Virtual Reality: A New Frontier of Physical Rehabilitation
Abstract
1. Introduction
Objective
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Research
2.3. Information Sources
2.4. Data Collection
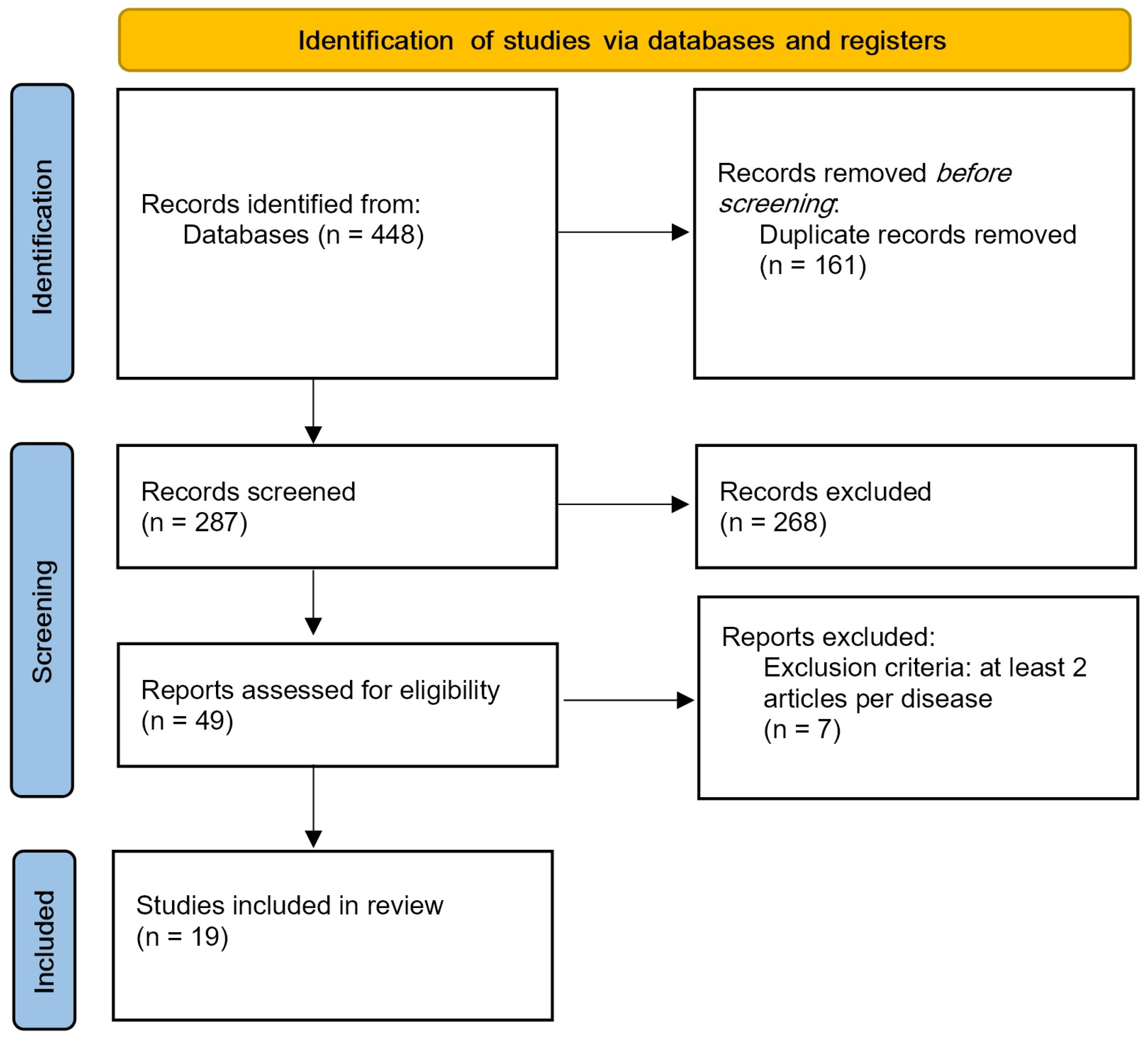
3. Results
4. Discussion
4.1. Acute/Subacute Stroke
4.2. Chronic Stroke
4.3. Parkinson
4.4. Amputation
4.5. Fibromyalgia
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARAT | Action Research Arm Test |
| AROM | Active Range Of Motion |
| BADL | Basic Activities of Daily Living |
| BBT | Box and Block Test |
| BPI | Brief Pain Inventory |
| CAHAI-13 | Chedoke–McMaster and Hand activity Inventory |
| CAHM | Confidence in Arm and Hand Movement scale |
| CSI | Composite Spasticity Index |
| CSQ-8 | Customer satisfaction questionnaire |
| DVPRS | The Defense and Veterans Pain Rating Scale |
| FIQ | Fibromyalgia Impact Questionnaire |
| FMA-UE | Fugl-Meyer Assessment for the Upper Extremity |
| FS | Fibromyalgia Syndrome |
| FSS | Fatigue Severity Scale |
| IMI | Intrinsic Motivation Inventory |
| IVR | Immersive Virtual Reality |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MFT | Manual Function Test |
| MMSE | Mini-Mental State Examination |
| MAL | Motor Activity Log |
| MS | Multiple Sclerosis |
| MSOT | Modified Sensory Organization Test |
| PCS | Pain Catastrophizing Scale |
| PD | Parkinson Disease |
| PGIC | Patient Global Impression of Change scale |
| PICOST | Population, Intervention, Comparator, Outcome, Study design, and Timeframe |
| PPT | Purdue pegboard coordination test |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| RPE | Rating of Perceived Exertion |
| SIS | Stroke Impact Scale |
| UEFMA | Upper Extremity Fugl–Meyer Assessment |
| UL | Upper Limb |
| VAS | Visual Analogic Scale |
| VR | Virtual Reality |
| WMFT | Wolf Motor Function Test |
References
- Pournajaf, S.; Morone, G.; Goffredo, M.; Bonaiuti, D.; Franceschini, M.; Pournajaf, S. Realtà Virtuale Applicata Alla Riabilitazione: Evidenze Cliniche e Prospettive Future. G. Ital. Med. Riabil. 2021, 35, 30–42. [Google Scholar]
- Elor, A.; Teodorescu, M.; Kurniawan, S. Project Star Catcher: A Novel Immersive Virtual Reality Experience for Upper Limb Rehabilitation. ACM Trans. Access. Comput. 2018, 11, 20. [Google Scholar] [CrossRef]
- Fedrizzi, E. Approccio del Gipci Alla Riabilitazione del Bambino con Paralisi Cerebrale Storia Naturale ed Esperienze di Intervento Basate Sulle Neuroscienze, 1st ed.; FrancoAngeli: Milan, Italy, 2019; ISBN 978-88-917-7912-0. [Google Scholar]
- Bourdin, P.; Martini, M.; Sanchez-Vives, M.V. Altered Visual Feedback from an Embodied Avatar Unconsciously Influences Movement Amplitude and Muscle Activity. Sci. Rep. 2019, 9, 19747. [Google Scholar] [CrossRef] [PubMed]
- Kokkinara, E.; Slater, M. Measuring the Effects through Time of the Influence of Visuomotor and Visuotactile Synchronous Stimulation on a Virtual Body Ownership Illusion. Perception 2014, 43, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Gerig, N.; Mayo, J.; Baur, K.; Wittmann, F.; Riener, R.; Wolf, P. Missing Depth Cues in Virtual Reality Limit Performance and Quality of Three Dimensional Reaching Movements. PLoS ONE 2018, 13, e0189275. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Malighetti, C.; Cipresso, P.; Pedroli, E.; Realdon, O.; Mantovani, F.; Riva, G. Changing Body Representation Through Full Body Ownership Illusions Might Foster Motor Rehabilitation Outcome in Patients with Stroke. Front. Psychol. 2020, 11, 1962. [Google Scholar] [CrossRef]
