Prediction and Fitting of Nonlinear Dynamic Grip Force of the Human Upper Limb Based on Surface Electromyographic Signals
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design for Subjects
2.2. Data Preprocessing
2.2.1. Outlier Removal and Filtering
- Baseline Drift and Motion Artifacts [21].
- 2.
- System Noise.
2.2.2. Wavelet Denoising
2.2.3. Removing Motion Artifacts
2.3. Data Fitting and Regression
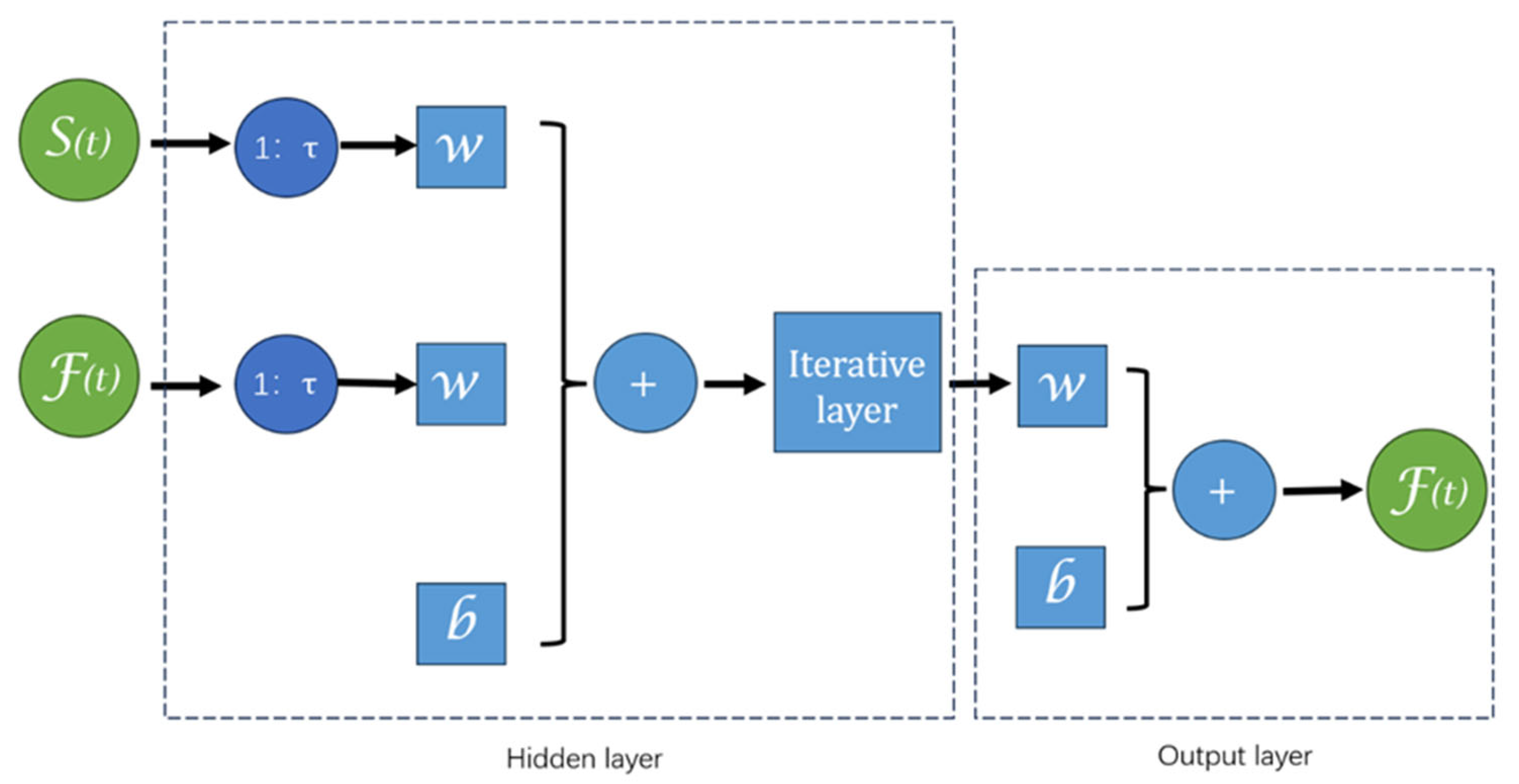
2.3.1. Neural Network Time Series Fitting
2.3.2. Comparison of Different Training Methods
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhury, R.H.; Reaz, M.B.I.; Ali, M.A.B.M.; Bakar, A.A.A.; Chellappan, K.; Chang, T.G. Surface Electromyography Signal Processing and Classification Techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Gannon, H.; Ferris, D.P. A biomechanical comparison of proportional electromyography control to biological torque control using a powered hip exoskeleton. Front. Bioeng. Biotechnol. 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Li, G.; Jiang, D.; Xu, S.; Chen, D. Grasping force prediction based on sEMG signals. Alex. Eng. J. 2020, 59, 1135–1147. [Google Scholar] [CrossRef]
- McCool, P.; Petropoulakis, L.; Soraghan, J.J.; Chatlani, N. Improved pattern recognition classification accuracy for surface myoelectric signals using spectral enhancement. Biomed. Signal Process. Control 2015, 18, 61–68. [Google Scholar] [CrossRef]
- NN Unanyan, A.A. Belov. Design of upper limb prosthesis using real-time motion detection method based on EMG signal processing. Biomed. Signal Process. Control 2021, 70, 103062. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Li, G.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted Muscle Reinnervation for Real-time Myoelectric Control of Multifunctional Artificial Arms. J. Am. Med. Assoc. (JAMA) 2009, 301, 619–628. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, J.; Zheng, B.; Jiang, X. Phase-Based Grasp Classification for Prosthetic Hand Control Using sEMG. Biosensors 2022, 12, 57. [Google Scholar] [CrossRef]
- Kim, S.; Chung, W.K.; Kim, K. SEMG-based static force estimation for human-robot interaction using deep learning. In Proceedings of the 2020 17th International Conference on Ubiquitous Robots (UR), Kyoto, Japan, 22–26 June 2020; pp. 81–86. [Google Scholar]
- Wu, Y.; Liang, S.; Yan, T.; Ao, J.; Zhou, Z.; Li, X. Classification and simulation of process of linear change for grip force at different grip speeds by using supervised learning based on sEMG. Expert Syst. Appl. 2022, 206, 117785. [Google Scholar] [CrossRef]
- Jiang, D.; Li, G.; Sun, Y.; Kong, J.; Tao, B. Gesture recognition based on skeletonization algorithm and CNN with ASL database. Multimed. Tools Appl. 2019, 78, 29953–29970. [Google Scholar] [CrossRef]
- Scheme, E.; Englehart, K. Electromyogram dynamical characteristics of sEMG signals of hand grasps via recurrence plot pattern recognition for control of powered upper-limb prostheses: State of the art and challenges for clinical use. J. Rehabil. Res. Dev. 2011, 48, 643–659. [Google Scholar] [CrossRef]
