The Effect of Filtering on Signal Features of Equine sEMG Collected During Overground Locomotion in Basic Gaits
Abstract
1. Introduction
2. Materials and Methods
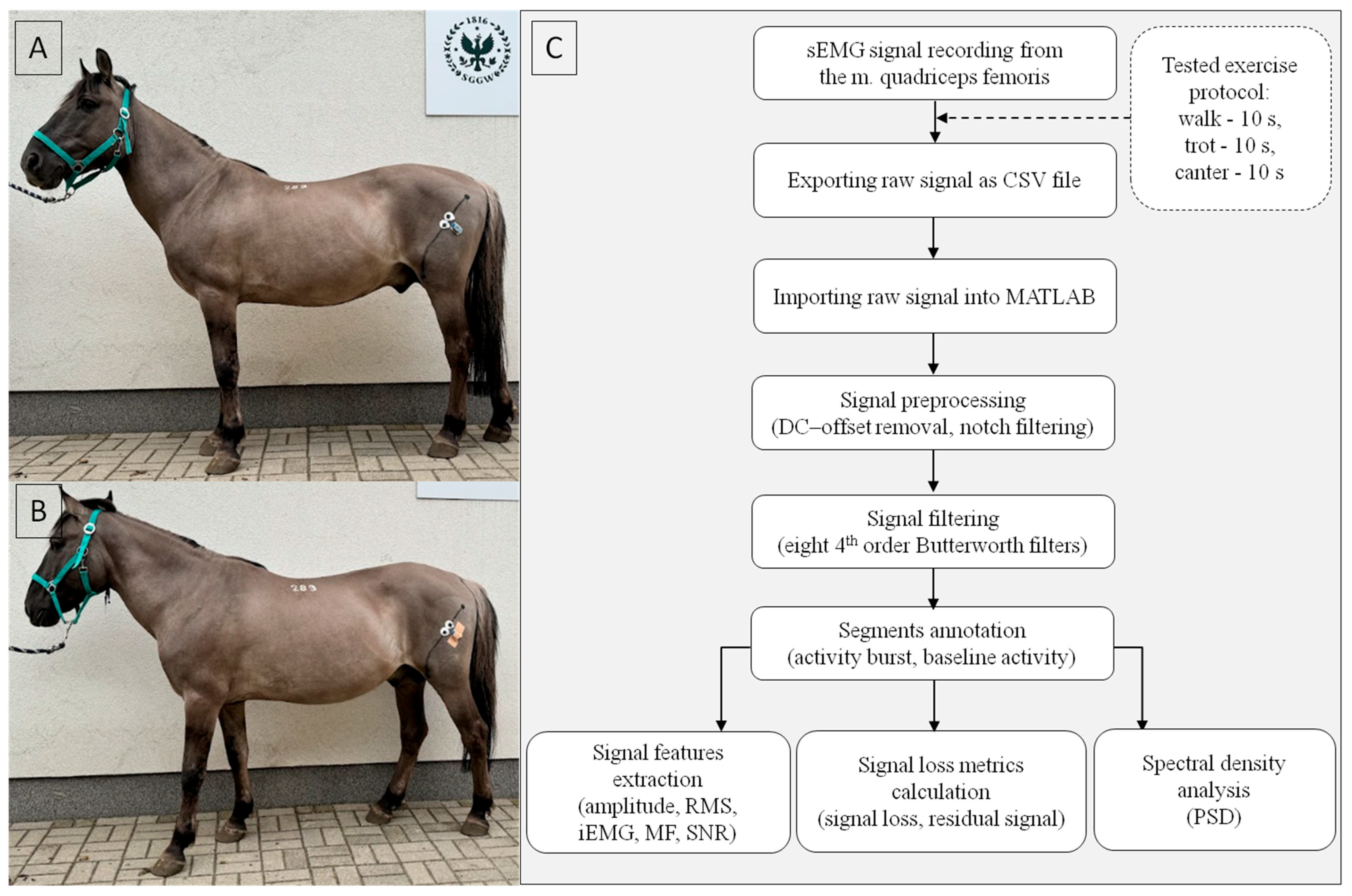
2.1. sEMG Signal Collection
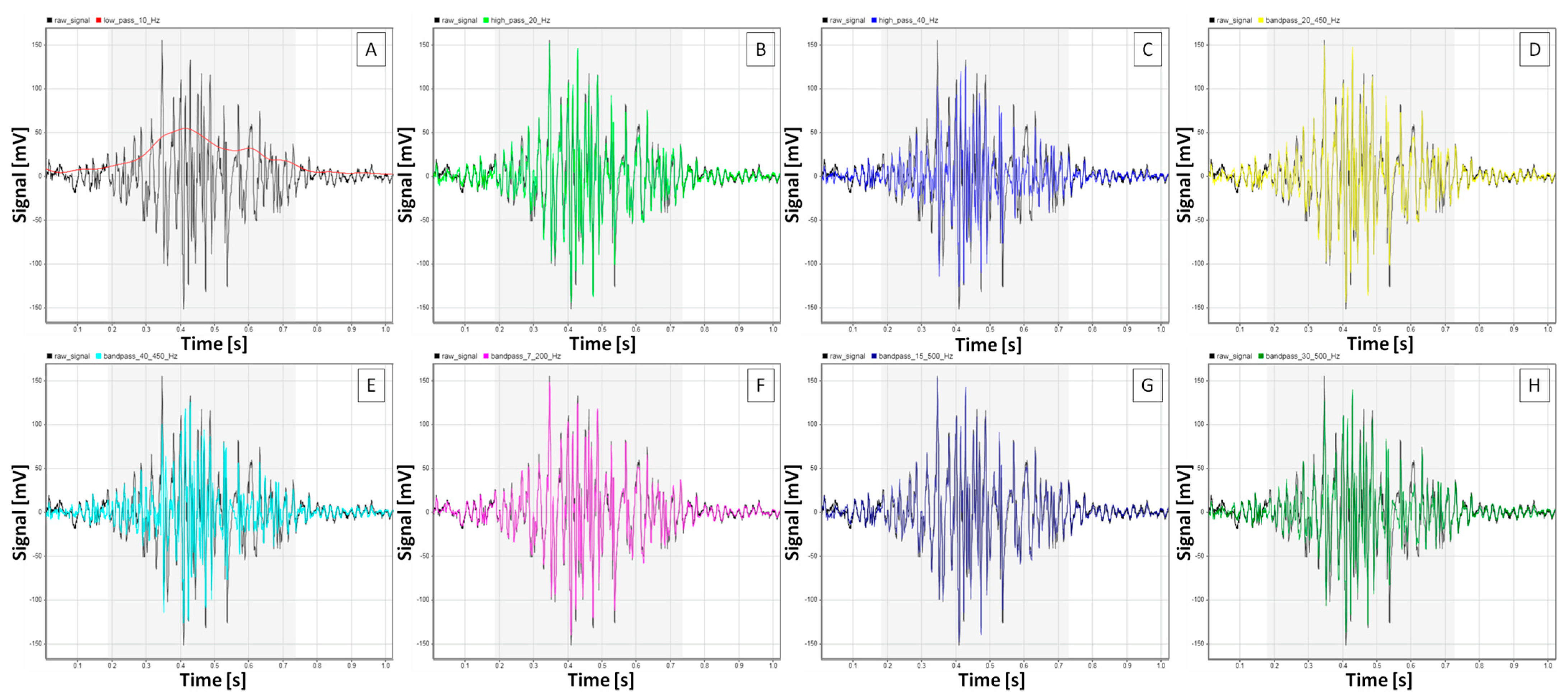
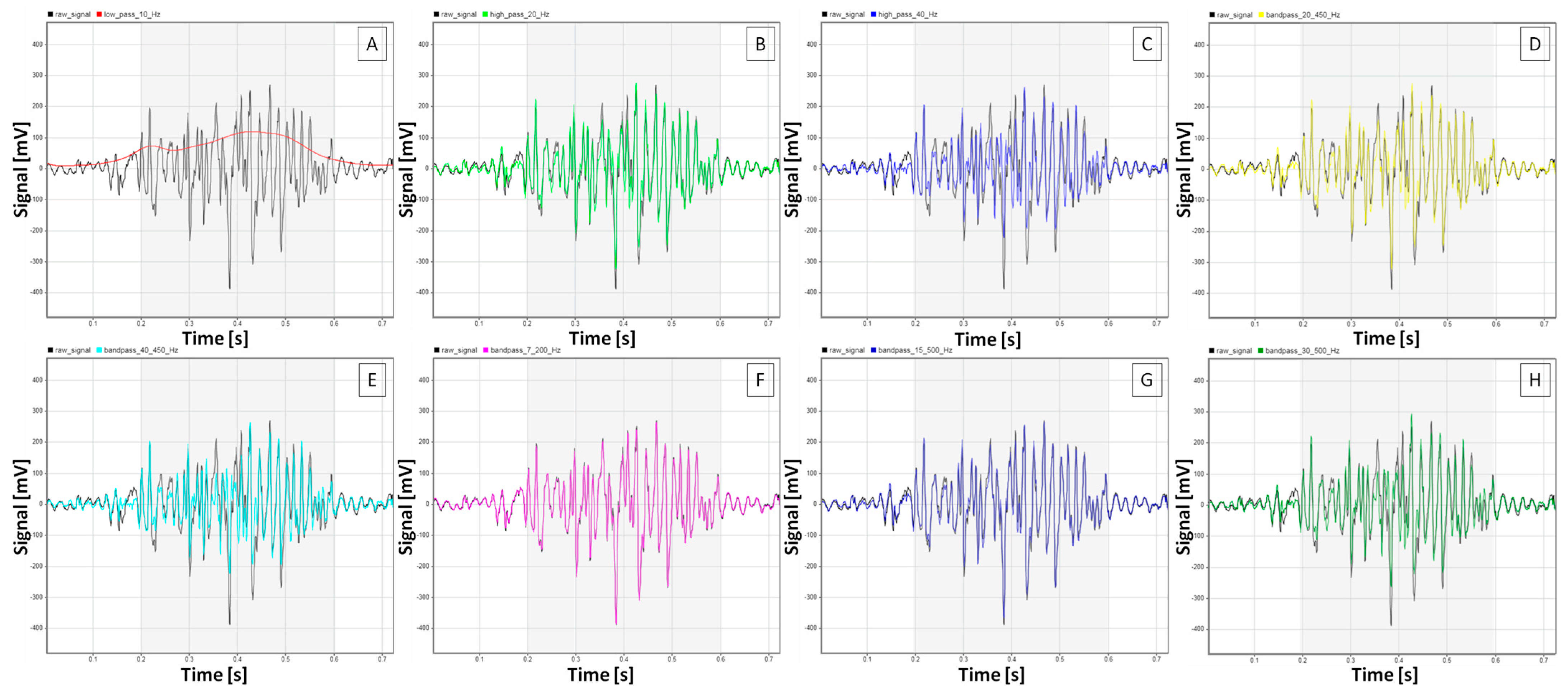
2.2. sEMG Signal Processing
2.3. sEMG Signal Features Extraction
2.4. sEMG Signal Loss Metrics Calculation
2.5. Power Spectral Density Calculation
2.6. Statistical Analysis
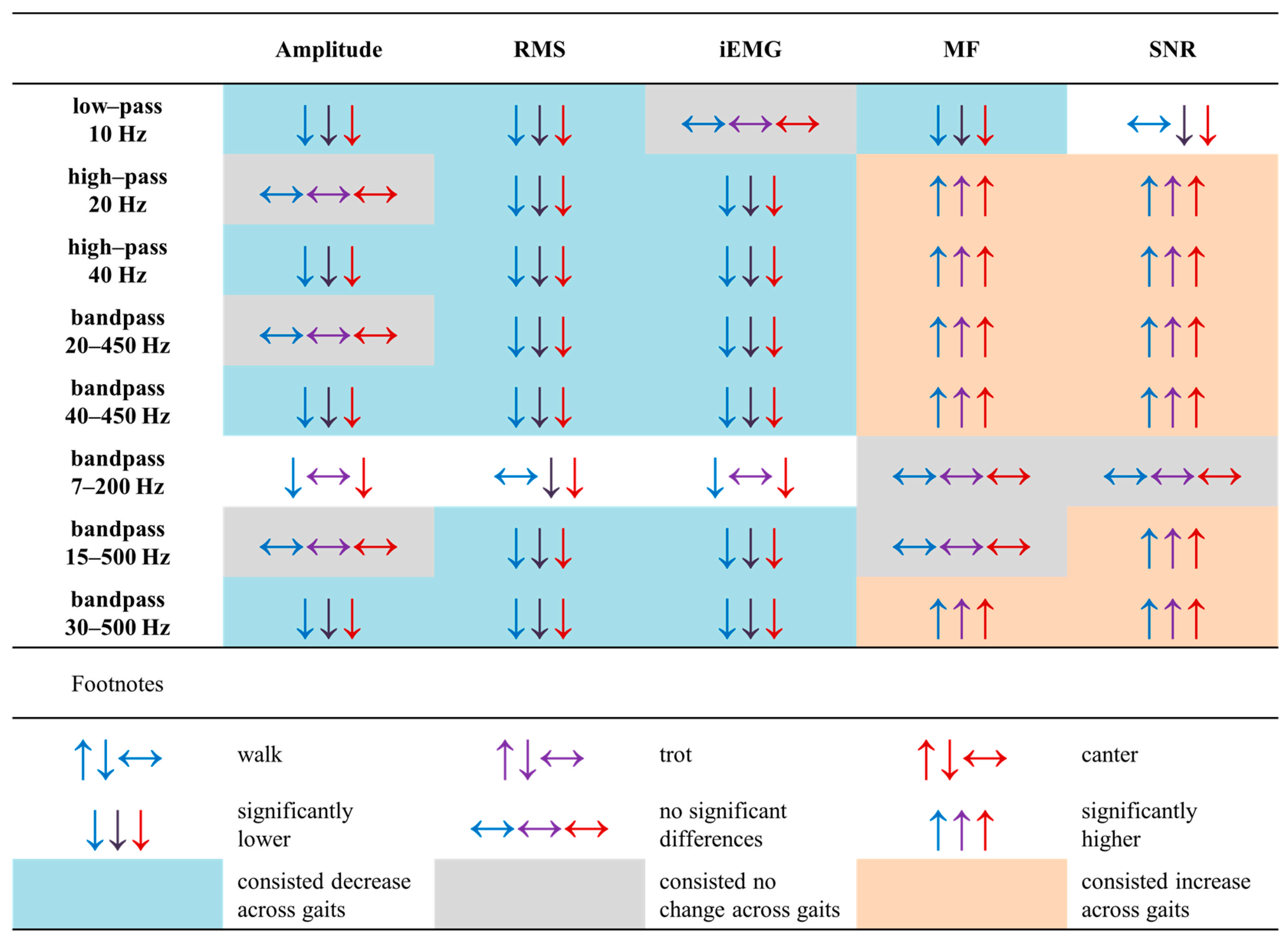
3. Results
3.1. sEMG Signal Characteristics
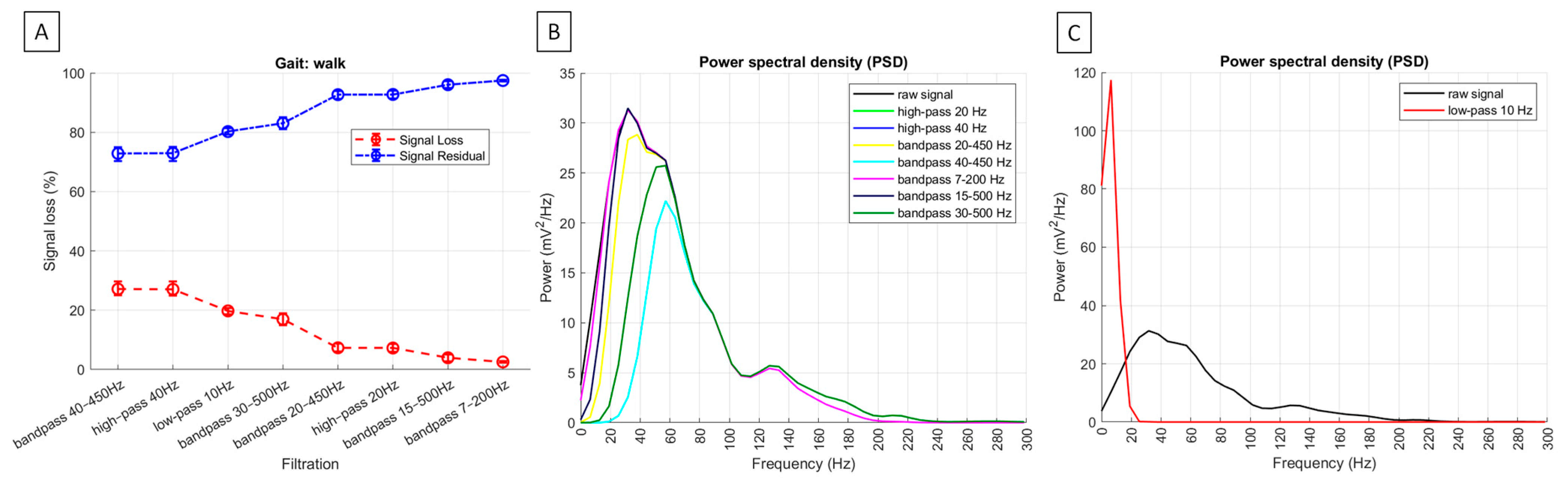
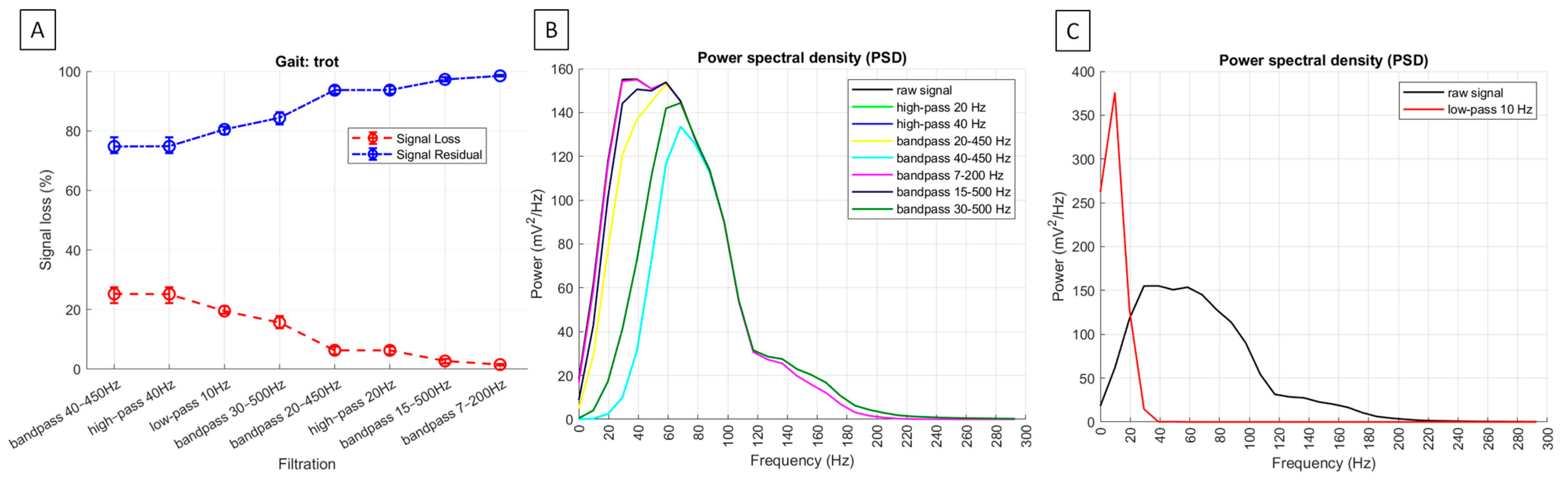
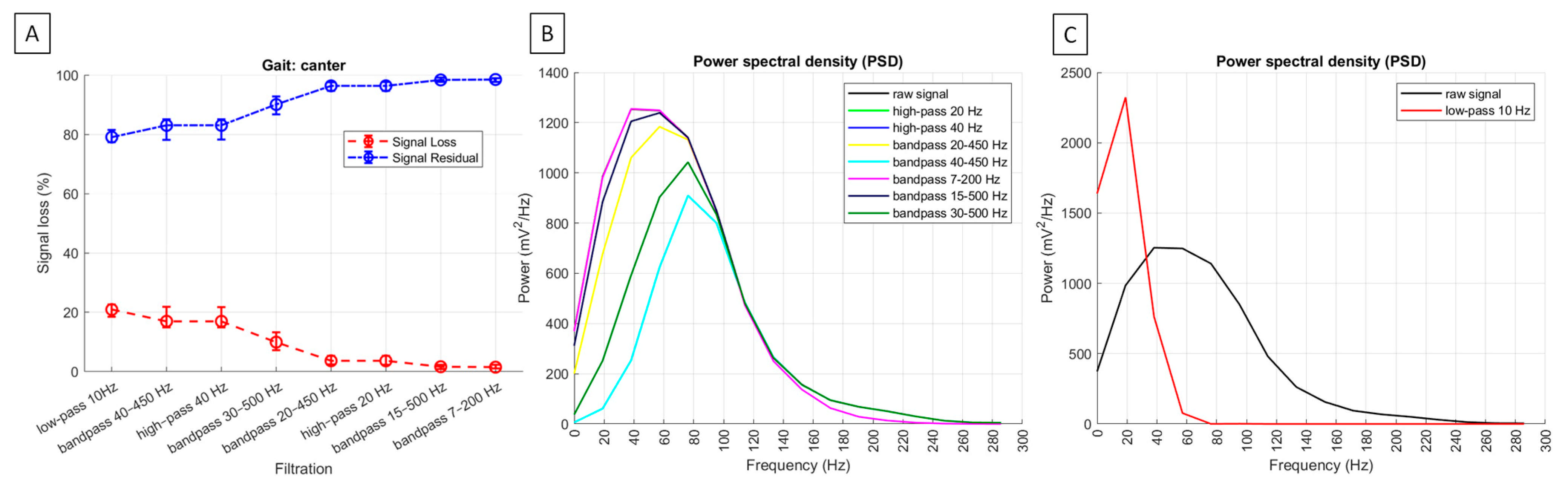
3.2. sEMG Signal Loss Metrics and Power Spectral Density
4. Discussion
4.1. Effect of Noise Attenuation Filtering Methods on Basic sEMG Signal Features
4.2. Effect of Specific Low–Pass Filtering at 10 Hz on Basic sEMG Signal Features
4.3. Recommendations for the Comparison of Equine sEMG Signal Features
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamucci, F.; Pallante, M.; Molle, S.; Merlo, E.; Bertuglia, A. A Preliminary Study on the Use of HD–sEMG for the Functional Imaging of Equine Superficial Muscle Activation during Dynamic Mobilization Exercises. Animals 2022, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Spoormakers, T.J.; St. George, L.; Smit, I.H.; Hobbs, S.J.; Brommer, H.; Clayton, H.M.; Richards, R.J.; Serra Bragança, F.M. Adaptations in equine axial movement and muscle activity occur during induced fore-and hindlimb lameness: A kinematic and electromyographic evaluation during in-hand trot. Equine Vet. J. 2023, 55, 1112–1127. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M. Electromyography in the horse: A useful technology? J. Equine Vet. Sci. 2018, 60, 43–58. [Google Scholar] [CrossRef]
- St. George, L.; Clayton, H.M.; Sinclair, J.; Richards, J.; Roy, S.H.; Hobbs, S.J. Muscle function and kinematics during submaximal equine jumping: What can objective outcomes tell us about athletic performance indicators? Animals 2021, 11, 414. [Google Scholar] [CrossRef]