- Phelan, I.; Furness, P.J.; Matsangidou, M.; Carrion-Plaza, A.; Dunn, H.; Dimitri, P.; Lindley, S.A. Playing Your Pain Away: Designing a Virtual Reality Physical Therapy for Children with Upper Limb Motor Impairment. Virtual Real. 2023, 27, 173–185. [Google Scholar] [CrossRef]
- Chau, B.; Phelan, I.; Ta, P.; Chi, B.; Loyola, K.; Yeo, E.; Dunn, J.; Humbert, S.; Hata, J.; Haglund, R.; et al. Immersive Virtual Reality for Pain Relief in Upper Limb Complex Regional Pain Syndrome: A Pilot Study. Innov. Clin. Neurosci. 2020, 17, 47–52. [Google Scholar]
- Hoffman, H.G.; Boe, D.A.; Rombokas, E.; Khadra, C.; LeMay, S.; Meyer, W.J.; Patterson, S.; Ballesteros, A.; Pitt, S.W. Virtual Reality Hand Therapy: A New Tool for Nonopioid Analgesia for Acute Procedural Pain, Hand Rehabilitation, and VR Embodiment Therapy for Phantom Limb Pain. J. Hand Ther. 2020, 33, 254–262. [Google Scholar] [CrossRef]
- Stanica, I.-C.; Moldoveanu, F.; Portelli, G.-P.; Dascalu, M.-I.; Moldoveanu, A.; Ristea, M.G. Flexible Virtual Reality System for Neurorehabilitation and Quality of Life Improvement. Sensors 2020, 20, 6045. [Google Scholar] [CrossRef]
- Amirthalingam, J.; Paidi, G.; Alshowaikh, K.; Iroshani Jayarathna, A.; Salibindla, D.B.A.M.R.; Karpinska-Leydier, K.; Ergin, H.E. Virtual Reality Intervention to Help Improve Motor Function in Patients Undergoing Rehabilitation for Cerebral Palsy, Parkinson’s Disease, or Stroke: A Systematic Review of Randomized Controlled Trials. Cureus 2021, 13, e16763. [Google Scholar] [CrossRef]
- Ahmed, N.; Mauad, V.A.Q.; Gomez-Rojas, O.; Sushea, A.; Castro-Tejada, G.; Michel, J.; Liñares, J.M.; Pedrosa Salles, L.; Candido Santos, L.; Shan, M.; et al. The Impact of Rehabilitation-Oriented Virtual Reality Device in Patients With Ischemic Stroke in the Early Subacute Recovery Phase: Study Protocol for a Phase III, Single-Blinded, Randomized, Controlled Clinical Trial. J. Cent. Nerv. Syst. Dis. 2020, 12, 117957351989947. [Google Scholar] [CrossRef]
- Erhardsson, M.; Alt Murphy, M.; Sunnerhagen, K.S. Commercial Head-Mounted Display Virtual Reality for Upper Extremity Rehabilitation in Chronic Stroke: A Single-Case Design Study. J. NeuroEng. Rehabil. 2020, 17, 154. [Google Scholar] [CrossRef]
- Clark, W.E.; Sivan, M.; O’Connor, R.J. Evaluating the Use of Robotic and Virtual Reality Rehabilitation Technologies to Improve Function in Stroke Survivors: A Narrative Review. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319863557. [Google Scholar] [CrossRef]
- Sánchez-Herrera-Baeza, P.; Cano-de-la-Cuerda, R.; Oña-Simbaña, E.D.; Palacios-Ceña, D.; Pérez-Corrales, J.; Cuenca-Zaldivar, J.N.; Gueita-Rodriguez, J.; Balaguer-Bernaldo De Quirós, C.; Jardón-Huete, A.; Cuesta-Gomez, A. The Impact of a Novel Immersive Virtual Reality Technology Associated with Serious Games in Parkinson’s Disease Patients on Upper Limb Rehabilitation: A Mixed Methods Intervention Study. Sensors 2020, 20, 2168. [Google Scholar] [CrossRef]
- Oña, E.D.; Jardón, A.; Cuesta-Gómez, A.; Sánchez-Herrera-Baeza, P.; Cano-de-la-Cuerda, R.; Balaguer, C. Validity of a Fully-Immersive VR-Based Version of the Box and Blocks Test for Upper Limb Function Assessment in Parkinson’s Disease. Sensors 2020, 20, 2773. [Google Scholar] [CrossRef]
