Temporal Stability of Signal Quality in Non-Contact Biopotential Electrodes
Abstract
1. Introduction
- Sensing surface, also known as detection disk, which represents the immediate interface toward the human body;
- Preamplifier, which transforms the high impedance of the front end into low impedance for further amplification and processing;
- Data acquisition system for digitization of the data, enabling further digital signal processing.
- Sweat/micro-sweat;
- Temperature or temperature gradients;
- Change in the pressure on the sensor (also called “pressure” for the sake of simplicity and brevity);
- Inherent issues of the non-contact electrode topology.
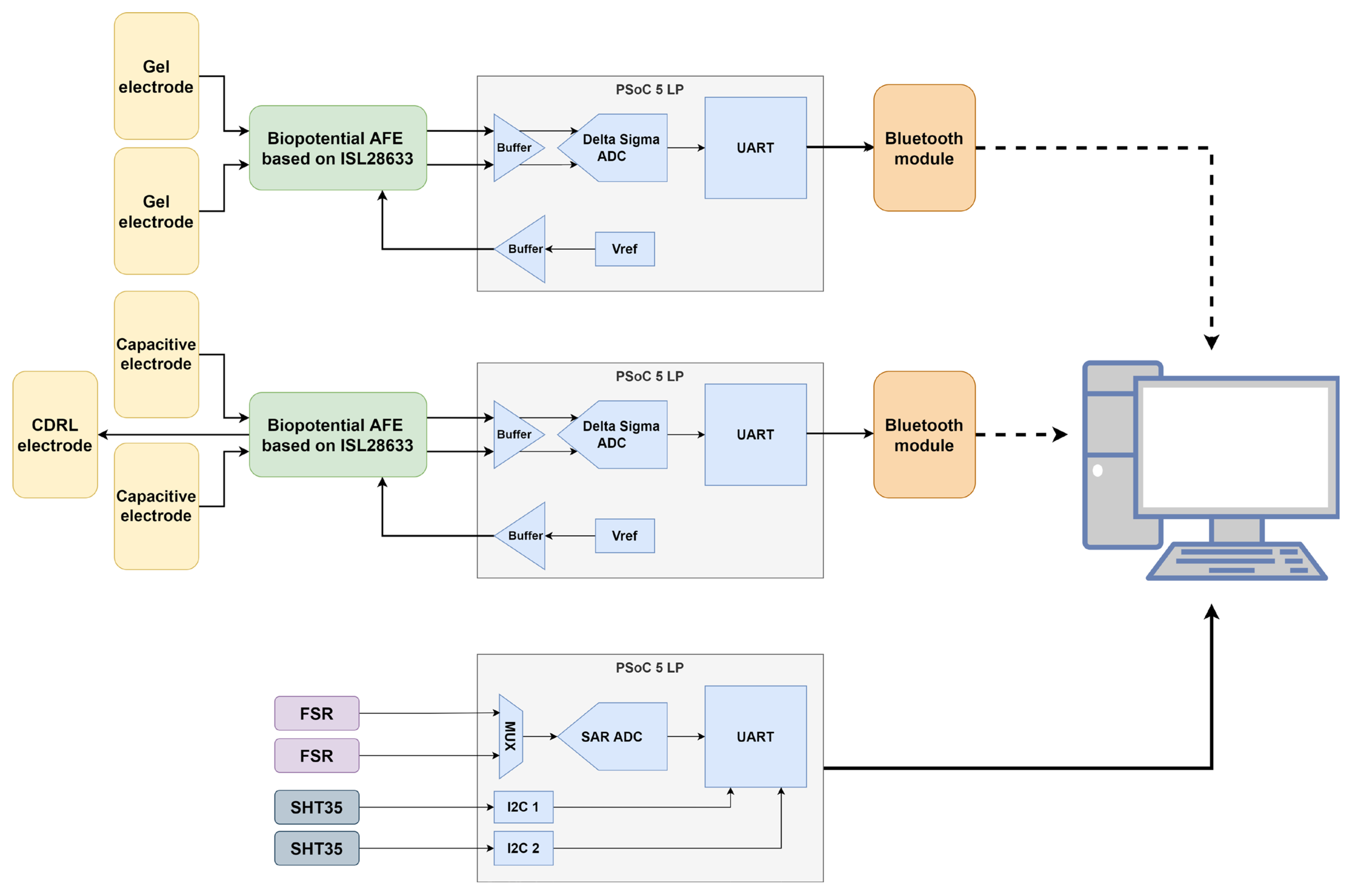
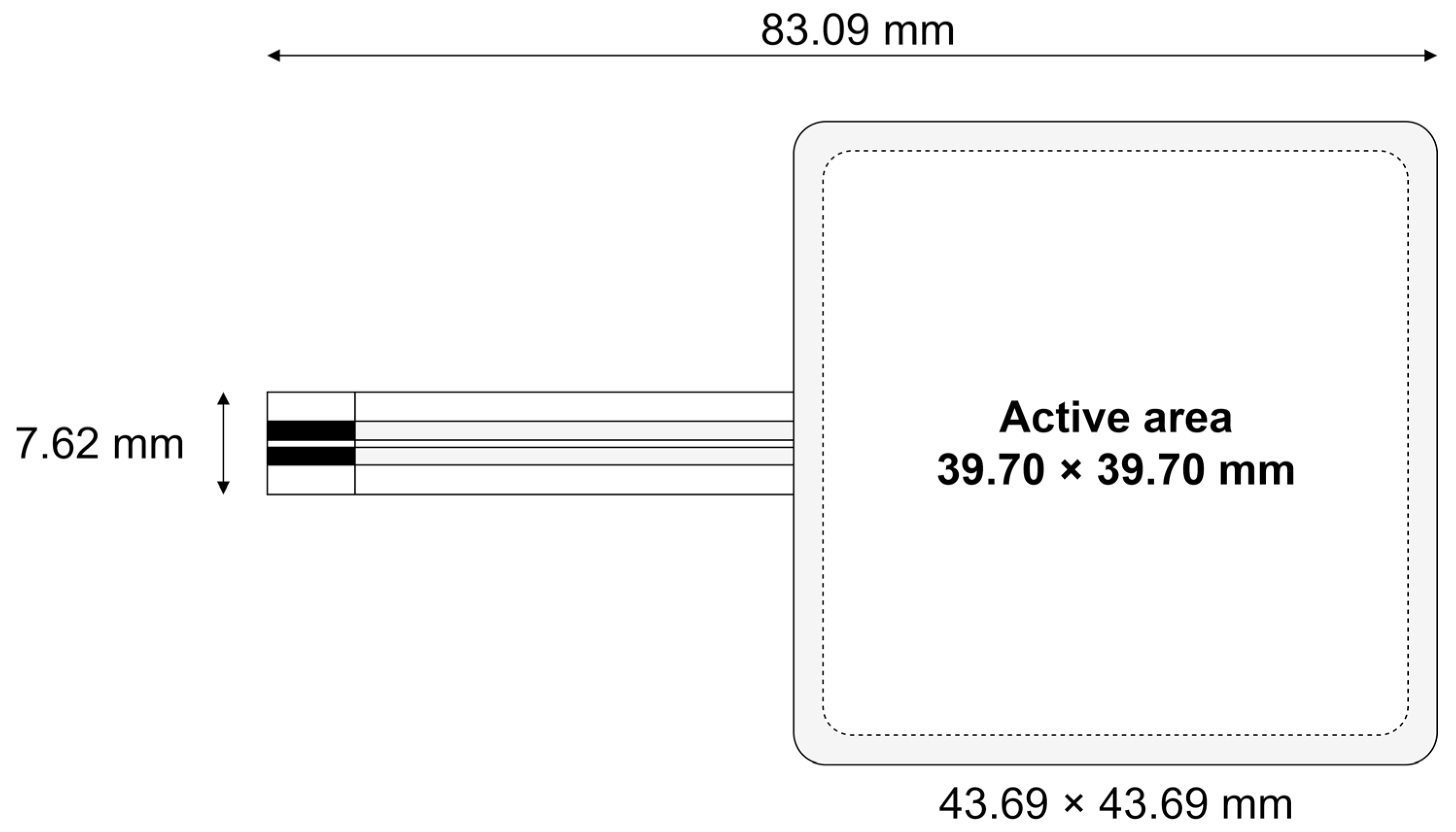
2. Materials and Methods
- An input differential low-pass filter—which serves as both a radiofrequency interference (RFI) filter and an anti-aliasing filter (AAF) with a corner frequency of 500 Hz;
- An ISL28633 (Renesas Electronics Corporation, Tokyo, Japan) fully differential instrumentation amplifier [39];
- A PSoC™ 5LP microcontroller (Infineon Technologies AG, Neubiberg, Germany) [40] that uses an internal Delta-Sigma analog-to-digital converter (ADC) for signal digitization.
- The subject is informed about the measurement procedure, reasons for collecting each of the recorded data and the whole context of the measurement, and gives consent if agrees with the presented conditions;
- The subject is seated in the seat with CDRL placed under the subject’s right leg;
- The skin on the subject’s wrists, where the gel electrodes will be placed, is cleaned and prepared for the adhesion of gel electrodes according to the common practice for such measurements;
- Gel electrodes are placed on each of the wrists of the subject;
- The subject places hands over the sensors according to Figure 7;
- The measurement assistant starts the measurement for each of the sensors and moves at least 2 m away from the subject to avoid influencing the measurements. The subject is instructed to remain as still as possible while breathing normally;
- After the effect has been clearly manifested (at least 10 min) and the subject starts to feel discomfort due to lack of movement, the measurement is completed, and electrodes are disposed of while the data are being saved. The pair of cloths was replaced for a new measurement.