- Busse, N.I.; Gonzalez, M.L.; Krason, M.L.; Johnson, S.E. β–Hydroxy β–methylbutyrate supplementation to adult Thoroughbred geldings increases type IIA fiber content in the gluteus medius. J. Anim. Sci. 2021, 99, skab264. [Google Scholar] [CrossRef]
- St. George, L.B.; Clayton, H.M.; Sinclair, J.K.; Richards, J.; Roy, S.H.; Hobbs, S.J. Electromyographic and Kinematic Comparison of the Leading and Trailing Fore–and Hindlimbs of Horses during Canter. Animals 2023, 13, 1755. [Google Scholar] [CrossRef]
- Wijnberg, I.D.; Back, W.; De Jong, M.; Zuidhof, M.C.; Van Den Belt, A.J.M.; Van der Kolk, J.H. The role of electromyography in clinical diagnosis of neuromuscular locomotor problems in the horse. Equine Vet. J. 2004, 36, 718–722. [Google Scholar] [CrossRef]
- St. George, L.B.; Spoormakers, T.J.; Smit, I.H.; Hobbs, S.J.; Clayton, H.M.; Roy, S.H.; van Weeren, P.R.; Richards, J.; Serra Bragança, F.M. Adaptations in equine appendicular muscle activity and movement occur during induced fore–and hindlimb lameness: An electromyographic and kinematic evaluation. Front. Vet. Sci. 2022, 9, 989522. [Google Scholar] [CrossRef]
- St. George, L.B.; Spoormakers, T.J.P.; Hobbs, S.J.; Clayton, H.M.; Roy, S.H.; Serra Braganca, F.M. Which sEMG variable best distinguishes between non–lame and induced lameness conditions in horses? Comp. Exerc. Physiol. 2023, 19, S16. [Google Scholar]
- Huntington, P.J.; Seneque, S.; Slocombe, R.F.; Jeffcott, L.B.; McLean, A.; Luff, A.R. Use of phenytoin to treat horses with Australian stringhalt. Aust. Vet. J. 1991, 68, 221–224. [Google Scholar] [CrossRef]
- Aman, J.E.; Valberg, S.J.; Elangovan, N.; Nicholson, A.; Lewis, S.S.; Konczak, J. Abnormal locomotor muscle recruitment activity is present in horses with shivering and Purkinje cell distal axonopathy. Equine Vet. J. 2018, 50, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Knaggs, H.; Tabor, G.; Williams, J.M. An initial investigation into the effects of the equine transeva technique (pulsating current electrotherapy) on the equine Gluteus superficialis. Comp. Exerc. Physiol. 2022, 18, 27–35. [Google Scholar] [CrossRef]