- Sun, Y.; Hunt, C.L.; Niu, W.; Li, Z.; Cyrino, G.; Cavalcante, R.; Lamounier, E.; Soares, A.B.; Thakor, N.V. A Comparison between Virtual Reality and Augmented Reality on Upper-Limb Prosthesis Control. In Proceedings of the 2021 International Symposium on Electrical, Electronics and Information Engineering, Seoul, Republic of Korea, 19–21 February 2021; pp. 521–528. [Google Scholar]
- Hashim, N.A.; Abd Razak, N.A.; Gholizadeh, H.; Abu Osman, N.A. Video Game–Based Rehabilitation Approach for Individuals Who Have Undergone Upper Limb Amputation: Case-Control Study. JMIR Serious Games 2021, 9, e17017. [Google Scholar] [CrossRef]
- Queiroz, L.P. Worldwide Epidemiology of Fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An Update on Clinical Characteristics, Aetiopathogenesis and Treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Ibancos-Losada, M.D.R.; Nieto-Escámez, F.A.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2021, 11, 1167. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Fluet, G.; Qiu, Q.; Yarossi, M.; Merians, A.; Tunik, E.; Adamovich, S. Intensive Virtual Reality and Robotic Based Upper Limb Training Compared to Usual Care, and Associated Cortical Reorganization, in the Acute and Early Sub-Acute Periods Post-Stroke: A Feasibility Study. J. NeuroEng. Rehabil. 2019, 16, 92. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Zhao, Z.; Wang, J.; Xu, B.; Zhang, L.; Cheng, R.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; et al. Proactive Motor Functional Recovery Following Immersive Virtual Reality–Based Limb Mirroring Therapy in Patients with Subacute Stroke. Neurotherapeutics 2020, 17, 1919–1930. [Google Scholar] [CrossRef]
- Park, W.; Kim, J.; Kim, M. Efficacy of Virtual Reality Therapy in Ideomotor Apraxia Rehabilitation: A Case Report. Medicine 2021, 100, e26657. [Google Scholar] [CrossRef]
- Schuster-Amft, C.; Eng, K.; Suica, Z.; Thaler, I.; Signer, S.; Lehmann, I.; Schmid, L.; McCaskey, M.A.; Hawkins, M.; Verra, M.L.; et al. Effect of a Four-Week Virtual Reality-Based Training versus Conventional Therapy on Upper Limb Motor Function after Stroke: A Multicenter Parallel Group Randomized Trial. PLoS ONE 2018, 13, e0204455. [Google Scholar] [CrossRef]
- Weber, L.M.; Nilsen, D.M.; Gillen, G.; Yoon, J.; Stein, J. Immersive Virtual Reality Mirror Therapy for Upper Limb Recovery After Stroke: A Pilot Study. Am. J. Phys. Med. Rehabil. 2019, 98, 783–788. [Google Scholar] [CrossRef]
- Song, Y.-H.; Lee, H.-M. Effect of Immersive Virtual Reality-Based Bilateral Arm Training in Patients with Chronic Stroke. Brain Sci. 2021, 11, 1032. [Google Scholar] [CrossRef]
- Mullick, A.A.; Baniña, M.C.; Tomita, Y.; Fung, J.; Levin, M.F. Obstacle Avoidance and Dual-Tasking During Reaching While Standing in Patients with Mild Chronic Stroke. Neurorehabil. Neural Repair 2021, 35, 915–928. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chiang, W.-C.; Yeh, Y.-C.; Fan, S.-C.; Yang, W.-H.; Kuo, H.-C.; Li, P.-C. Effects of Virtual Reality-Based Motor Control Training on Inflammation, Oxidative Stress, Neuroplasticity and Upper Limb Motor Function in Patients with Chronic Stroke: A Randomized Controlled Trial. BMC Neurol. 2022, 22, 21. [Google Scholar] [CrossRef]
- Cikajlo, I.; Peterlin Potisk, K. Advantages of Using 3D Virtual Reality Based Training in Persons with Parkinson’s Disease: A Parallel Study. J. NeuroEng. Rehabil. 2019, 16, 119. [Google Scholar] [CrossRef]