3. Results
3.1. Stability of Gel Electrodes vs. Non-Contact Electrodes
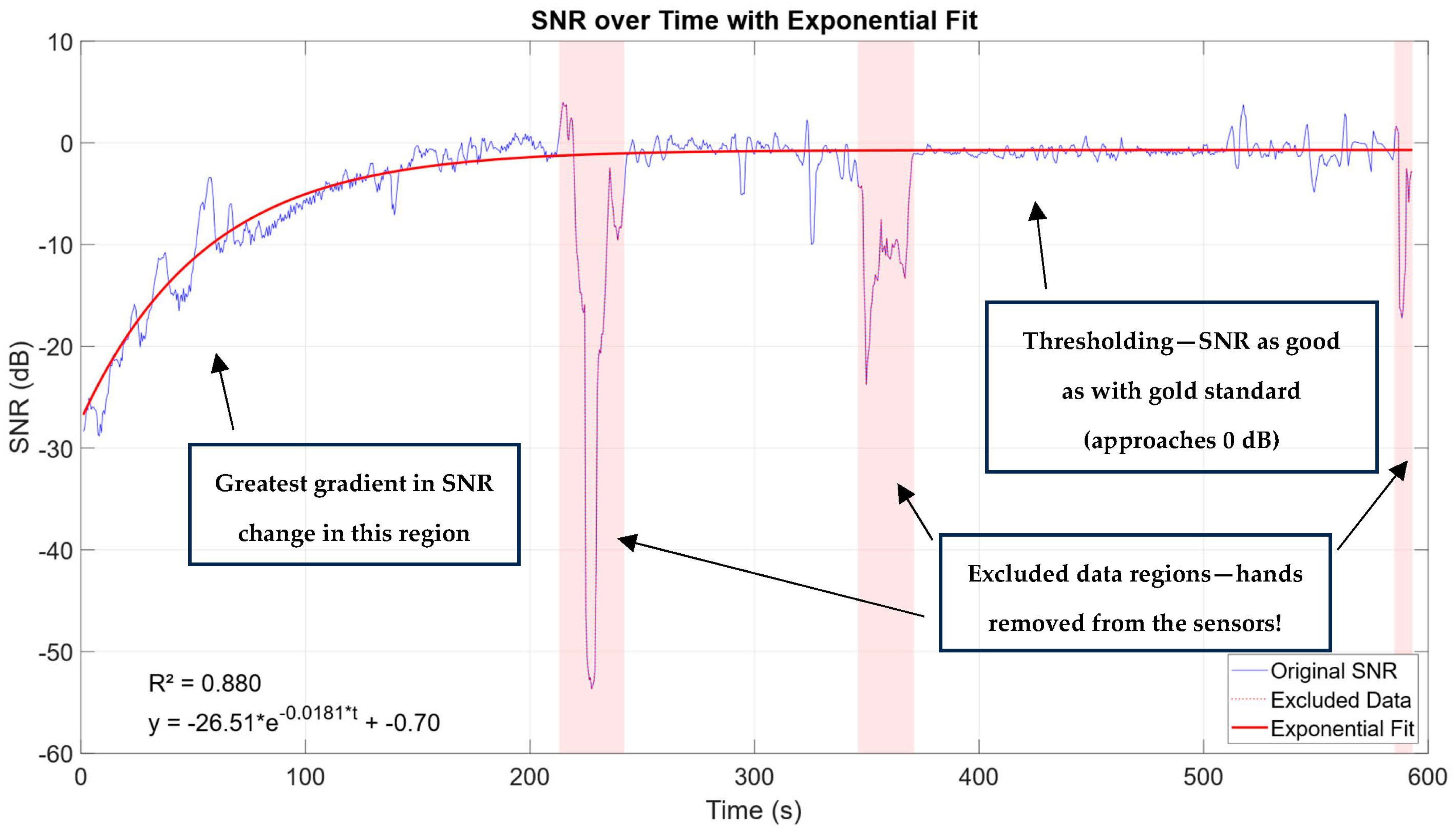
3.2. SNR Computation
3.3. SNR vs. Temperature
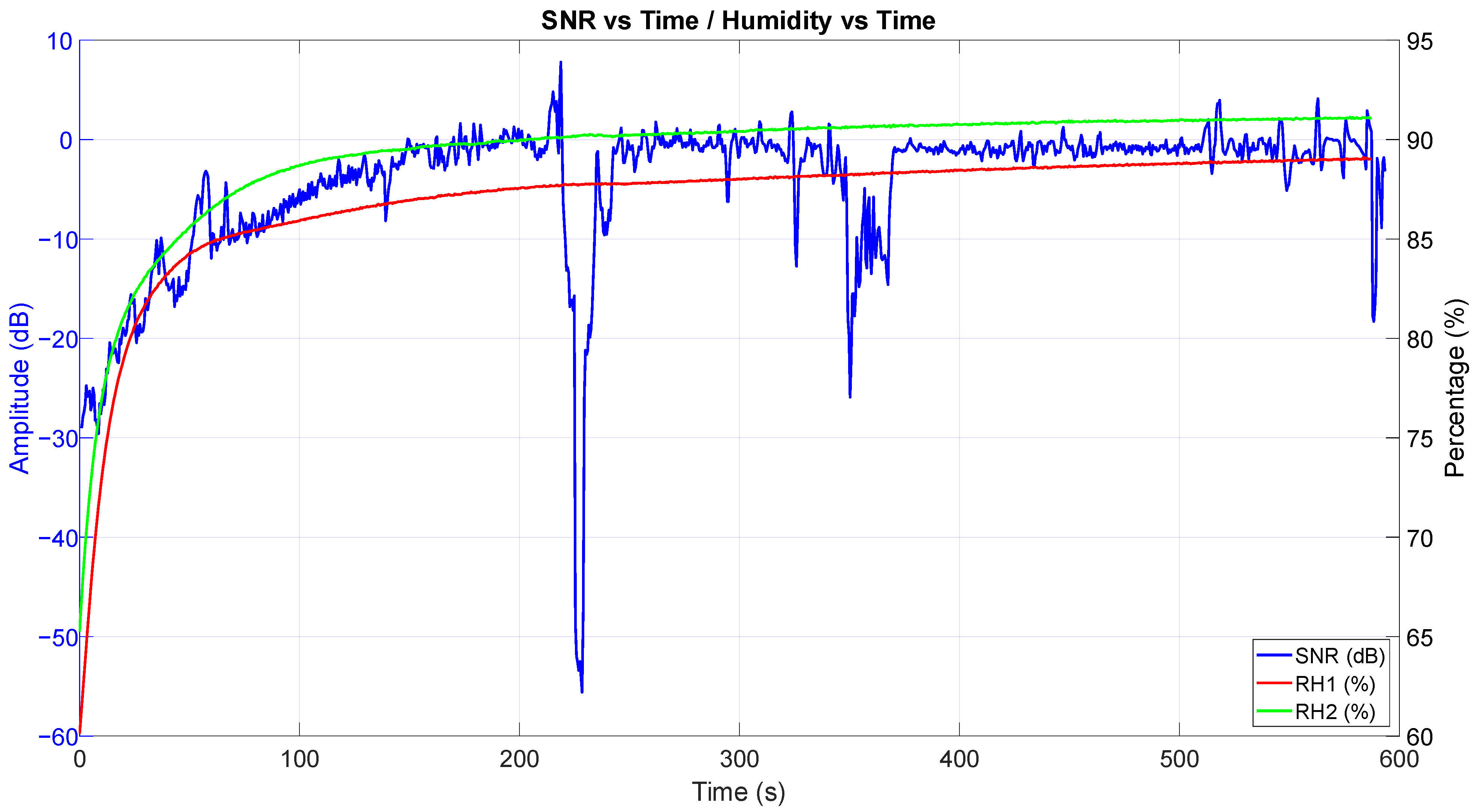
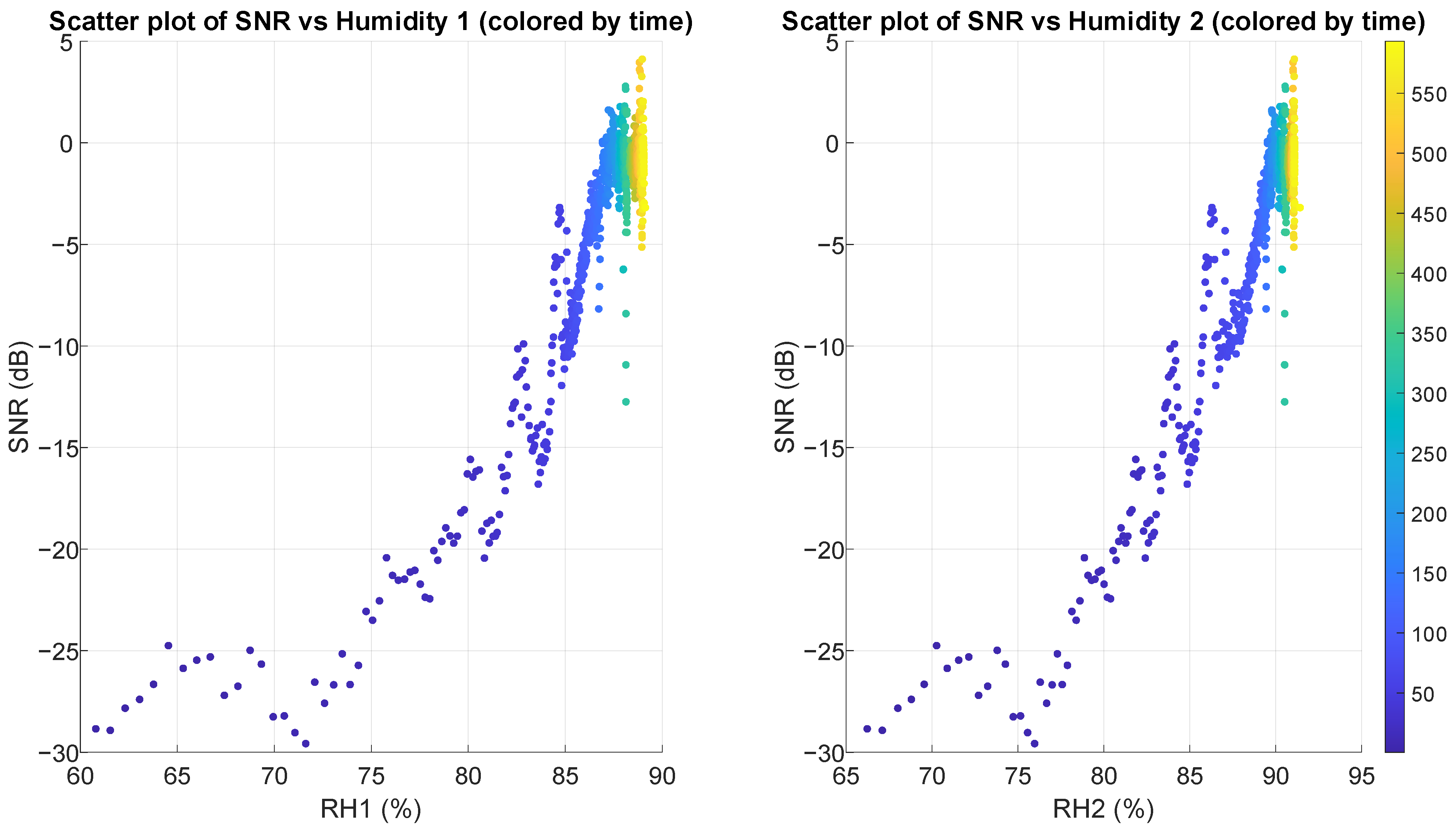
3.4. SNR vs. Relative Humidity
3.5. SNR vs. Pressure on the Sensor
3.6. Scatter Plots and Correlations
3.7. Parameter Isolation Tests
- Test of non-hygroscopic dielectric;
- Changing the initially used cloth for a new, dry cloth after the measurement has entered the steady state (SNR at 0 dB or better, after approx. 10 min);
- Testing SNR of a slightly damp cloth.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECG | electrocardiography |
| SNR | signal-to-noise ratio |
| cECG | capacitive electrocardiogram |
| CMVR | common-mode voltage range |
| CDRL | capacitive driven right leg |
| CMRR | common-mode rejection ratio |
| AFE | analog frontend |
| RFI | radiofrequency interference |
| AAF | anti-aliasing filter |
| ADC | analog-to-digital converter |
| FSR | force sensing resistor |
| DAC | digital-to-analog converters |
| SAR | successive approximation register |
| PCB | printed circuit board |
| RH | relative humidity |
| DFN | dual flat no-leads (package) |
| PVC | polyvinyl chloride |
| SPP | (Bluetooth) Serial Port Profile |
| COM | communication port |
References
- Chiang, H. Sen ECG-Based Mental Stress Assessment Using Fuzzy Computing and Associative Petri Net. J. Med. Biol. Eng. 2015, 35, 833–844. [Google Scholar] [CrossRef]