- Valentin, S.; Zsoldos, R.R. Surface electromyography in animal biomechanics: A systematic review. J. Electromyogr. Kinesiol. 2016, 28, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Smit, I.H.; Parmentier, J.I.; Rovel, T.; van Dieen, J.; Bragança, F.S. Towards standardisation of surface electromyography measurements in the horse: Bipolar electrode location. J. Electromyogr. Kinesiol. 2024, 76, 102884. [Google Scholar] [CrossRef]
- St. George, L.S.; Hobbs, S.J.; Richards, J.; Sinclair, J.; Holt, D.; Roy, S.H. The effect of cut–off frequency when high–pass filtering equine sEMG signals during locomotion. J. Electromyogr. Kinesiol. 2018, 43, 28–40. [Google Scholar] [CrossRef]
- Robert, C.; Valette, J.P.; Degueurce, C.; Denoix, J.M. Correlation between surface electromyography and kinematics of the hindlimb of horses at trot on a treadmill. Cells Tissues Organs 1999, 165, 113–122. [Google Scholar] [CrossRef]
- Robert, C.; Valette, J.P.; Pourcelot, P.; Audigie, F.; Denoix, J.M. Effects of trotting speed on muscle activity and kinematics in saddlehorses. Equine Vet. J. 2002, 34, 295–301. [Google Scholar] [CrossRef]
- Levionnois, O.L.; Menge, M.; Thormann, W.; Mevissen, M.; Spadavecchia, C. Effect of ketamine on the limb withdrawal reflex evoked by transcutaneous electrical stimulation in ponies anaesthetised with isoflurane. Vet. J. 2010, 186, 304–311. [Google Scholar] [CrossRef]
- Peham, C.; Licka, T.F.; Scheidl, M. Evaluation of a signal–adapted filter for processing of periodic electromyography signals in horses walking on a treadmill. Am. J. Vet. Res. 2001, 62, 1687–1689. [Google Scholar] [CrossRef]
- St. George, L.S.; Williams, J.M. Electromyographic evaluation of approach stride, jump stride and intermediate stride in selected superficial muscles of the jumping horse: A preliminary study. Comp. Exerc. Physiol. 2013, 9, 23–32. [Google Scholar] [CrossRef]
- Zellner, A.; Bockstahler, B.; Peham, C. The effects of Kinesio Taping on the trajectory of the forelimb and the muscle activity of the Musculus brachiocephalicus and the Musculus extensor carpi radialis in horses. PLoS ONE 2017, 12, e0186371. [Google Scholar] [CrossRef] [PubMed]