- Bardizbanian, B.; Zhu, Z.; Li, J.; Huang, X.; Dai, C.; Martinez-Luna, C.; McDonald, B.E.; Farrell, T.R.; Clancy, E.A. Efficiently training two-DoF hand-wrist EMG-force models. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 369–373. [Google Scholar]
- Sharma, T.; Sharma, K.P.; Sharma, T. Identification and Classification of Myoelectric Signal Features Related to Hand Motions. Neurophysiology 2024, 53, 164–174. [Google Scholar] [CrossRef]
- Huang, R.; Xue, X.; Xiao, R.; Bu, F. A novel method for ecg signal compression and reconstruction: Down-sampling operation and signal-referenced network. Electronics 2023, 12, 1760. [Google Scholar] [CrossRef]
- Lieber, R.L.; Jacobson, M.D.; Fazeli, B.M.; Abrams, R.A.; Botte, M.J. Architecture of selected muscles of the arm and forearm: Anatomy and implications for tendon transfer. J. Hand Surg. 1992, 17, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Palastanga, N.; Field, D.; Soames, R. Anatomy and Human Movement: Structure and Function; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Schieber, M.H. Muscular production of individuated finger movements: The roles of extrinsic finger muscles. J. Neurosci. 1995, 15, 284–297. [Google Scholar] [CrossRef]
- Ma, N.; Kumar, D.K.; Pah, N. Classification of hand direction using multi-channel electromyography by neural network. In Proceedings of the Seventh Australian and New Zealand Intelligent Information Systems Conference, Perth, Australia, 18–21 November 2001; pp. 405–410. [Google Scholar]
- Kuran, B. Functional assessment in hand with flexor and extensor tendon injuries. In Hand Function: A Practical Guide to Assessment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 109–124. [Google Scholar]
- Peleg, D.; Braiman, E.; Yom-Tov, E.; Inbar, G.F. Classification of finger activation for use in a robotic prosthesis arm. IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 290–293. [Google Scholar] [CrossRef]
- Huang, R.; Woo, S.-W.; Hong, K.-S. Real-time Motion Artifacts and Low-Frequency Drift Correction for Functional Near-infrared Spectroscopy. In Proceedings of the 2020 12th International Conference on Computer and Automation Engineering (ICCAE 2020), Association for Computing Machinery, New York, NY, USA, 14–16 February 2020; pp. 60–64. [Google Scholar]
- Chu, J.U.; Moon, I.; Lee, Y.J.; Kim, S.K.; Mun, M.S. A supervised feature-projection-based real-time EMG pattern recognition for multifunction myoelectric hand control. IEEE-ASME Trans. Mechatron. 2007, 12, 282–290. [Google Scholar] [CrossRef]
- Vijayvargiya, A.; Singh, B.; Kumar, R.; Tavares, J.M.R. Human lower limb activity recognition techniques, databases, challenges and its applications using sEMG signal: An overview. Biomed. Eng. Lett. 2022, 12, 343–358. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Gao, L.; Miao, Q. Current progress on weak signal detection. In Proceedings of the 2013 International Conference on Quality, Reliability, Risk, Maintenance, and Safety Engineering (QR2MSE), Chengdu, China, 15–18 July 2013; pp. 1812–1818. [Google Scholar]