- Huang, S.; Li, J.; Zhang, P.; Zhang, W. Detection of Mental Fatigue State with Wearable ECG Devices. Int. J. Med. Inform. 2018, 119, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.; Kim, N.; Kim, S.; Lee, C.; Park, S.M. Deep ECG-Respiration Network (DeepER Net) for Recognizing Mental Stress. Sensors 2019, 19, 3021. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Wang, Y. Modeling and Recognition of Driving Fatigue State Based on R-R Intervals of ECG Data. IEEE Access 2019, 7, 175584–175593. [Google Scholar] [CrossRef]
- Choi, G.H.; Ko, H.; Pedrycz, W.; Singh, A.K.; Pan, S.B. Recognition System Using Fusion Normalization Based on Morphological Features of Post-Exercise ECG for Intelligent Biometrics. Sensors 2020, 20, 7130. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Chen, Y.; Xia, Y.; Qi, X.; Wan, X.; Jin, Y.; Liu, J.; He, Q.; Li, K.; et al. Robust, Self-adhesive, and Low-contact Impedance Polyvinyl Alcohol/Polyacrylamide Dual-network Hydrogel Semidry Electrode for Biopotential Signal Acquisition. SmartMat 2023, 5, e1173. [Google Scholar] [CrossRef]
- Li, G.L.; Wu, J.T.; Xia, Y.H.; He, Q.G.; Jin, H.G. Review of Semi-Dry Electrodes for EEG Recording. J. Neural Eng. 2020, 17, 051004. [Google Scholar] [CrossRef]
- Baek, H.J.; Chung, G.S.; Kim, K.K.; Park, K.S. A Smart Health Monitoring Chair for Nonintrusive Measurement of Biological Signals. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 150–158. [Google Scholar] [CrossRef]
- Uguz, D.U.; Dettori, R.; Napp, A.; Walter, M.; Marx, N.; Leonhardt, S.; Antink, C.H. Car Seats with Capacitive Ecg Electrodes Can Detect Cardiac Pacemaker Spikes. Sensors 2020, 20, 6288. [Google Scholar] [CrossRef]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECG Recording on a Bed during Sleep without Direct Skin-Contact. IEEE Trans. Biomed. Eng. 2007, 54, 718–725. [Google Scholar] [CrossRef]