- Crook, T.C.; Wilson, A.; Hodson-Tole, E. The effect of treadmill speed and gradient on equine hindlimb muscle activity. Equine Vet. J. 2010, 42, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.K.; Warren, L.K.; Lawrence, L.M.; Thompson, K.N. Electromyographic activity of the long digital extensor muscle in the exercising Thoroughbred horse. Equine Vet. J. 1998, 30, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Spadavecchia, C.; Spadavecchia, L.; Andersen, O.K.; Arendt–Nielsen, L.; Leandri, M.; Schatzmann, U. Quantitative assessment of nociception in horses by use of the nociceptive withdrawal reflex evoked by transcutaneous electrical stimulation. Am. J. Vet. Res. 2002, 63, 1551–1556. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Arendt–Nielsen, L.; Andersen, O.K.; Spadavecchia, L.; Doherr, M.; Schatzmann, U. Comparison of nociceptive withdrawal reflexes and recruitment curves between the forelimbs and hind limbs in conscious horses. Am. J. Vet. Res. 2003, 64, 700–707. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Andersen, O.K.; Arendt–Nielsen, L.; Spadavecchia, L.; Doherr, M.; Schatzmann, U. Investigation of the facilitation of the nociceptive withdrawal reflex evoked by repeated transcutaneous electrical stimulations as a measure of temporal summation in conscious horses. Am. J. Vet. Res. 2004, 65, 901–908. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Levionnois, O.; Kronen, P.; Andersen, O.K. The effects of isoflurane minimum alveolar concentration on withdrawal reflex activity evoked by repeated transcutaneous electrical stimulation in ponies. Vet. J. 2010, 183, 337–344. [Google Scholar] [CrossRef]
- St. George, L.S.; Roy, S.H.; Richards, J.; Sinclair, J.; Hobbs, S.J. Surface EMG signal normalisation and filtering improves sensitivity of equine gait analysis. Comp. Exerc. Physiol. 2019, 15, 173–186. [Google Scholar] [CrossRef]
- Rankins, E.M.; Salem, K.; Manso Filho, H.C.; Malinowski, K.; McKeever, K.H. Effect of Clenbuterol on Muscle Activity During Exercise in Standardbred Horses. J. Equine Vet. Sci. 2022, 118, 104126. [Google Scholar] [CrossRef]
