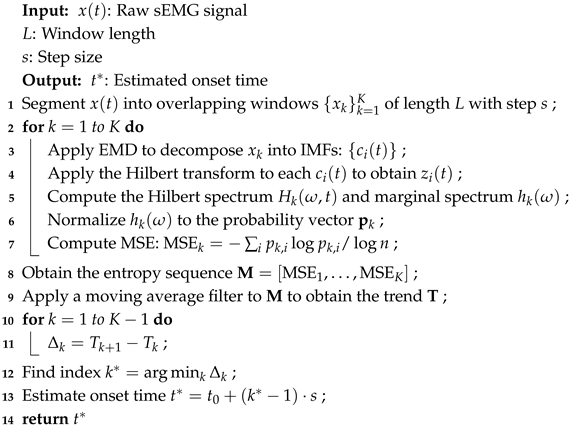
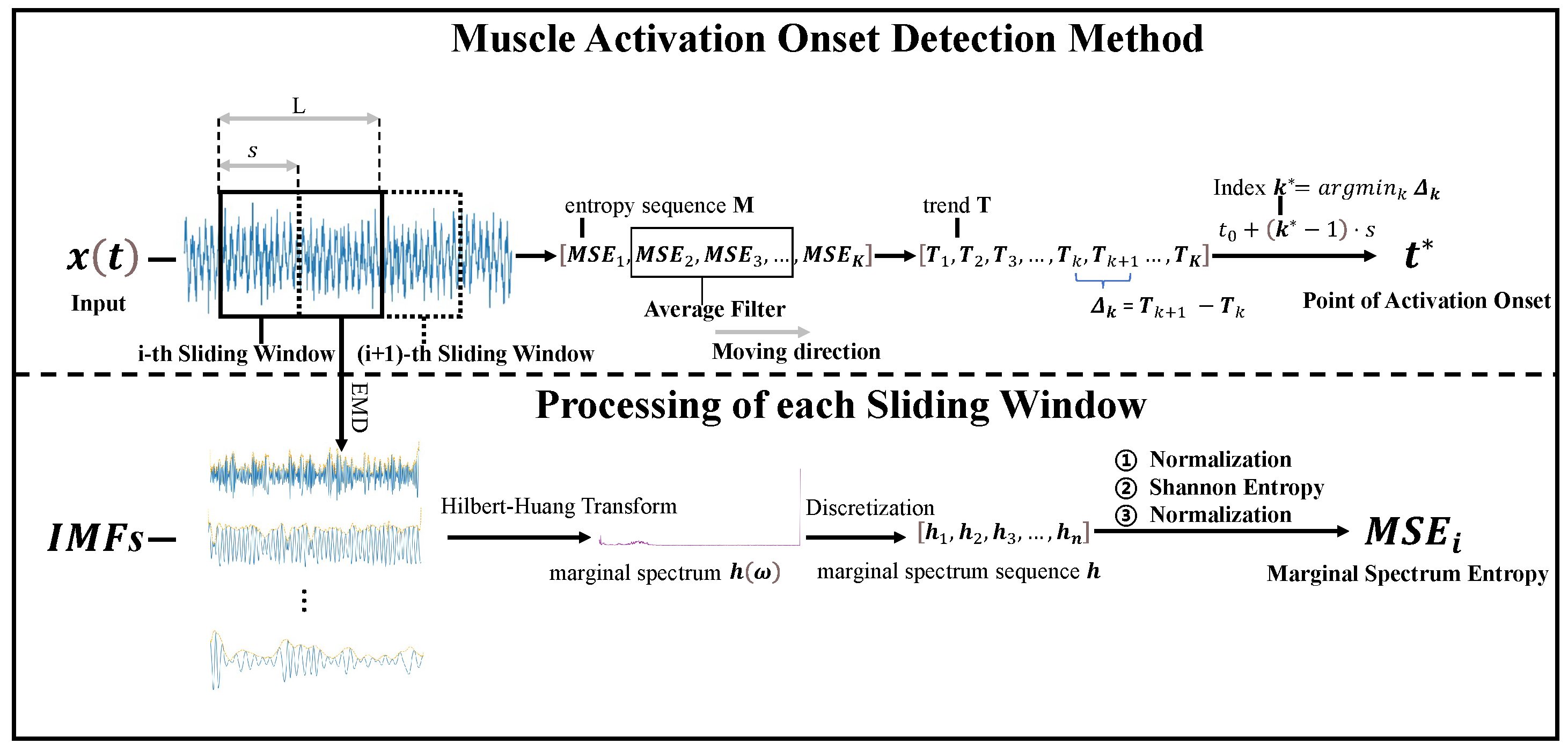
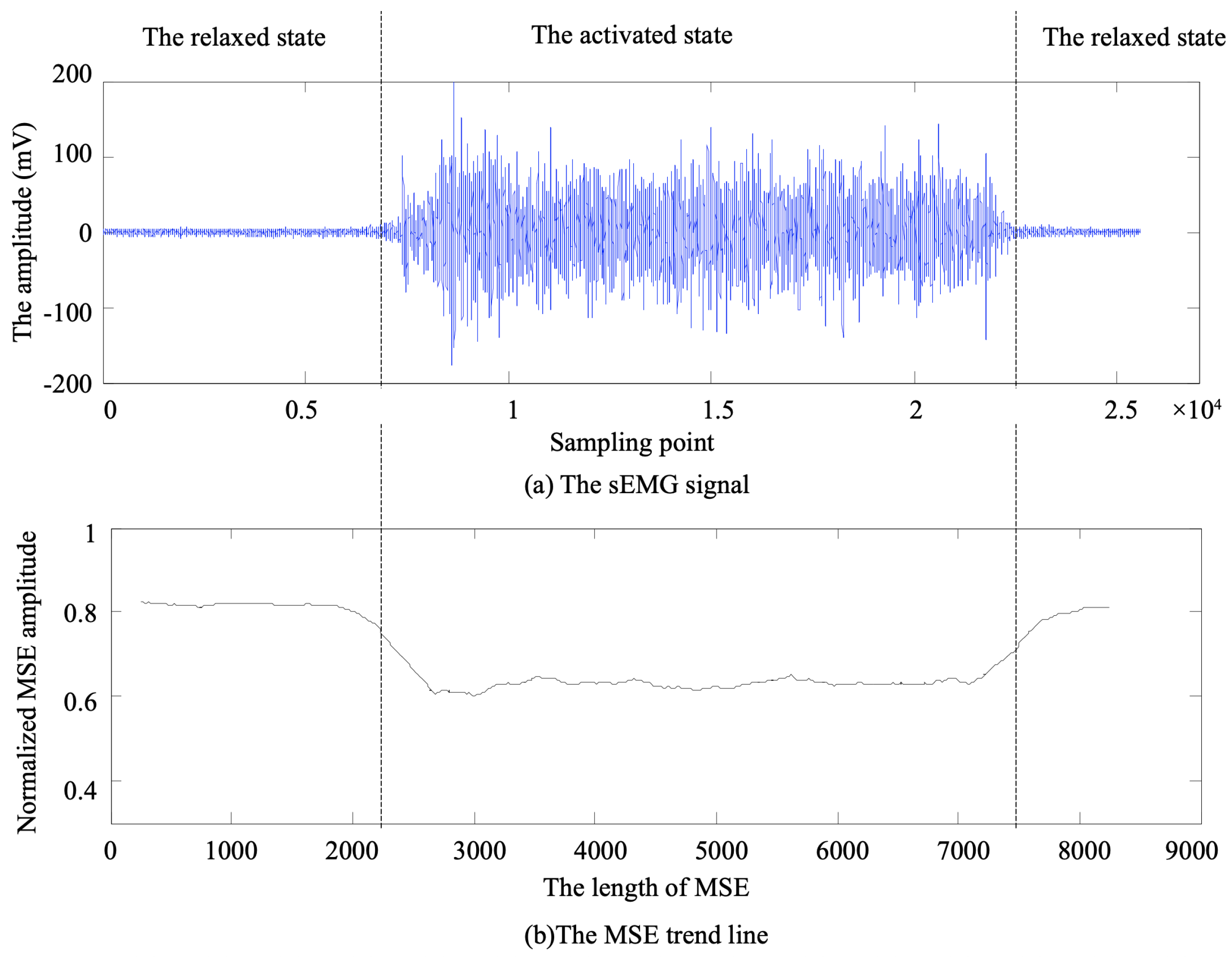
4.1. Performance Evaluation of Five Methods for sEMG Onset Detection
To evaluate the effectiveness of the proposed muscle activation onset detection method based