limb | |
| ITV_BFRRWa | Inter-Task Variability (measured by BFRR) in the W matrix ITV_BFRRW is the average across the BFRRW values resulting from the = 28 pairwise comparison of the task-specific muscle synergies of the 8 tasks. |
| ITV_BFRRCa | Inter-Task Variability (measured by BFRR) in the C matrix ITV_BFRRC is the average across the BFRRC values resulting from the = 28 pairwise comparison of the task-specific activation profiles of the 8 tasks. |
| ITV_BFRRC(mod)a | Inter-Task Variability (measured by BFRR) in the modified C matrix ITV_BFRRC(mod) is the average across the BFRRC(mod) values resulting from the = 28 pairwise comparison of the task-specific modified activation profiles of the 8 tasks. |
| Acupuncture | Sham Acupuncture | No Acupuncture | All Groups | |
|---|---|---|---|---|
| Change in clinical scores (mean ± SD) after 2 weeks | ||||
| FMA(A) | 2.05 ± 2.19 ** | 2.62 ± 3.05 ** | 1.41 ± 1.50 ** | 2.07 ± 2.43 ** |
| FMA(UE) | 3.95 ± 5.14 ** | 3.62 ± 4.62 ** | 2.82 ± 2.28 ** | 3.51 ± 4.33 ** |
| WMFT | 5.62 ± 5.07 ** | 4.71 ± 4.69 ** | 3.35 ± 2.89 ** | 4.64 ± 4.49 ** |
| BI(UE) | 5.43 ± 5.20 ** | 3.29 ± 5.41 * | 2.88 ± 3.61 ** | 3.93 ± 5.00 ** |
| BS | 0.86 ± 0.83 ** | 0.57 ± 0.73 ** | 0.35 ± 0.84 | 0.61 ± 0.82 ** |
| Change in clinical scores (mean ± SD) after 4 weeks | ||||
| FMA(A) | 4.62 ± 4.33 ** | 5.14 ± 4.80 ** | 5.71 ± 6.18 ** | 5.12 ± 5.11 ** |
| FMA(UE) | 9.76 ± 8.27 ** | 8.76 ± 7.32 ** | 9.94 ± 6.68 ** | 9.46 ± 7.52 ** |
| WMFT | 12.05 ± 8.40 ** | 10.33 ± 8.71 ** | 7.47 ± 5.13 ** | 10.12 ± 7.94 ** |
| BI(UE) | 10.86 ± 8.29 ** | 6.24 ± 7.65 ** | 8.41 ± 6.80 ** | 8.51 ± 7.90 ** |
| BS | 1.52 ± 1.22 ** | 1.24 ± 1.23 ** | 1.29 ± 1.45 ** | 1.36 ± 1.30 ** |
| Change in muscle synergy indexes (mean ± SD) after 4 weeks | ||||
| DevDO | −0.81 ± 1.56 * | 0.19 ± 1.30 | 0.00 ± 0.97 | −0.22 ± 1.39 |
| DevDA | −0.21 ± 1.30 | −0.15 ± 0.90 | 0.07 ± 0.76 | −0.11 ± 1.03 |
| BFRRW | 0.00 ± 0.03 | 0.00 ± 0.02 | −0.01 ± 0.03 | 0.00 ± 0.03 |
| MI # | 0.01 ± 0.02 * | −0.01 ± 0.03 | 0.00 ± 0.03 | 0.00 ± 0.03 |
| FI # | −0.02 ± 0.03 * | 0.00 ± 0.03 | 0.01 ± 0.04 | 0.00 ± 0.04 |
| BFRRC | 0.04 ± 0.09 * | 0.02 ± 0.07 | 0.03 ± 0.06 | 0.03 ± 0.07 ** |
| BFRRC (mod) | 0.05 ± 0.12 † | 0.03 ± 0.08 | 0.03 ± 0.08 | 0.04 ± 0.10 ** |
| DO | −0.12 ± 0.25 * | 0.03 ± 0.20 | 0.03 ± 0.20 | −0.02 ± 0.23 |
| MEA ^ | 0.04 ± 0.11 | −0.02 ± 0.08 | −0.03 ± 0.08 | 0.00 ± 0.10 |
| ITV_BFRRW | −0.03 ± 0.16 | −0.06 ± 0.13 † | 0.00 ± 0.15 | −0.03 ± 0.15 |
| ITV_BFRRC | 0.06 ± 0.16 | 0.00 ± 0.12 | 0.05 ± 0.10 * | 0.04 ± 0.13 * |
| ITV_BFRRC (mod) | 0.07 ± 0.19 | −0.01 ± 0.14 | 0.06 ± 0.10 * | 0.04 ± 0.16 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwok, F.T.; Pan, R.; Ling, S.; Dong, C.; Xie, J.J.; Chen, H.; Cheung, V.C.K. Can EMG-Derived Upper Limb Muscle Synergies Serve as Markers for Post-Stroke Motor Assessment and Prediction of Rehabilitation Outcome? Sensors 2025, 25, 3170. https://doi.org/10.3390/s25103170
Kwok FT, Pan R, Ling S, Dong C, Xie JJ, Chen H, Cheung VCK. Can EMG-Derived Upper Limb Muscle Synergies Serve as Markers for Post-Stroke Motor Assessment and Prediction of Rehabilitation Outcome? Sensors. 2025; 25(10):3170. https://doi.org/10.3390/s25103170
Chicago/Turabian StyleKwok, Fung Ting, Ruihuan Pan, Shanshan Ling, Cong Dong, Jodie J. Xie, Hongxia Chen, and Vincent C. K. Cheung. 2025. "Can EMG-Derived Upper Limb Muscle Synergies Serve as Markers for Post-Stroke Motor Assessment and Prediction of Rehabilitation Outcome?" Sensors 25, no. 10: 3170. https://doi.org/10.3390/s25103170
APA StyleKwok, F. T., Pan, R., Ling, S., Dong, C., Xie, J. J., Chen, H., & Cheung, V. C. K. (2025). Can EMG-Derived Upper Limb Muscle Synergies Serve as Markers for Post-Stroke Motor Assessment and Prediction of Rehabilitation Outcome? Sensors, 25(10), 3170. https://doi.org/10.3390/s25103170