- Takahashi, Y.; Mukai, K.; Matsui, A.; Ohmura, H.; Takahashi, T. Electromyographic changes in hind limbs of Thoroughbreds. Am. J. Vet. Res. 2018, 79, 828–835. [Google Scholar] [CrossRef]
- Takahashi, Y.; Mukai, K.; Ohmura, H.; Takahashi, T. Do muscle activities of M. splenius and M. brachiocephalicus decrease because of exercise–induced fatigue in Thoroughbred horses? J. Equine Vet. Sci. 2020, 86, 102901. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.W.; Housh, T.J.; Cramer, J.T.; Malek, M.H.; Mielke, M.; Hendrix, R.; Weir, J.P. Electrode shift and normalization reduce the innervation zone’s influence on EMG. Med. Sci. Sports Exerc. 2008, 40, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Huigen, E.; Peper, A.; Grimbergen, C.A. Investigation into the origin of the noise of surface electrodes. Med. Biol. Eng. Comput. 2002, 40, 332–338. [Google Scholar] [CrossRef]
- Basmajian, J.; De Luca, C. Muscles Alive: Their Function Revealed by Electromyography; Williams and Wilkins: Baltimore, MD, USA, 1985. [Google Scholar]
- Mewett, D.T.; Reynolds, K.J.; Nazeran, H. Reducing power line interference in digitised electromyogram recordings by spectrum interpolation. Med. Biol. Eng. Comput. 2004, 42, 524–531. [Google Scholar] [CrossRef]
- Clancy, E.A.; Morin, E.L.; Hajian, G.; Merletti, R. Tutorial. Surface electromyogram (sEMG) amplitude estimation: Best practices. J. Electromyogr. Kinesiol. 2023, 72, 102807. [Google Scholar] [CrossRef]
- Youngworth, R.N.; Gallagher, B.B.; Stamper, B.L. An overview of power spectral density (PSD) calculations. Opt. Manuf. Test. VI 2005, 5869, 206–216. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst–Klug, C.; Rau, G. Development of recommendations for semg sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Domino, M.; Borowska, M.; Stefanik, E.; Domańska-Kruppa, N.; Turek, B. The effect of cut-off frequency on signal features when filtering equine semg signal from selected extensor muscles. Appl. Sci. 2025, 15, 4737. [Google Scholar] [CrossRef]
- The MathWorks Inc. Signal Analyser in MATLAB 2024. Available online: https://www.mathworks.com/help/signal/ref/signalanalyzer–app.html (accessed on 12 December 2024).