- Kadochnikova, A.; Zhu, Y.; Lang, Z.Q.; Kadirkamanathan, V. Integrated Identification of the Nonlinear Autoregressive Models with Exogenous Inputs (NARX) for Engineering Systems Design. In IEEE Transactions on Control Systems Technology; IEEE: New York, NY, USA, 2023; Volume 31, pp. 394–401. [Google Scholar]
- Serikov, T.; Zhetpisbayeva, A.; Mirzakulova, S.; Zhetpisbayev, K.; Ibrayeva, Z.; Tolegenova, A.; Soboleva, L.; Zhumazhanov, B. Application of the NARX neural network for predicting a one-dimensional time series. East. Eur. J. Enterp. Technol. 2021, 5, 113. [Google Scholar] [CrossRef]
- Truong, T.T.; Airao, J.; Hojati, F.; Ilvig, C.F.; Azarhoushang, B.; Karras, P.; Aghababaei, R. Data-driven prediction of tool wear using Bayesian regularized artificial neural networks. Measurement 2024, 238, 115303. [Google Scholar] [CrossRef]
- Ranganathan, A. The levenberg-marquardt algorithm. Tutoral LM Algorithm 2004, 11, 101–110. [Google Scholar]
- Nazareth, J.L. Conjugate gradient method. WIREs Comput. Stat. 2009, 1, 348–353. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Guan, X.; Liang, B. Research on overfitting of deep learning. In Proceedings of the 2019 15th International Conference on Computational Intelligence and Security (CIS), Macao, China, 13–16 December 2019; pp. 78–81. [Google Scholar]
- Ghasemian, A.; Hosseinmardi, H.; Clauset, A. Evaluating overfit and underfit in models of network community structure. IEEE Trans. Knowl. Data Eng. 2019, 32, 1722–1735. [Google Scholar] [CrossRef]
- Aliferis, C.; Simon, G. Overfitting, Underfitting and General Model Overconfidence and Under-Performance Pitfalls and Best Practices in Machine Learning and AI. In Artificial Intelligence and Machine Learning in Health Care and Medical Sciences: Best Practices and Pitfalls; Springer International Publishing: Cham, Switzerland, 2024; pp. 477–524. [Google Scholar]
- Suganthi, J.R.; Rajeswari, K. Evaluation of Muscle Fatigue based on SEMG using Deep Learning Techniques. In Proceedings of the 2023 5th International Conference on Inventive Research in Computing Applications (ICIRCA), Coimbatore, India, 3–5 August 2023; pp. 1–6. [Google Scholar]
- Beck, T.W.; Stock, M.S.; DeFreitas, J.M. Time-Frequency Analysis of Surface Electromyographic Signals During Fatiguing Isokinetic Muscle Actions. J. Strength Cond. Res. 2012, 26, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, S. Long Short-Term Memory; Neural Computation MIT-Press: San Diego, CA, USA, 1997. [Google Scholar]
- Cho, K.; Van Merriënboer, B.; Gulcehre, C.; Bahdanau, D.; Bougares, F.; Schwenk, H.; Bengio, Y. Learning phrase representations using RNN encoder-decoder for statistical machine translation. arXiv 2014, arXiv:1406.1078. [Google Scholar]
- Ying, X. An overview of overfitting and its solutions. J. Phys. Conf. Ser. 2019, 1168, 022022. [Google Scholar] [CrossRef]
- Rizzo, R.; Garcia-Retortillo, S.; Ivanov, P.C. Dynamic networks of physiologic interactions of brain waves and rhythms in muscle activity. Hum. Mov. Sci. 2022, 84, 102971. [Google Scholar] [CrossRef]