on marginal spectrum entropy (MSE), a series of experiments was conducted using surface electromyography (sEMG) signals collected from the soleus muscle during natural walking. A total of 20 valid sEMG sequences were analyzed and compared against four widely used baseline methods, as follows:
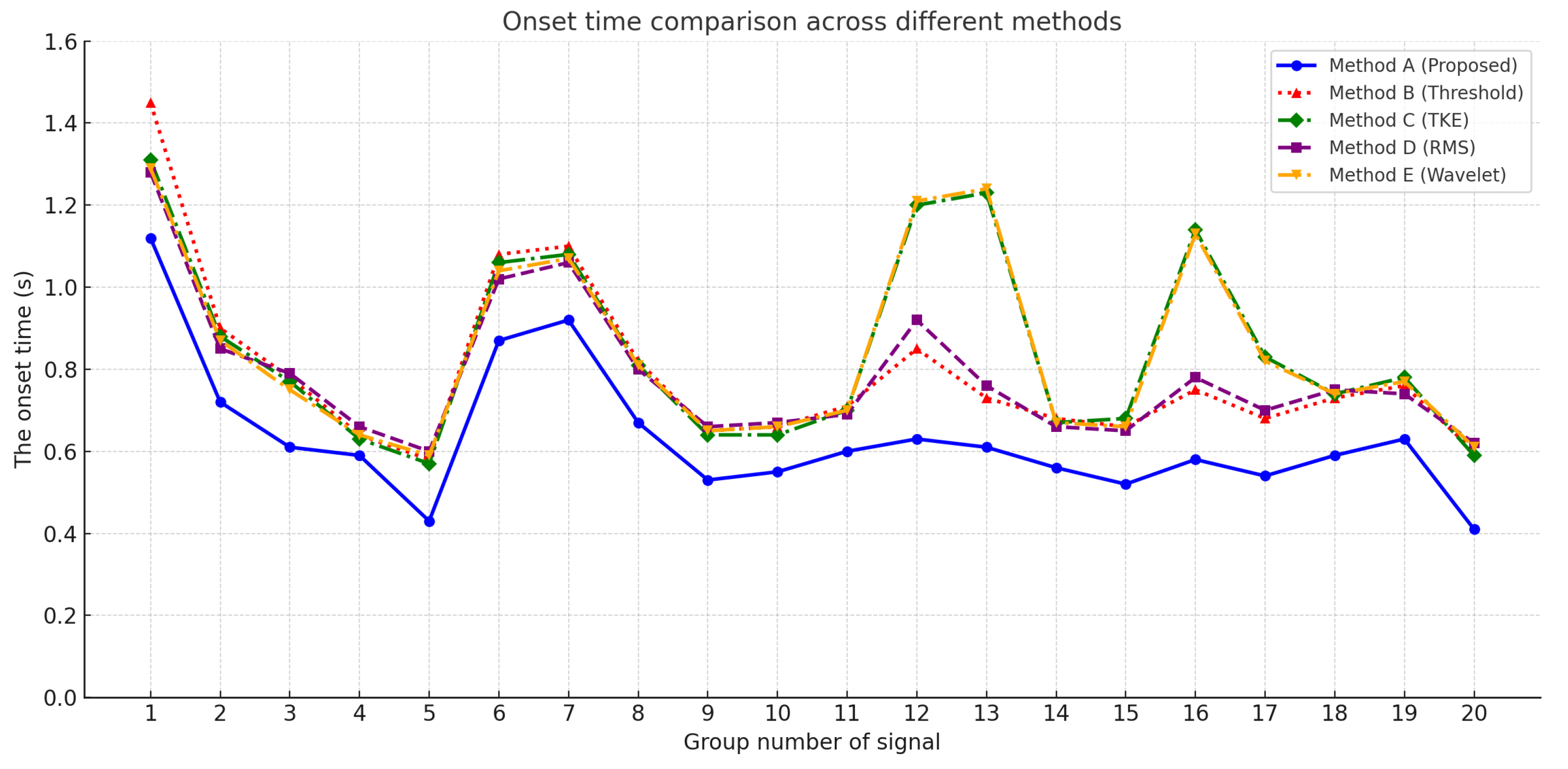
As shown in
Figure 6, the onset times detected by all five methods are plotted across 20 signal groups. It is evident that the proposed method consistently achieves earlier activation detection in most cases compared to the alternatives.