- Licka, T.; Peham, C.; Frey, A. Electromyographic activity of the longissimus dorsi muscles in horses during trotting on a treadmill. Am. J. Vet. Res. 2004, 65, 155–158. [Google Scholar] [CrossRef]
- Zaneb, H.; Kaufmann, V.; Stanek, C.; Peham, C.; Licka, T. Quantitative differences in activities of back and pelvic limb muscles during walking and trotting between chronically lame and nonlame horses. Am. J. Vet. Res. 2009, 70, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Zsoldos, R.; Kotschwar, A.; Kotschwar, A.B.; Rodriguez, C.; Peham, C.; Licka, T. Activity of the equine rectus abdominus and oblique external abdominal muscles measured my surface EMG during walk and trot on the treadmill. Equine Vet. J. 2010, 42, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Merlo, A.; Campanini, I. Technical aspects of surface electromyography for clinicians. Open J. Occup. Ther. 2010, 3, 98–109. [Google Scholar] [CrossRef]
- Merletti, R.; Cerone, G.L. Tutorial. Surface EMG detection, conditioning and pre–processing: Best practices. J. Electromyogr. Kinesiol. 2020, 54, 102440. [Google Scholar] [CrossRef]
- Zedka, M.; Kumar, S.; Narayan, Y. Comparison of surface EMG signals between electrode types, interelectrode distances and electrode orientations in isometric exercise of the erector spinae muscle. Electromyogr. Clin. Neurophysiol. 1997, 37, 439–447. [Google Scholar]
- Beck, T.W.; Housh, T.J.; Johnson, G.O.; Weir, J.P.; Cramer, J.T.; Coburn, J.W.; Malek, M.H. The effects of interelectrode distance on electromyographic amplitude and mean power frequency during isokinetic and isometric muscle actions of the biceps brachii. J. Electromyogr. Kinesiol. 2005, 15, 482–495. [Google Scholar] [CrossRef]
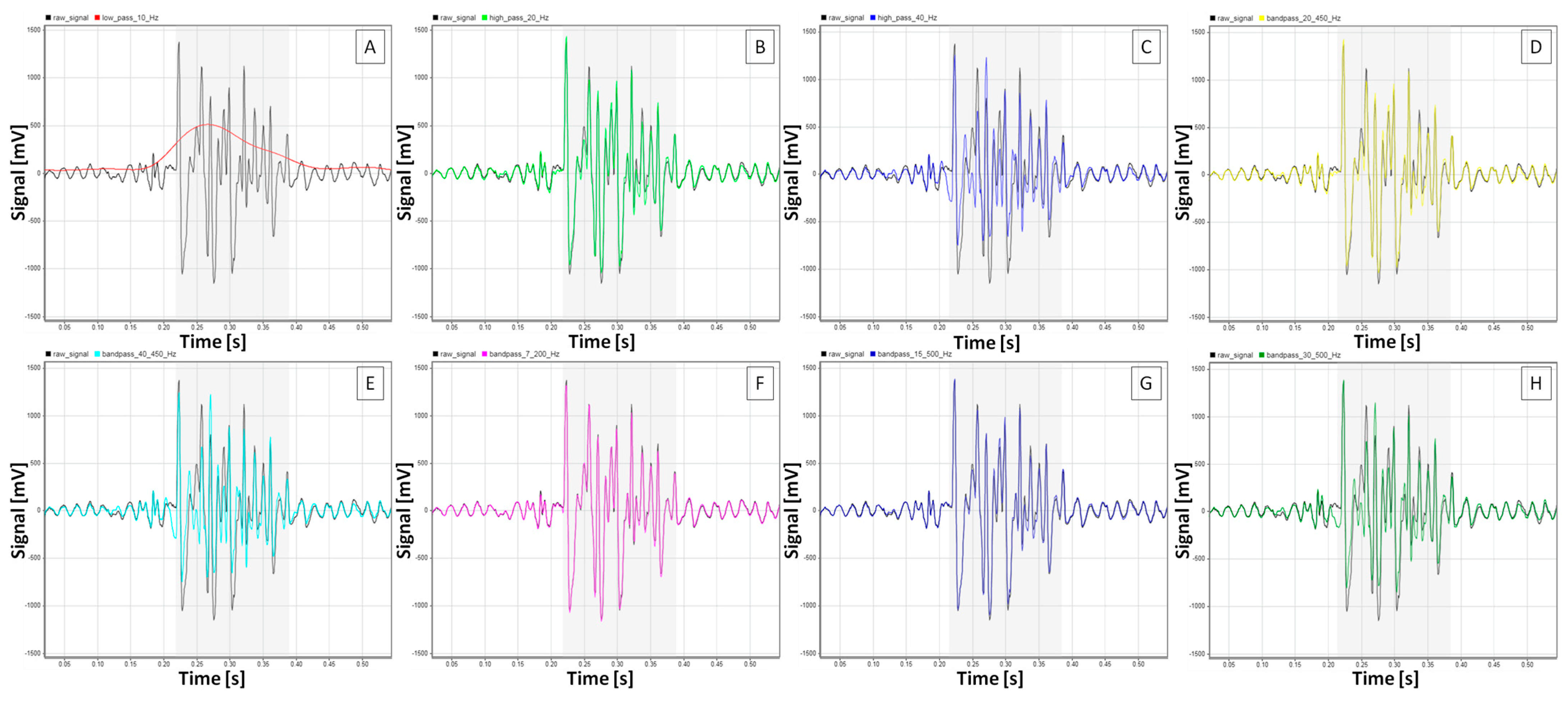
| Amplitude [mV] | RMS [mV] | iEMG [mV×s] | MF [Hz] | SNR [dB] | |
|---|---|---|---|---|---|
| raw signal | 206.2 (170.0; 257.1) | 46.4 (40.9; 54.1) | 33.0 (29.4; 38.2) | 48.8 (44.1; 54.4) | 12.1 (10.8; 13.7) |
| low–pass 10 Hz | 67.1 * (61.6; 84.2) | 37.4 * (32.8; 43.6) | 33.1 (29.3; 38.1) | 2.9 * (2.7; 3.1) | 12.5 (10.7; 13.9) |
| high–pass 20 Hz | 197.2 (170.2; 240.8) | 43.3 * (38.2; 50.6) | 30.4 * (26.9; 34.9) | 57.0 * (52.0; 62.5) | 13.5 * (12.6; 14.8) |
| high–pass 40 Hz | 173.8 * (149.6; 210.2) | 33.4 * (30.9; 41.2) | 22.7 * (20.8; 27.5) | 80.7 * (74.2; 88.1) | 13.6 * (12.4; 14.8) |
| bandpass 20–450 Hz | 196.9 (169.7; 241.1) | 43.3 * (38.2; 50.5) | 30.4 * (26.9; 34.9) | 56.9 * (52.0; 62.4) | 13.5 * (12.6; 14.8) |
| bandpass 40–450 Hz | 172.2 * (150.3; 212.8) | 33.4 * (30.8; 41.1) | 22.7 * (20.8; 27.5) | 80.6 * (74.1; 87.9) | 13.6 * (12.5; 14.8) |
| bandpass 7–200 Hz | 197.5 * (166.5; 246.6) | 45.4 (40.4; 53.2) | 32.4 * (28.9; 37.5) | 47.5 (43.1; 51.9) | 12.2 (11.1; 13.9) |
| bandpass 15–500 Hz | 198.5 (168.1; 256.3) | 45.0 * (39.5; 52.6) | 31.7 * (28.0; 36.3) | 52.6 (47.4; 58.3) | 13.3 * (12.0; 14.6) |
| bandpass 30–500 Hz | 188.2 * (163.0; 224.8) | 38.3 * (34.2; 45.4) | 26.2 * (24.3; 21.4) | 68.5 * (63.5; 74.7) | 13.8 * (12.5; 15.0) |
| Amplitude [mV] | RMS [mV] | iEMG [mV×s] | MF [Hz] | SNR [dB] | |