- Henriksen, B.; Nielsen, R.; Kraus, M.; Geng, B. A Virtual Reality System for Treatment of Phantom Limb Pain Using Game Training and Tactile Feedback. In Proceedings of the Virtual Reality International Conference—Laval Virtual 2017, Laval, France, 22–24 March 2017; pp. 1–4. [Google Scholar]
- Gulsen, C.; Soke, F.; Eldemir, K.; Apaydin, Y.; Ozkul, C.; Guclu-Gunduz, A.; Akcali, D.T. Effect of Fully Immersive Virtual Reality Treatment Combined with Exercise in Fibromyalgia Patients: A Randomized Controlled Trial. Assist. Technol. 2022, 34, 256–263. [Google Scholar] [CrossRef]
- Christensen, S.W.M.; Almsborg, H.; Vain, T.S.; Vaegter, H.B. The Effect of Virtual Reality on Cold Pain Sensitivity in Patients with Fibromyalgia and Pain-Free Individuals: A Randomized Crossover Study. Games Health J. 2023, 12, 295–301. [Google Scholar] [CrossRef]
- Darnall, B.D.; Krishnamurthy, P.; Tsuei, J.; Minor, J.D. Self-Administered Skills-Based Virtual Reality Intervention for Chronic Pain: Randomized Controlled Pilot Study. JMIR Form. Res. 2020, 4, e17293. [Google Scholar] [CrossRef]
- Tuck, N.; Pollard, C.; Good, C.; Williams, C.; Lewis, G.; Hames, M.; Aamir, T.; Bean, D. Active Virtual Reality for Chronic Primary Pain: Mixed Methods Randomized Pilot Study. JMIR Form. Res. 2022, 6, e38366. [Google Scholar] [CrossRef]
- Hoolahan, K. Exploratory Research on the Gamification of Exercise for Fibromyalgia Using Virtual Reality. In Proceedings of the Virtual Reality International Conference, Virtual, 20–22 April 2019. [Google Scholar]
- Shahrbanian, S.; Ma, X.; Aghaei, N.; Korner-Bitensky, N.; Moshiri, K.; Simmonds, M.J. Use of Virtual Reality (Immersive vs. Non Immersive) for Pain Management in Children and Adults: A Systematic Review of Evidence from Randomized Controlled Trials. Eur. J. Exp. Biol. 2012, 2, 1408–1422. [Google Scholar]
- Chen, L.; Chen, Y.; Fu, W.B.; Huang, D.F.; Lo, W.L.A. The Effect of Virtual Reality on Motor Anticipation and Hand Function in Patients with Subacute Stroke: A Randomized Trial on Movement-Related Potential. Neural Plast. 2022, 2022, 7399995. [Google Scholar] [CrossRef]
- Salatino, A.; Zavattaro, C.; Gammeri, R.; Cirillo, E.; Piatti, M.L.; Pyasik, M.; Serra, H.; Pia, L.; Geminiani, G.; Ricci, R. Virtual Reality Rehabilitation for Unilateral Spatial Neglect: A Systematic Review of Immersive, Semi-Immersive and Non-Immersive Techniques. Neurosci. Biobehav. Rev. 2023, 152, 105248. [Google Scholar] [CrossRef]
- Campo Prieto, P.; Santos García, D.; Cancela Carral, J.M.; Rodríguez Fuentes, G. Estado actual de la realidad virtual inmersiva como herramienta de rehabilitación física y funcional en pacientes con enfermedad de Parkinson: Revisión sistemática. Rev. Neurol. 2021, 73, 358. [Google Scholar] [CrossRef]
- Vassantachart, A.Y.; Yeo, E.; Chau, B. Virtual and Augmented Reality-Based Treatments for Phantom Limb Pain: A Systematic Review. Innov. Clin. Neurosci. 2022, 19, 48–57. [Google Scholar]
- KoçyïĞïT, B.F.; Akaltun, M.S. Kinesiophobia Levels in Fibromyalgia Syndrome and the Relationship Between Pain, Disease Activity, Depression. Arch. Rheumatol. 2020, 35, 214–219. [Google Scholar] [CrossRef]
- Masmoudi, M.; Zenati, N.; Izountar, Y.; Benbelkacem, S.; Haicheur, W.; Guerroudji, M.A.; Oulefki, A.; Hamitouche, C. Assessing the Effectiveness of Virtual Reality Serious Games in Post-Stroke Rehabilitation: A Novel Evaluation Method. Multimed. Tools Appl. 2024, 83, 36175–36202. [Google Scholar] [CrossRef]