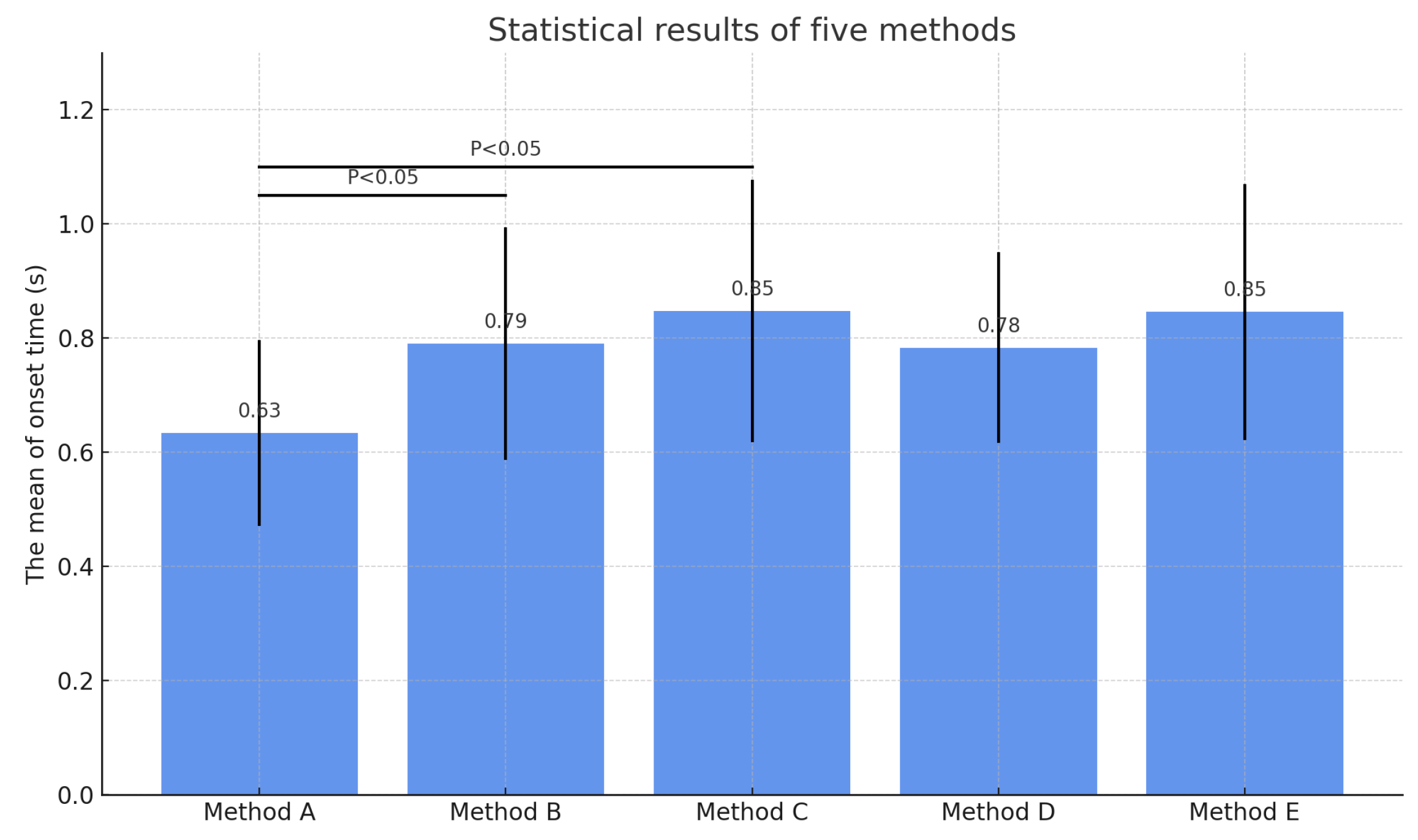
To further compare the methods in terms of average performance, a statistical bar chart was generated, as shown in
Figure 7. Each bar represents the mean onset time for the corresponding method, with error bars indicating standard deviations. Statistical significance annotations (
) were added to highlight differences between the proposed method and other methods.
As shown in
Figure 7, the proposed method not only achieves the earliest detection on average but also exhibits the smallest variance, indicating higher stability and consistency. In order to quantitatively assess detection performance, multiple evaluation metrics were computed, including sensitivity, specificity, false negative rate (FNR), false positive rate (FPR), root mean square error (RMSE), and detection advance. The results are summarized in
Table 5.
The proposed method outperforms all baselines across the listed metrics, especially in FNR and FPR, indicating a significantly lower probability of both missed detections and false positives. Moreover, the lowest RMSE suggests enhanced temporal precision in estimating onset time. From a clinical perspective, although an advance of 0.14–0.16 s may appear small in absolute terms, it can be crucial for real-time rehabilitation systems. For instance, in post-stroke gait training or wearable exoskeleton control, early identification of muscle activation enables faster system response, more synchronized actuation, and reduction of fatigue or coordination delays. The proposed approach thus holds practical value for both engineering applications and clinical interventions. While Method B and Method C can, in theory, perform real-time detection based on instantaneous features, the proposed MSE method is currently designed for offline analysis with a full signal window.
4.2. Ablation Study Analysis