- Atallah, L.; Serteyn, A.; Meftah, M.; Schellekens, M.; Vullings, R.; Bergmans, J.W.M.; Osagiator, A.; Oetomo, S.B. Unobtrusive ECG Monitoring in the NICU Using a Capacitive Sensing Array. Physiol. Meas. 2014, 35, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, C.W.; Hsieh, C.W. Noise-Resistant Cecg Using Novel Capacitive Electrodes. Sensors 2020, 20, 2577. [Google Scholar] [CrossRef]
- Bednar, T.; Carnecka, L.; Babusiak, B. Bioamplifier Using Active Electrodes. In Proceedings of the 2020 IEEE 21st International Conference on Computational Problems of Electrical Engineering, CPEE 2020, (Online Conference), Poland, 16–19 September 2020. [Google Scholar]
- Chi, Y.M.; Cauwenberghs, G. Wireless Non-Contact EEG/ECG Electrodes for Body Sensor Networks. In Proceedings of the 2010 International Conference on Body Sensor Networks, BSN 2010, Singapore, 7–9 June 2010. [Google Scholar]
- Portelli, A.J.; Nasuto, S.J. Design and Development of Non-Contact Bio-Potential Electrodes for Pervasive Health Monitoring Applications. Biosensors 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, S.Y.; Chang, W.Y. Novel Stable Capacitive Electrocardiogram Measurement System. Sensors 2021, 21, 3668. [Google Scholar] [CrossRef]
- Mao, P.; Lv, H.; Peng, H. Non-Contact Electrocardiogram Dry Electrode Design. In Proceedings of the Proceedings—2020 3rd International Conference on Advanced Electronic Materials, Computers and Software Engineering, AEMCSE 2020, Shenzhen, China, 24–26 April 2020. [Google Scholar]