|---|---|---|---|---|---|
| raw signal | 429.6 (373.0; 515.8) | 114.5 (103.3; 130.8) | 84.2 (77.8; 98.4) | 51.8 (46.7; 55.7) | 16.1 (14.4; 17.9) |
| low-pass 10 Hz | 140.6 * (121.7; 168.1) | 90.5 * (82.3; 103.0) | 84.0 (77.5; 98.4) | 3.9 * (3.6; 4.2) | 15.3 * (13.8; 17.1) |
| high–pass 20 Hz | 420.2 (357.3; 531.5) | 108.9 * (95.4; 123.3) | 79.0 * (72.4; 91.6) | 57.7 * (52.8; 63.9) | 17.0 * (14.5; 19.1) |
| high–pass 40 Hz | 383.1 * (2295.2; 449.2) | 86.8 * (78.7; 96.6) | 63.9 * (57.2; 71.2) | 77.7 * (70.9; 81.8) | 16.7 * (14.8; 19.2) |
| bandpass 20–450 Hz | 420.4 (356.7; 531.4) | 108.9 * (95.3; 123.2) | 79.0 * (72.3; 91.6) | 57.7 * (52.8; 63.8) | 17.0 * (14.5; 19.1) |
| bandpass 40–450 Hz | 383.2 * (294.9; 450.2) | 86.7 * (78.7; 96.6) | 63.9 * (57.1; 71.1) | 77.7 * (70.9; 81.1) | 16.7 * (14.8; 19.2) |
| bandpass 7–200 Hz | 417.9 (354.6; 505.9) | 112.8 * (101.6; 129.2) | 83.5 (76.9; 97.6) | 50.9 (45.6; 54.8) | 16.4 (14.4; 18.0) |
| bandpass 15–500 Hz | 421.5 (362.0; 507.0) | 112.2 * (99.8; 128.5) | 82.0 * (75.1; 95.1) | 55.0 (48.4; 58.7) | 16.7 * (14.6; 18.8) |
| bandpass 30–500 Hz | 405.2 * (318.4; 483.3) | 98.4 * (86.5; 109.3) | 71.4 * (64.6; 81.6) | 66.5 * (61.3; 72.0) | 16.8 * (14.6; 19.0) |
| Amplitude [mV] | RMS [mV] | iEMG [mV×s] | MF [Hz] | SNR [dB] | |
|---|---|---|---|---|---|
| raw signal | 1223.0 (1056.0; 1487.0) | 353.8 (322.8; 402.4) | 262.0 (233.3; 293.8) | 61.0 (55.3; 69.1) | 18.1 (16.2; 19.2) |
| low-pass 10 Hz | 425.5 * (371.8; 487.0) | 286.4 * (248.9; 317.1) | 261.9 (231.1; 293.8) | 8.5 * (8.2; 9.1) | 17.0 * (15.1; 18.7) |
| high-pass 20 Hz | 1203.0 (975.2; 1454.0) | 337.0 * (305.2; 388.9) | 250.8 * (221.9; 282.0) | 64.9 * (59.8; 72.8) | 18.3 * (16.2; 19.9) |
| high-pass 40 Hz | 1029.0 * (859.0; 1330.0) | 293.3 * (245.0; 333.3) | 211.0 * (187.2; 245.1) | 81.1 * (75.8; 86.6) | 18.2 * (16.3; 20.2) |
| bandpass 20–450 Hz | 1204.0 (967.1; 1451.0) | 337.0 * (305.1; 388.8) | 250.7 * (221.8; 282.0) | 64.9 * (59.8; 72.8) | 18.3 * (16.2; 19.9) |
| bandpass 40–450 Hz | 1030.0 * (851.1; 1328.0) | 293.1 * (245.0; 333.3) | 210.8 * (187.1; 245.0) | 81.0 * (75.8; 86.6) | 18.2 * (16.3; 20.2) |
| bandpass 7–200 Hz | 1134.0 * (997.9; 137.0) | 347.8 * (315.2; 397.8) | 258,4 * (229.3; 290.6) | 60.3 (53.9; 67.3) | 18.2 (16.1; 19.3) |
| bandpass 15–500 Hz | 1232.0 (986.2; 1515.0) | 348.9 * (315.0; 396.3) | 259.2 * (227.7; 289.0) | 62.8 (57.8; 71.4) | 18.4 * (16.2; 19.9) |
| bandpass 30–500 Hz | 1124.0 * (921.8; 1376.0) | 313.8 * (282.3; 362.6) | 231.4 * (204.3; 264.6) | 72.5 * (66.7; 79.6) | 18.0 * (15.8; 19.9) |
| raw signal | low–pass 10 Hz | high–pass 20 Hz | high–pass 40 Hz | bandpass 20–450 Hz | bandpass 40–450 Hz | bandpass 7–200 Hz | bandpass 15–500 Hz | bandpass 30–500 Hz | |
| signal loss | no applicable | 19.8% (18.5; 20.8) | 7.3% a (6.0; 8.7) | 27.0% b (24.9; 29.8) | 7.3% a (6.1; 8.8) | 27.1% b (25.0; 29.9) | 2.5% c (2.2; 2.9) | 4.0% d (2.8; 5.1) | 16.9% b (15.0; 19.1) |
| residual signal | no applicable | 80.3% (79.2; 81.5) | 92.8% a (91.4; 94.0) | 73.0% b (70.2; 75.1) | 92.7% a (91.3; 93.9) | 72.9% b (70.1; 75.1) | 97.5% c (97.1; 97.8) | 96.1% d (94.9; 97.3) | 83.1% b (80.9; 85.1) |