To further evaluate the effectiveness of each core component within the proposed model, two ablation experiments were conducted. These experiments were designed to assess the contributions of the marginal spectrum entropy (MSE) module and the slope-based trend analysis module to the overall detection performance. In the first ablation setting, the MSE computation was removed and replaced by a simple moving average of signal energy within the same sliding window. The activation onset was then identified using the slope analysis of the energy signal. In the second ablation setting, the slope-based detection component was eliminated, and the onset was directly determined by identifying the minimum point on the MSE curve, without calculating the temporal slope. The simplified variants were compared against the complete model to evaluate the independent contributions of each module.
As shown in
Table 6, the full model achieved the best performance across all metrics, including the highest sensitivity (
), specificity (
), and the lowest false negative rate (FNR =
) and false positive rate (FPR =
). The full configuration also attained the lowest root mean square error (RMSE =
s) and the most advanced average detection lead time (
s).
To provide a more intuitive comparison of the effects of different ablation configurations,
Figure 8 illustrates the variations in detection advance and RMSE across the three configurations. As observed, the full model (denoted as Full) consistently outperformed the two simplified variants in both metrics, with a detection advance of
s and a minimal RMSE of
s.
Based on the combined analysis of
Table 6 and
Figure 8, both the MSE and slope modules are found to be critical to the model’s effectiveness. Removing the MSE component results in a substantial drop in sensitivity and an increase in detection error, indicating that spectral entropy plays a vital role in capturing the frequency complexity changes associated with slow muscle activations. Similarly, eliminating the slope-based detection module leads to a degradation in temporal localization accuracy, with RMSE increasing from
to
s, suggesting that the trend analysis contributes significantly to precise onset estimation. The integration of both modules substantially enhances the model’s responsiveness and robustness, particularly in handling gradually activating muscle signals.