- Moreno-Garcíaa, E.; Mújica-Ascenciob, S.; De La Rosa-Vázquezb, J.M.; Stolik-Isakina, S. A Capacitive Bioelectrode for Recording Electrophysiological Signals. Rev. Cuba. Fis. 2013, 30, 89–92. [Google Scholar]
- Shahandashti, P.F.; Pourkheyrollah, H.; Jahanshahi, A. Performance of PDMS-Based Inexpensive Stretchable Dry Electrodes in Long-Term SEMG Monitoring. In Proceedings of the 2020 28th Iranian Conference on Electrical Engineering, ICEE 2020, Tabriz, Iran, 4–6 August 2020. [Google Scholar]
- Liu, S.; Liu, X.; Jiang, Y.; Wang, X.; Huang, P.; Wang, H.; Zhu, M.; Tan, J.; Li, P.; Lin, C.; et al. Flexible Non-Contact Electrodes for Bioelectrical Signal Monitoring. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Honolulu, HI, USA, 18–21 July 2018. [Google Scholar]
- Jiang, Y.; Liu, L.; Chen, L.; Zhang, Y.; He, Z.; Zhang, W.; Zhao, J.; Lu, D.; He, J.; Zhu, H.; et al. Flexible and Stretchable Dry Active Electrodes with Pdms and Silver Flakes for Bio-Potentials Sensing Systems. IEEE Sens. J. 2021, 21, 12255–12268. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhu, M.; Jiang, Y.; Wang, D.; Wang, X.; Yang, W.; Chen, S.; Li, G. Flexible Non-Contact Electrodes for Minimizing Artifacts in Electrocardiograms Measurement during Motion. In Proceedings of the IET Conference Publications, Qinhuangdao, China, 18–21 November 2019; Volume 2019. [Google Scholar]
- Lim, Y.G.; Lee, J.S.; Lee, S.M.; Lee, H.J.; Park, K.S. Capacitive Measurement of ECG for Ubiquitous Healthcare. Ann. Biomed. Eng. 2014, 42, 2218–2227. [Google Scholar] [CrossRef]
- Texas Instruments. LMP7721—Ultra-Low Input Bias Current Operational Amplifier. Available online: https://www.ti.com/lit/gpn/lmp7721 (accessed on 26 March 2025).