- Diriba Kenea, C.; Gemechu Abessa, T.; Lamba, D.; Bonnechère, B. Technological Features of Immersive Virtual Reality Systems for Upper Limb Stroke Rehabilitation: A Systematic Review. Sensors 2024, 24, 3546. [Google Scholar] [CrossRef]
- Weech, S.; Kenny, S.; Barnett-Cowan, M. Presence and Cybersickness in Virtual Reality Are Negatively Related: A Review. Front. Psychol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Kim, H.; Tobisawa, S.; Park, H.; Kim, J.; Lee, J.; Shin, D. Aging-Induced Degradation in Tracking Performance in Three-Dimensional Movement. SICE J. Control Meas. Syst. Integr. 2024, 17, 239–246. [Google Scholar] [CrossRef]
| PICOST | Questions | Area of Interest | Search Terms | Exclusion Criteria | |
|---|---|---|---|---|---|
| P | patient population problem | how would I describe a group of patients similar to mine? | ≥18 years old | “adult” or “elder” | <18 years old |
| I | intervention prognostic factor or exposure | which main intervention, prognostic factor or exposure am I considering? | motor rehabilitation with virtual reality. Dysfunctions of the upper limb. | (“re-education” or “rehabilitation” or “physical activity”) and (“virtual reality” or “vr” or “immersive virtual reality” or “HMD” or “head-mounted display” or “IVR”) and “upper limb” or fibromyalgia syndrome | non-immersive virtual reality |
| C | comparison or intervention | what is the main alternative to compare with the intervention? | no intervention | ||
| O | outcome you would like to measure or achieve | what can I hope to achieve, measure, influence? | 1—improvement of residual capacities and recovery of lost or damaged motor functions | ||
| 2—perceived quality of life | “Quality of life” or “QoL” | ||||
| 3—activities of daily living | “ADL” or “activity of daily living” | ||||
| S | study types | what is the best type/design? | RCT’s experimental analytical studies case report | ||
| T | time | are there any time restrictions? | filter: 2018–2023 | ||
| Article | Objective | Subjects | Tool | Duration | Activity | Outcome | Results | Limits |
|---|---|---|---|---|---|---|---|---|
| Patel et al. (2019) [24] | testing whether +8 h of intensive training with IVR improves impairment and changes in cortical reorganization compared to usual therapy | 13 subjects 30–80 years old | IVR + usual therapy vs. t. usual | 8 sessions of 1 h intensive training (200–300 movements) + 3 h usual therapy | hand activities | UEFMA; Wrist AROM; WMFT; surface EMG | changes in both groups in cortical reorganization. IVR results in better UEFMA and Wrist AROM of the pulse and in the amusement scale. Control group less tension. | small sample |
| Mekbib et al. (2020) [25] | check cortical and physical changes with unilat. and bilat. mirroring ex. with IVR | 8 subacute stroke and 13 healthy controls | IVR | 1 h VR and 1 h conventional therapy per day, 4 days/week for 2 weeks | catching and moving a ball with unilateral and bilateral mirroring es | FMA-UE; MRI | improvements in motor function and bilateral M1 connectivity | small population lack of control with usual therapy |
| Park et al. (2021) [26] | check whether IVR improves apraxic symptoms | 1 acute subject 56 years old | IVR vs. VR vs. AR vs. T.O. | 20 min a day, 5 days a week, for 4 weeks | reaching and grasping; consecutive grasping and releasing gestures | MMSE; UEFMA Modified Barthel Index; upper limb apraxia score test | improvements in apraxia with IVR and consecutive grasp and release gestures | large-scale studies needed |