4.2.1. Validation of the Sliding Window Mechanism
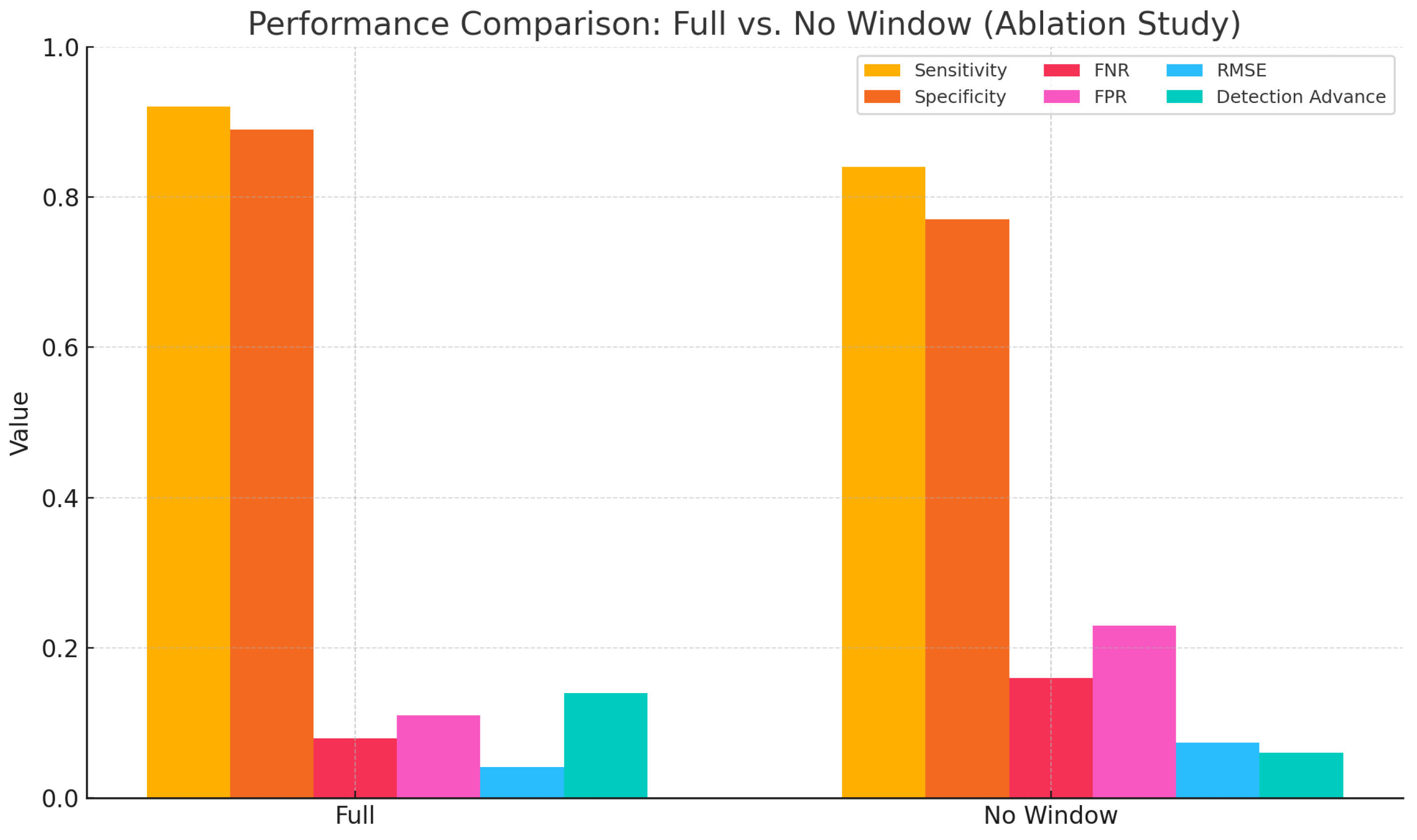
To further evaluate the impact of the sliding window mechanism on detection stability, an ablation experiment was conducted. In this configuration, the sliding window used for computing both marginal spectrum entropy (MSE) and the trend slope was removed. Instead, single-point values were extracted globally for decision-making. Compared to the complete model, this simplified version lacked local smoothing and statistical support, which may result in detection fluctuations and increased sensitivity to noise. As shown in
Table 7, the removal of the sliding window led to a noticeable degradation in performance. Specifically, the root mean square error (RMSE) increased to
s, the detection advance decreased to
s, and both the false negative rate (FNR) and false positive rate (FPR) worsened. These results indicate that the sliding window plays a critical role in suppressing local signal fluctuations and enhancing the stability of feature representation. To further illustrate the contribution of the sliding window across various performance metrics, a comparative bar chart is presented in
Figure 9. The figure clearly demonstrates that the removal of the sliding window led to overall declines in detection robustness and accuracy.