- Haberman, M.A.; Spinelli, E.M.; García, P.A.; Guerrero, F.N. Capacitive Driven-Right-Leg Circuit Design. Int. J. Biomed. Eng. Technol. 2015, 17, 115. [Google Scholar] [CrossRef]
- Ueno, A.; Akabane, Y.; Kato, T.; Hoshino, H.; Kataoka, S.; Ishiyama, Y. Capacitive Sensing of Electrocardiographic Potential through Cloth from the Dorsal Surface of the Body in a Supine Position: A Preliminary Study. IEEE Trans. Biomed. Eng. 2007, 54, 759–766. [Google Scholar] [CrossRef]
- Eilebrecht, B.; Wartzek, T.; Willkomm, J.; Schommartz, A.; Walter, M.; Leonhardt, S. Motion Artifact Removal from Capacitive ECG Measurements by Means of Adaptive Filtering. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2011; Volume 37. [Google Scholar]
- Bhardwaj, R.; Balasubramanian, V. Viability of Cardiac Parameters Measured Unobtrusively Using Capacitive Coupled Electrocardiography (CECG) to Estimate Driver Performance. IEEE Sens. J. 2019, 19, 4321–4330. [Google Scholar] [CrossRef]
- Klaic, L.; Stanesic, A.; Cifrek, M. Numerical Modelling of Capacitive Electrodes for Biomedical Signals Measurement. In Proceedings of the 2021 44th International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 27 September–1 October 2021; pp. 367–370. [Google Scholar]
- Cindric, D.; Stanesic, A.; Cifrek, M. Analog Frontend for ECG/EMG Capacitive Electrodes. In Proceedings of the 2021 44th International Convention on Information, Communication and Electronic Technology, MIPRO 2021—Proceedings, Opatija, Croatia, 27 September–1 October 2021. [Google Scholar]
- Klaic, L.; Stanesic, A.; Cifrek, M. Numerical Modelling of Multi-Layered Capacitive Electrodes for Biomedical Signals Measurement. In Proceedings of the 2022 45th Jubilee International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 23–27 May 2022; pp. 339–343. [Google Scholar]
- Cindrić, D.; Klaić, L.; Stanešić, A.; Cifrek, M. Non-Contact Biopotential Amplifier with Capacitive Driven Right Leg Circuit. In Mediterranean Conference on Medical and Biological Engineering and Computing; Springer: Cham, Switzerland, 2024; pp. 180–188. [Google Scholar]
- Fong, E.-M.; Chung, W.-Y. A Hygroscopic Sensor Electrode for Fast Stabilized Non-Contact ECG Signal Acquisition. Sensors 2015, 15, 19237–19250. [Google Scholar] [CrossRef] [PubMed]
- Torreblanca González, J.; García Ovejero, R.; Lozano Murciego, Á.; Villarrubia González, G.; De Paz, J.F. Effects of Environmental Conditions and Composition on the Electrical Properties of Textile Fabrics. Sensors 2019, 19, 5145. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zheng, X.; Wang, K.; Chen, H.; Chen, W.; Chen, C.; Luo, J. Exploring Key Factors on Capacitive ECG Signal Quality Under Practical Considerations. IEEE Trans. Instrum. Meas. 2024, 73, 4011614. [Google Scholar] [CrossRef]
- Leicht, L.; Eilebrecht, B.; Weyer, S.; Leonhardt, S.; Teichmann, D. Closed-Loop Control of Humidification for Artifact Reduction in Capacitive ECG Measurements. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 300–313. [Google Scholar] [CrossRef]
- Kusche, R.; Kaufmann, S.; Ryschka, M. Dry Electrodes for Bioimpedance Measurements—Design, Characterization and Comparison. Biomed. Phys. Eng. Express 2018, 5, 015001. [Google Scholar] [CrossRef]
- Takano, M.; Yamagishi, S.; Ohmuta, T.; Fukuoka, Y.; Ueno, A. Non-Contact Simultaneous Measurements of Electrocardiogram and Respiratory Movements Using Capacitive Sheet Electrodes. Adv. Biomed. Eng. 2017, 6, 28–36. [Google Scholar] [CrossRef]
- Renesas ISL28533, ISL28534, ISL28535, ISL28633, ISL28634, ISL28635 Datasheet, FN8364. Available online: https://www.renesas.com/en/document/dst/isl28533-isl28534-isl28535-isl28633-isl28634-isl28635-datasheet?r=493636 (accessed on 31 March 2025).
- Cypress Semiconductor Corporation PSoC® 5LP: CY8C58LP Family Datasheet 001-84932 Rev. *O. 2024. Available online: https://www.infineon.com/dgdl/Infineon-PSoC_5LP_CY8C58LP_Family_Datasheet_Programmable_System-on-Chip_(PSoC_)-DataSheet-v15_00-EN.pdf?fileId=8ac78c8c7d0d8da4017d0ec547013ab9 (accessed on 26 March 2025).
- Klaić, L.; Čuljak, I.; Stanešić, A.; Vrhoci, I.; Šajinović, P.; Cifrek, M.; Džapo, H. Wireless Device for Biomedical Signal Acquisition with Dry Electrodes on an E-Bike. In European Congress on Biomedical and Veterinary Engineering; Springer: Cham, Switzerland, 2024; pp. 60–72. [Google Scholar]
- Stanešić, A.; Klaić, L.; Cindrić, D.; Cifrek, M. Analog Frontend for Capacitive Electrodes in Biomedical Applications. In Proceedings of the 2025 48th International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 2–6 June 2025. [Google Scholar]
- Jackson, J.D. Electrodynamics, Classical. In Digital Encyclopedia of Applied Physics; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Sensirion Datasheet SHT3x-DIS. Available online: https://sensirion.com/media/documents/213E6A3B/63A5A569/Datasheet_SHT3x_DIS.pdf (accessed on 26 March 2025).
- Ohmite FSR Series Force Sensing Resistor. Available online: https://www.ohmite.com/assets/images/res-fsr-rev-4.23.pdf (accessed on 26 March 2025).