| Article | Objective | Subjects | Tool | Duration | Activity | Outcome | Results | Limits |
|---|---|---|---|---|---|---|---|---|
| Schuster-Amft et al. (2018) [27] | the aim of this study was to compare virtual reality-based training with conventional therapy | 54 subjects | IVR vs. conventional therapy | 16 sessions, 45 min, 4 weeks | e.g., reaching, grasping, releasing. With Bi-Manua Trainer | BBT; CAHAI-13; SIS | similar effects between groups. Better IVR group | number of patients in each group |
| Erhardsson et al. (2020) [14] | exploring the potential of virtual reality for chronic stroke rehabilitation | 7 subjects | IVR | 10 weeks between 200 and 900 min | 5 commercial games | ARAT; BBT; questionnaire ABILHAND; FMA-UE | improvement of the Action Research Arm Test participants with more training has higher results | have a researcher, an expert in rehabilitation and VR games, on site for all training sessions |
| Weber et al. (2019) [28] | using IVR for mirror therapy | 10 subjects 25–68 years | IVR | 12 sessions of 3 treatment blocks for 5 min performed twice for a total of 30 min per session | 1st block: global limb movements 2nd block: lifting and moving rocks 3rd block: daily activities | FMA-UE; ARAT | non-significant improvement | small sample, relatively severely impaired subjects and insufficient intensity |
| Song et al. (2021) [29] | to determine the effect of an intervention tool combining an immersive VR system with bilateral upper limb training on EEG measurements in stroke patients with chronic hemiplegia | 12 subjects with hemiplegia | IVR bilateral arm training vs. normal bilateral | 5 times a week, 4 weeks, 30 min | daily activities, such as switching on lights, organizing a chest of drawers, organizing a kitchen, watering plants and buying items in a convenience store | UL function EMG; MFT; sensory function testing of the upper limb | increase in limb function in the IVR group, non-significant improvement in sensory function test, improvement in intrinsic sensory function in the standard group. No significant improvement in superior limb muscle activity | small sample, lack of prior studies with ECG |
| Mullick et al. (2021) [30] | identifying whether and to what extent cognitive–motor deficits in well-healed stroke individuals affect the ability to adapt | 13 strokes 63.9 ± 8.1 years 11 healthy 63.7 ± 10.9 years) | IVR | 4 experimental blocks consisting of 15, 60, 15 and 60 exercises, respectively. Rest 2 to 5 min between | reaching a bottle with obstacle avoidance in single and dual tasks with memorization activities | FMA-UE; elbow flexor spasticity CSI; WMFT; MAL; CAHM | better results in healthy subjects and positive correlation between confidence in arm strength and exercise success | results are limited to subjects with chronic mild stroke. The sample size was too small |
| Huang et al. (2022) [31] | to investigate the effects of VRT on serum markers of inflammation, oxidative stress and neuroplasticity, and on upper limb motor function in chronic stroke patients | 30 subjects | IVR vs. conventional occupational therapy | 16 sessions, 60 min, 2/3 sessions per week | commercial games | levels of: heme oxygenase 1, 8-hydroxy-2-deoxyguanosine, brain-derived neurotrophic factor, interleukin-6; FMA-UE; AROM, ARAT, RPE | positive results supporting IVR for biomarkers and FMA-EU and AROM and RPE by an average of 12 | subjects only with chronic stroke, lack of differences with subj. healthy, period too short, small sample |