These findings further demonstrate that the sliding window not only enhances the smoothness of feature extraction but also maintains temporal resolution while improving model robustness under low-amplitude, fluctuating signals. Its structural support is particularly critical when dealing with slow activations and non-abrupt myoelectric patterns.
4.2.2. Discussion
A novel muscle activation onset detection method based on the fusion of marginal spectrum entropy (MSE) and trend slope analysis was proposed, and its superior sensitivity and temporal accuracy under slow activation conditions were demonstrated through comparative experiments with mainstream methods. Existing techniques, such as fixed thresholding, Teager–Kaiser energy operator (TKE), root mean square (RMS) averaging, and wavelet-based energy decomposition, generally rely on fixed amplitude thresholds, instantaneous energy variations, or frequency domain energy distributions. These methods are typically effective in detecting abrupt or rapid activations. However, under conditions involving gradually increasing activations, slowly rising signals, or low signal-to-noise ratios, such approaches often suffer from delayed triggering or missed detections. In contrast, the proposed method leverages the sensitivity of MSE to frequency complexity and the precise tracking ability of trend slope analysis for slow-varying patterns. This fundamental distinction in methodological design enhances robustness and adaptability in non-abrupt activation scenarios, making the approach particularly suitable for progressive control tasks such as gait initiation, rehabilitation training, and functional electrical stimulation. Despite the promising results, several limitations regarding the generalizability of the method should be acknowledged. The evaluation was conducted on only 20 sEMG signal samples, all collected from the soleus muscle during natural walking conditions. The soleus, as a primary plantar flexor, exhibits relatively slow activation dynamics, rendering it a suitable test case for the proposed approach. However, significant differences exist across muscle groups in terms of morphology, fiber composition, and activation rhythms. Therefore, whether the proposed method maintains its performance on other muscles—such as the rectus femoris, biceps femoris, or forearm muscle groups—remains to be further investigated. Moreover, the limited sample size restricts the assessment of the method’s generalization under broader variations, including inter-subject diversity, changes in posture, and external load conditions. Future work may involve expanding the dataset to include multiple muscles and tasks, and incorporating transfer learning strategies across subjects to enhance the model’s adaptability and practical scope. Although the proposed method achieves higher precision and lower FNR, its offline nature limits its use in closed-loop real-time applications. Future work will explore an incremental entropy update mechanism to enable low-latency deployment.