| PSD [mV2/Hz] | 36.8 a (28.3; 60.0) | 114.5 * (87.2; 158.6) | 31.5 b (24.0; 51.8) | 18.8 c (13.6; 26.5) | 31.5 b (24.0; 51.8) | 18.8 c (13.6; 26.5) | 36.8 a (28.2; 60.0) | 37.0 a (26.3; 55.6) | 23.6 d (18.1; 35.7) |
| raw signal | low–pass 10 Hz | high–pass 20 Hz | high–pass 40 Hz | bandpass 20–450 Hz | bandpass 40–450 Hz | bandpass 7–200 Hz | bandpass 15–500 Hz | bandpass 30–500 Hz | |
| signal loss | no applicable | 19.5% (18.6; 21.2) | 6.3% a (4.9; 7.5) | 25.2% b (22.1; 27.6) | 6.4% a (4.9; 7.6) | 25.3% b (22.1; 27.6) | 1.5% c (1.2; 1.7) | 2.7% c (1.9; 3.4) | 15.6% d (13.7; 17.9) |
| residual signal | no applicable | 80.5% (78.8; 81.4) | 93.8% a (92.5; 95.1) | 74.9% b (72.5; 78.0) | 93.7% a (92.4; 95.1) | 74.8% b (72.5; 77.9) | 98.5% c (98.3; 98.8) | 97.3% c (96.6; 98.1) | 84.4% d (82.2; 86.3) |
| PSD [mV2/Hz] | 200.2 a (160.9; 259.6) | 435.3 * (361.2; 569.5) | 175.8 b (142.0; 234.9) | 118.1 c (85.8; 154.6) | 175.8 b (142.0; 234.9) | 118.1 c (85.8; 154.6) | 199.8 a (161.2; 259.2) | 191.7 ab (153.7; 254.7) | 153.5 d (116.8; 185.9) |
| raw signal | low–pass 10 Hz | high–pass 20 Hz | high–pass 40 Hz | bandpass 20–450 Hz | bandpass 40–450 Hz | bandpass 7–200 Hz | bandpass 15–500 Hz | bandpass 30–500 Hz | |
| signal loss | no applicable | 20.9% (18.3; 22.6) | 3.7% a (2.3; 5.3) | 17.0% b (14.6; 21.9) | 3.7% a (2.4; 5.3) | 17.0% b (14.6; 21.9) | 1.5% c (1.0; 2.3) | 1.6% c (0.9; 2.3) | 9.9% d (7.2; 13.3) |
| residual signal | no applicable | 79.1% (77.4; 81.7) | 96.4% a (94.7; 97.8) | 83.1% b (78.2; 85.5) | 96.4% a (94.7; 97.7) | 83.1% b (78.2; 85.5) | 98.5% c (97.7; 99.0) | 98.4% c (97.7; 99.1) | 90.1% d (86.7; 92.8) |
| PSD [mV2/Hz] | 1417 a (1075; 1889) | 2274 * (1725; 2827) | 1379 b (954; 1849) | 1034 c (756; 1352) | 1379 b (954; 1849) | 1034 c (756; 1352) | 1416 a (1070; 1886) | 1404 a (1024; 1880) | 1200 d (860; 1594) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domino, M.; Borowska, M.; Stefanik, E.; Domańska-Kruppa, N.; Skibniewski, M.; Turek, B. The Effect of Filtering on Signal Features of Equine sEMG Collected During Overground Locomotion in Basic Gaits. Sensors 2025, 25, 2962. https://doi.org/10.3390/s25102962
Domino M, Borowska M, Stefanik E, Domańska-Kruppa N, Skibniewski M, Turek B. The Effect of Filtering on Signal Features of Equine sEMG Collected During Overground Locomotion in Basic Gaits. Sensors. 2025; 25(10):2962. https://doi.org/10.3390/s25102962
Chicago/Turabian StyleDomino, Małgorzata, Marta Borowska, Elżbieta Stefanik, Natalia Domańska-Kruppa, Michał Skibniewski, and Bernard Turek. 2025. "The Effect of Filtering on Signal Features of Equine sEMG Collected During Overground Locomotion in Basic Gaits" Sensors 25, no. 10: 2962. https://doi.org/10.3390/s25102962
APA StyleDomino, M., Borowska, M., Stefanik, E., Domańska-Kruppa, N., Skibniewski, M., & Turek, B. (2025). The Effect of Filtering on Signal Features of Equine sEMG Collected During Overground Locomotion in Basic Gaits. Sensors, 25(10), 2962. https://doi.org/10.3390/s25102962