| Article | Objective | Subjects | Tool | Duration | Activity | Outcome | Results | Limits |
|---|---|---|---|---|---|---|---|---|
| Cikajlo et al. (2019) [32] | study the functional improvements, motivational aspects and clinical effectiveness of IVR compared to non-immersive VR | 20 subjects | IVR vs. VR non-immersive | 10 sessions 3 weeks | grasping, moving the object and releasing | modified IMI; BBT; UPDRS | IVR better in handling time, tremor in UPDRS test and fun. The laptop group had fewer errors and less pressure/voltage. Both improved BBT | small sample |
| Sánchez-Herrera-Baeza et al. (2020) [16] | assessing quantitative and qualitative effects in IVR treatment | 6 subjects 69–80 years old | IVR | 30 min, 3 times a week, 6 weeks | upper limb function exercises + cognitive exercises | Jamar hydraulic hand dynamometer; BBT; PPT; ARAT; CSQ-8 | significant improvements in strength, fine movement and coarse co-ordination dexterity, and speed movements on the affected side, and high satisfaction but a mental challenge | fatigue reduced pause, professional monitoring required, small sample, cannot be generalized to all subjects with PD |
| Oña et al. (2020) [17] | to evaluate the validity, feasibility and psychometric properties of a fully immersive VR-BBT to assess manual dexterity in PD patients | 20 subjects mean age 74.38 ± 0.94 years | IVR BBT vs. Real BBT | 3 trials | shifting of cubes | physical BBT; virtual BBT; satisfaction questionnaire | correlation between VR-BBT and BBT; correlation between VR-BBT score with PD severity as measured by the Hoehn and Yahr scale | the sample included only patients with mild to moderate stage PD |
| Article | Objective | Subjects | Tool | Duration | Activity | Outcome | Results | Limits |
|---|---|---|---|---|---|---|---|---|
| Hashim et al. (2021) [19] | examining the impact of IVR in muscle training, coordination and motivation | 5 amputees, 5 able-bodied subjects | IVR | 4 weeks, 10 sessions, 1 h | games: Crate Whacker, Race the Sun, Fruit Ninja e Kaiju Carnage | physical BBT; virtual BBT; EMG; IMI | increased muscle strength and coordination for all. High scores for interest, perceived competence, choice and usefulness, but low for pressure and tension | an uninspiring game lowered motivation to finish it |
| Henriksen et al. (2017) [33] | creation of the illusion of the reacquisition of a limb | 3 amputees with phantom limb pain | IVR + electrostimulation | 15 sessions in 5 weeks of 60/90 min | 1 bending game 2 frequency discrimination game 3 position discrimination game | 7-point Likert scale questionnaire | two participants increased control of the amputated limb | lack of quantitative tests and small sample |
| Article | Objective | Subjects | Tool | Duration | Activity | Outcome | Results | Limits |
|---|---|---|---|---|---|---|---|---|
| Gulsen et al., 2022 [34] | evaluate effects of VR combined with exercise training in fibromyalgia patients | 20 fibromyalgic women (age 18–65) | IVR simulation | 2 sessions per week, 20’ each, for 8 weeks | football game (countering balls from different directions) + dungeon game (tilting the trunk to avoid guillotines) | pain VAS; MSOT; Tampa scale for kinesiophobia; FIQ; FSS | IVR had positive effects in reducing pain, kinesiophobia, fatigue and improving emotional aspect of life quality | only women included, small sample |