- Bronzino, J.D.; Neuman, M.R. Biopotential Electrodes. In Medical Instrumentation: Application and Design, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]












| Age | Sex | BMI [kg/m2] | |
|---|---|---|---|
| Subject A | 29 | M | 23.9 |
| Subject B | 29 | F | 23.1 |
| Subject C | 55 | M | 28.7 |
| Subject D | 54 | F | 24.0 |
| Subject E | 58 | F | 22.5 |
| Subject F | 66 | M | 30.0 |
| Subject H | 21 | F | 20.7 |
| RH 1 | RH 2 | Temp 1 | Temp 2 | FSR1 | FSR2 | |
|---|---|---|---|---|---|---|
| Pearson r | 0.930 | 0.955 | 0.833 | 0.799 | −0.500 | −0.002 |
| Pearson p | 0 | 0 | 0 | 0 | <0.0001 | 0.947 |
| Spearman r | 0.758 | 0.757 | 0.758 | 0.758 | −0.400 | 0.117 |
| Spearman p | 0 | 0 | 0 | 0 | <0.0001 | 0.0003 |
| RH 1 | RH 2 | Temp 1 | Temp 2 | FSR1 | FSR2 | ||
|---|---|---|---|---|---|---|---|
| Subject A | Pearson r | 0.930 | 0.955 | 0.833 | 0.799 | −0.500 | −0.002 |
| Pearson p | 0 | 0 | 0 | 0 | <0.0001 | 0.947 | |
| Spearman r | 0.758 | 0.757 | 0.758 | 0.758 | −0.400 | 0.117 | |
| Spearman p | 0 | 0 | 0 | 0 | <0.0001 | 0.0003 | |
| Subject B | Pearson r | 0.785 | 0.804 | 0.969 | 0.957 | −0.155 | −0.668 |
| Pearson p | 0 | 0 | 0 | 0 | <0.0001 | 0 | |
| Spearman r | 0.820 | 0.820 | 0.820 | 0.820 | −0.266 | −0.636 | |
| Spearman p | 0 | 0 | 0 | 0 | <0.0001 | 0 | |
| Subject C | Pearson r | 0.946 | 0.976 | 0.911 | 0.892 | −0.601 | −0.234 |
| Pearson p | 0 | 0 | 0 | 0 | 0 | <0.0001 | |
| Spearman r | 0.755 | 0.703 | 0.754 | 0.756 | −0.534 | −0.333 | |
| Spearman p | 0 | 0 | 0 | 0 | <0.0001 | <0.0001 | |
| Subject D | Pearson r | 0.958 | 0.896 | 0.916 | 0.920 | 0.138 | −0.395 |
| Pearson p | 0 | 0 | 0 | 0 | <0.0001 | <0.0001 | |
| Spearman r | 0.793 | 0.704 | 0.793 | 0.793 | 0.043 | −0.414 | |
| Spearman p | 0 | 0 | 0 | 0 | 0.147 | <0.0001 | |
| Subject E | Pearson r | 0.815 | 0.829 | 0.856 | 0.866 | 0.085 | −0.135 |
| Pearson p | 0 | 0 | 0 | 0 | 0.005 | <0.0001 | |
| Spearman r | 0.867 | 0.873 | 0.872 | 0.873 | 0.194 | −0.117 | |
| Spearman p | 0 | 0 | 0 | 0 | <0.0001 | 0.0001 | |
| Subject F | Pearson r | 0.883 | 0.812 | 0.904 | 0.920 | −0.392 | −0.167 |
| Pearson p | 0 | 0 | 0 | 0 | <0.0001 | <0.0001 | |
| Spearman r | 0.809 | 0.782 | 0.811 | 0.813 | −0.749 | −0.131 | |
| Spearman p | 0 | 0 | 0 | 0 | 0 | <0.0001 | |
| Subject G | Pearson r | 0.888 | 0.982 | 0.940 | 0.820 | −0.237 | −0.115 |
| Pearson p | 0 | 0 | 0 | 0 | <0.0001 | 0.0009 | |
| Spearman r | 0.688 | 0.640 | 0.688 | 0.641 | −0.265 | −0.171 | |
| Spearman p | 0 | 0 | 0 | 0 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanešić, A.; Klaić, L.; Cindrić, D.; Cifrek, M. Temporal Stability of Signal Quality in Non-Contact Biopotential Electrodes. Sensors 2025, 25, 3077. https://doi.org/10.3390/s25103077
Stanešić A, Klaić L, Cindrić D, Cifrek M. Temporal Stability of Signal Quality in Non-Contact Biopotential Electrodes. Sensors. 2025; 25(10):3077. https://doi.org/10.3390/s25103077
Chicago/Turabian StyleStanešić, Antonio, Luka Klaić, Dino Cindrić, and Mario Cifrek. 2025. "Temporal Stability of Signal Quality in Non-Contact Biopotential Electrodes" Sensors 25, no. 10: 3077. https://doi.org/10.3390/s25103077
APA StyleStanešić, A., Klaić, L., Cindrić, D., & Cifrek, M. (2025). Temporal Stability of Signal Quality in Non-Contact Biopotential Electrodes. Sensors, 25(10), 3077. https://doi.org/10.3390/s25103077