- Penn, A.A.; Shatz, C.J. Brain waves and brain wiring: The role of endogenous and sensory-driven neural activity in development. Pediatr. Res. 1999, 45, 447–458. [Google Scholar] [CrossRef]
- Parajuli, N.; Sreenivasan, N.; Bifulco, P.; Cesarelli, M.; Savino, S.; Niola, V.; Esposito, D.; Hamilton, T.J.; Naik, G.R.; Gunawardana, U.; et al. Real-time EMG based pattern recognition control for hand prostheses: A review on existing methods, challenges and future implementation. Sensors 2019, 19, 4596. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, B.; Chen, B.; Torrens, P.M.; Atashzar, S.F.; Lin, D.; Sun, Q. Force-aware interface via electromyography for natural VR/AR interaction. ACM Trans. Graph. (TOG) 2022, 41, 268. [Google Scholar] [CrossRef]




| Training Method | MSE (N2) | RMSE (N)_ | MAE (N) | R-Value | Training Time | ||||
|---|---|---|---|---|---|---|---|---|---|
| Training Set | Testing Set | Training Set | Testing Set | Training Set | Testing Set | Training Set | Testing Set | ||
| BRSGD | 0.0045 | 0.2335 | 0.0670 | 0.4831 | 0.0328 | 0.2448 | 0.9999 | 0.9941 | 45 min 25 s |
| LM | 6.616 × 10−18 | 85.3096 | 2.572 × 10−9 | 9.2363 | 4.827 × 10−10 | 5.1816 | 1.0000 | 0.3510 | 9 min 30 s |
| CG | 0.0162 | 0.2181 | 0.1272 | 0.4670 | 0.0842 | 0.2330 | 0.9996 | 0.9944 | 5 s |
| Delay | Number of Neural Network Layers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | |||||||||
| MSE (N2) | MAE (N) | R | MSE (N2) | MAE (N) | R | MSE (N2) | MAE (N) | R | MSE (N2) | MAE (N) | R | |
| 5 | 0.17 | 0.72 | 0.9991 | 2.42 | 1.62 | 0.9940 | 3.09 | 1.83 | 0.9924 | 13.1 | 2.40 | 0.9673 |
| 10 | 0.28 | 0.87 | 0.9994 | 0.82 | 1.37 | 0.9980 | 1.60 | 1.86 | 0.9960 | 1.72 | 1.87 | 0.9957 |
| 20 | 0.87 | 1.36 | 0.9977 | 1.13 | 1.72 | 0.9970 | 0.96 | 1.79 | 0.9977 | 0.87 | 1.52 | 0.9980 |
| 30 | 0.34 | 1.34 | 0.9992 | 0.93 | 1.93 | 0.9978 | 1.70 | 2.20 | 0.9952 | 2.80 | 2.42 | 0.9925 |
| 40 | 1.23 | 1.97 | 0.9966 | 0.93 | 1.79 | 0.9971 | 1.47 | 2.03 | 0.9958 | 4.50 | 2.74 | 0.9886 |
| Average value | 0.58 | 1.25 | 0.9985 | 1.25 | 1.69 | 0.9968 | 1.76 | 1.94 | 0.9954 | 5.10 | 2.19 | 0.9884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Qu, M.; Han, M.; Wu, Z.; Wu, T.; Liu, M.; Yu, H. Prediction and Fitting of Nonlinear Dynamic Grip Force of the Human Upper Limb Based on Surface Electromyographic Signals. Sensors 2025, 25, 13. https://doi.org/10.3390/s25010013
Cai Z, Qu M, Han M, Wu Z, Wu T, Liu M, Yu H. Prediction and Fitting of Nonlinear Dynamic Grip Force of the Human Upper Limb Based on Surface Electromyographic Signals. Sensors. 2025; 25(1):13. https://doi.org/10.3390/s25010013
Chicago/Turabian StyleCai, Zixiang, Mengyao Qu, Mingyang Han, Zhijing Wu, Tong Wu, Mengtong Liu, and Hailong Yu. 2025. "Prediction and Fitting of Nonlinear Dynamic Grip Force of the Human Upper Limb Based on Surface Electromyographic Signals" Sensors 25, no. 1: 13. https://doi.org/10.3390/s25010013
APA StyleCai, Z., Qu, M., Han, M., Wu, Z., Wu, T., Liu, M., & Yu, H. (2025). Prediction and Fitting of Nonlinear Dynamic Grip Force of the Human Upper Limb Based on Surface Electromyographic Signals. Sensors, 25(1), 13. https://doi.org/10.3390/s25010013