| Christensen et al., 2023 [35] | investigate VR effects on cold pain threshold, tolerability, intensity in fibromyalgia patients and pain-free subjects; explore correlations between VR and pain catastrophization | 22 fibromyalgic women + 22 healthy women (average 47,6) | IVR simulation | one session, 50’ with 20’ rest | birthday party simulation (while dominant foot is placed into ice water tub) | pain VAS, cold pain tolerance, PCS | IVR had positive effects in pain threshold of pain-free subjects but not among fibromyalgia patients. No correlation has been found regarding pain catastrophyzation | only women included, small sample, experimental pain and not clinic pain |
| Darnall et al., 2020 [36] | evaluate feasibility and efficacy of a self-administered VR program for chronic pain, compare the VR treatment with an audio-only treatment | 97 fibromyalgia and/or chronic pain patients (age 18–75) | IVR simulation | 12 to 24 sessions, 15’ each, in 21 days | visual biofeedback that amplifies the environment responding to the users’ physiological behavior during breathing exercises | DVPRS; average Pain Intensity 11-points scale; PCS; PGIC | IVR had positive effects in reducing pain intensity, stress and improving mood of participants; better than audio only | no pain meds are evaluated in this study, pain is self-measured |
| Tuck et al., 2022 [37] | test efficacy of VR in a chronic pain treatment center and assess the acceptability of an active VR treatment program | 29 fibromyalgia and/or chronic pain patients (age 18–70) | IVR simulation | 2 sessions per week for 6 weeks | commercially available games encouraging full-body movements (Fruit Ninja, Holodance et al.) | BPI; Tampa scale for kinesiophobia; PGIC | IVR had positive effects in reducing pain intensity, improving scores and fun in patients | no control group, possible placebo effect |
| Hoolahan et al., 2019 [38] | evaluate if VR can be used as physical activity approach in fibromyalgia patients | 8 adults | IVR simulation | 2 sessions, 5’ each | picking up, throwing and dodging snowballs | RPE, satisfaction questionnaire | IVR was perceived as motivating, reducing perceived effort. All subjects except one wanted to continue playing | no control group, possible placebo effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capriotti, A.; Moret, S.; Del Bello, E.; Federici, A.; Lucertini, F. Virtual Reality: A New Frontier of Physical Rehabilitation. Sensors 2025, 25, 3080. https://doi.org/10.3390/s25103080
Capriotti A, Moret S, Del Bello E, Federici A, Lucertini F. Virtual Reality: A New Frontier of Physical Rehabilitation. Sensors. 2025; 25(10):3080. https://doi.org/10.3390/s25103080
Chicago/Turabian StyleCapriotti, Alessandro, Sarah Moret, Eleonora Del Bello, Ario Federici, and Francesco Lucertini. 2025. "Virtual Reality: A New Frontier of Physical Rehabilitation" Sensors 25, no. 10: 3080. https://doi.org/10.3390/s25103080
APA StyleCapriotti, A., Moret, S., Del Bello, E., Federici, A., & Lucertini, F. (2025). Virtual Reality: A New Frontier of Physical Rehabilitation. Sensors, 25(10), 3080. https://doi.org/10.3390/s25103080