From a quantitative perspective, the proposed method enables muscle onset detection 0.14∼0.16 s earlier, on average, than the baseline methods. Although this lead time appears modest in engineering terms, it holds critical importance in real-time control systems. For instance, in neurorehabilitation robotics, even a s increase in system response delay may cause gait asynchrony or incorrect muscle stimulation, thereby negatively affecting patient recovery. In movement feedback control, earlier intention recognition significantly enhances system responsiveness and naturalness. Such advantages are particularly relevant in time-sensitive clinical applications such as early-stage post-stroke rehabilitation, assistance for muscular weakness, and brain-computer interface-based interventions. Additionally, a preliminary interpretation of “slow activation” in sEMG signals was explored. In practical acquisition scenarios, slow activation does not merely refer to gradual amplitude changes but rather describes an activation process characterized by smooth transitions in both time and frequency domains, lacking clear-cut boundaries or abrupt features. These types of signals are more vulnerable to background noise, asynchronous activations, and inter-individual variability, which pose challenges for traditional detection algorithms. By combining spectral entropy with slope analysis, the proposed method captures weak yet continuous trends embedded in the signal, thereby enabling the identification of subtle precursors to activation onset. This mechanism not only proved effective in the soleus muscle scenario presented here but also holds the potential for extension to more complex conditions involving neuromuscular impairments or fatigue.
4.2.3. Parameter Sensitivity and Justification
To validate the rationality of the parameter settings used in this study, a sensitivity analysis was conducted focusing on two key parameters, that is, the sliding window length and the resolution of marginal spectrum entropy (MSE) computation. In the main experiments, the window length was set to 128 ms with a 50% overlap. This choice was guided by previous literature recommending similar values for extracting mid-to-low-frequency features in EMG signals, as it balances temporal resolution with sufficient spectral information. To assess robustness, we varied the window length between 96 ms and 192 ms and observed that the model yielded the most stable performance around 128 ms, with the lowest RMSE and false negative rate (FNR), confirming its effectiveness in capturing gradual signal transitions. For MSE calculation, we employed a Hilbert–Huang transform (HHT)-based marginal entropy measure. The process involved decomposing the signal using empirical mode decomposition (EMD), computing the instantaneous spectrum, and estimating the marginal spectral density, followed by normalized entropy calculation. The number of modes was limited to a maximum of six, and frequency components were discretized using equal-width binning for entropy estimation. Experimental results demonstrated that this configuration offered strong trend-tracking capabilities for slowly activated signals and provided better stability and interpretability than traditional multiscale entropy or short-time Fourier-based approaches. These parameter choices are clarified in the Methods section and further analyzed in the Results section.
4.2.4. Limitation and Future Work
Although the proposed muscle activation onset detection method based on marginal spectrum entropy (MSE) has demonstrated promising performance in slow activation scenarios, several limitations may affect its generalizability and broader applicability. First, the dataset used in this study is relatively limited in both sample size and diversity. The current experiments include only 20 sEMG sequences collected from 10 male and 10 female healthy adults, with recordings performed during natural walking and limited to the right soleus muscle. While the soleus, a muscle rich in slow-twitch fibers, is well-suited for evaluating the detection performance under slow activation conditions, focusing exclusively on this muscle restricts the extrapolation of results to other muscle groups or activation patterns. Second, the experimental task—level-ground walking—represents a relatively simple locomotor activity and does not cover more complex or varied movement types, such as upper limb motions, high-speed activities (e.g., running), or postural transitions. As such, the applicability of the proposed method in scenarios involving rapid muscle recruitment or co-contraction remains to be further validated. Third, all participants were healthy, right-foot dominant, and free from any history of neuromuscular disorders, which may introduce selection bias and limit the clinical relevance of the findings to populations such as stroke survivors, elderly individuals with sarcopenia, or patients with neurological impairments. To address these limitations, future work will focus on three main directions. First, the dataset will be expanded to include additional muscle groups (e.g., rectus femoris, biceps brachii, tibialis anterior) and a wider range of motor tasks (e.g., sit-to-stand transitions, reaching, stair climbing), in order to comprehensively evaluate the anatomical and functional generalizability of the method. Second, the subject population will be broadened to include elderly individuals, patients with neuromotor impairments, and clinical populations at different stages of rehabilitation, to assess robustness and translational potential. Third, domain adaptation and transfer learning techniques will be explored to systematically enhance cross-subject generalization, reduce reliance on subject-specific calibration, and improve performance in real-world deployment settings.