Microwave Flow Cytometric Detection and Differentiation of Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Sample Preparation
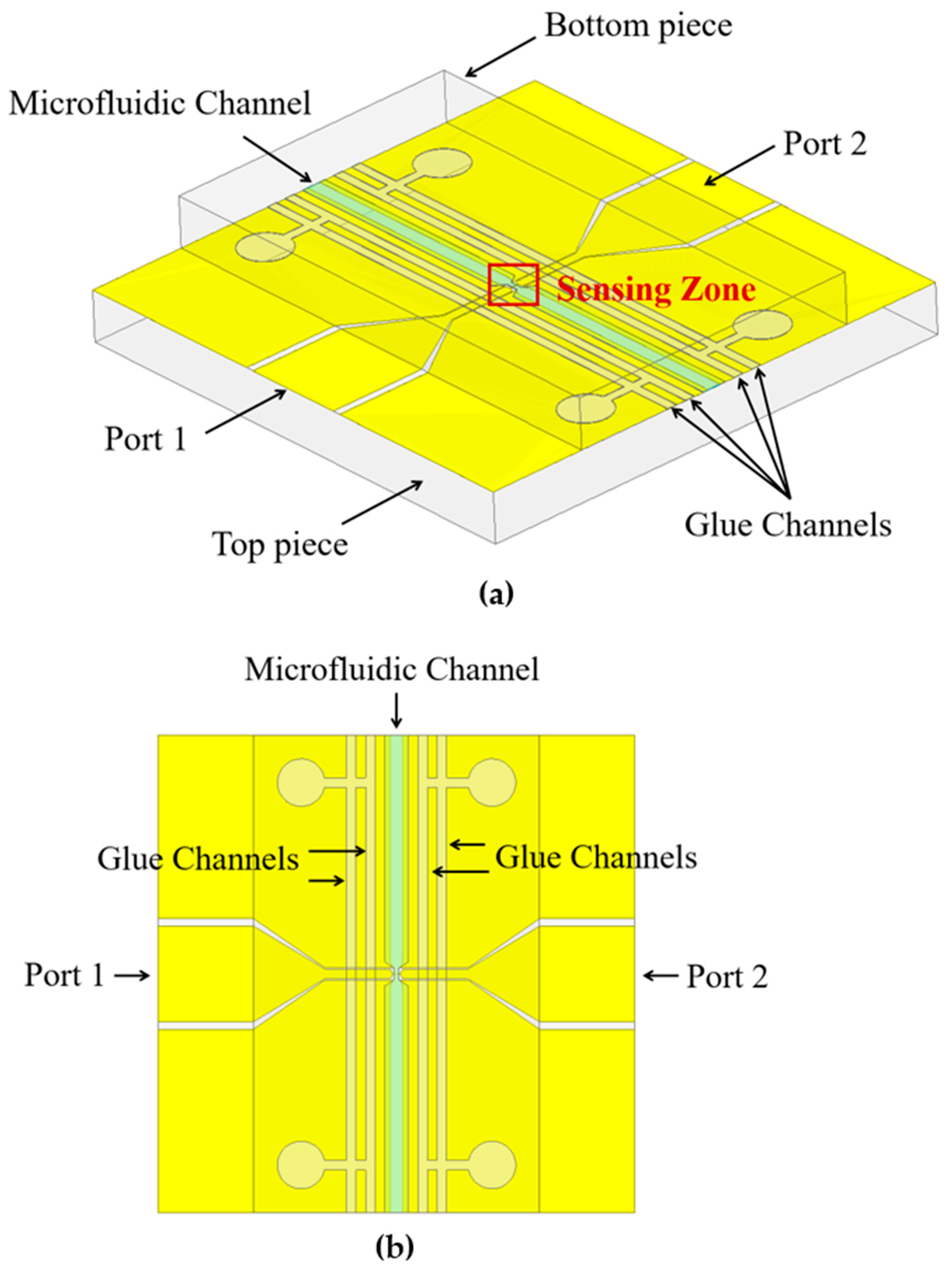
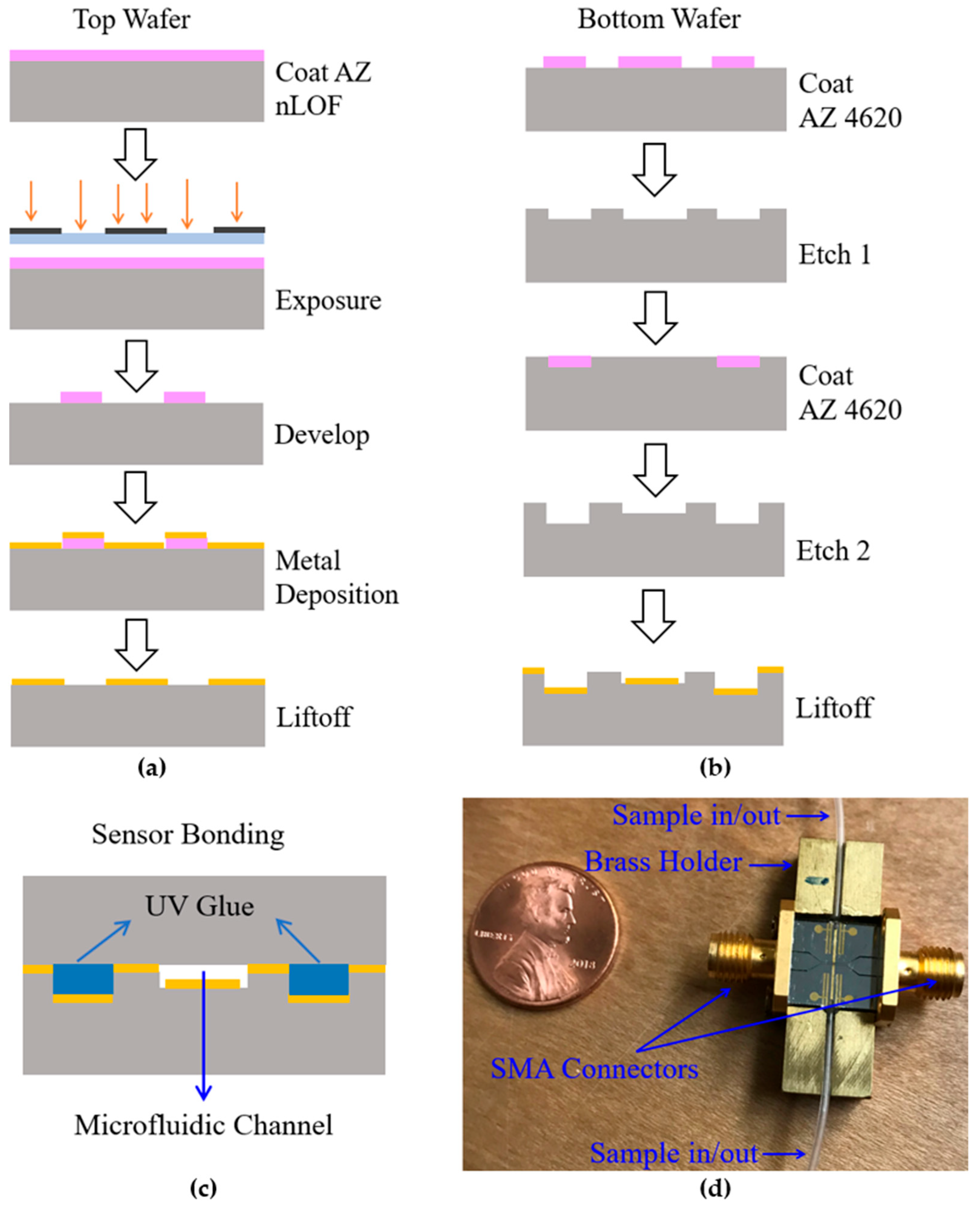
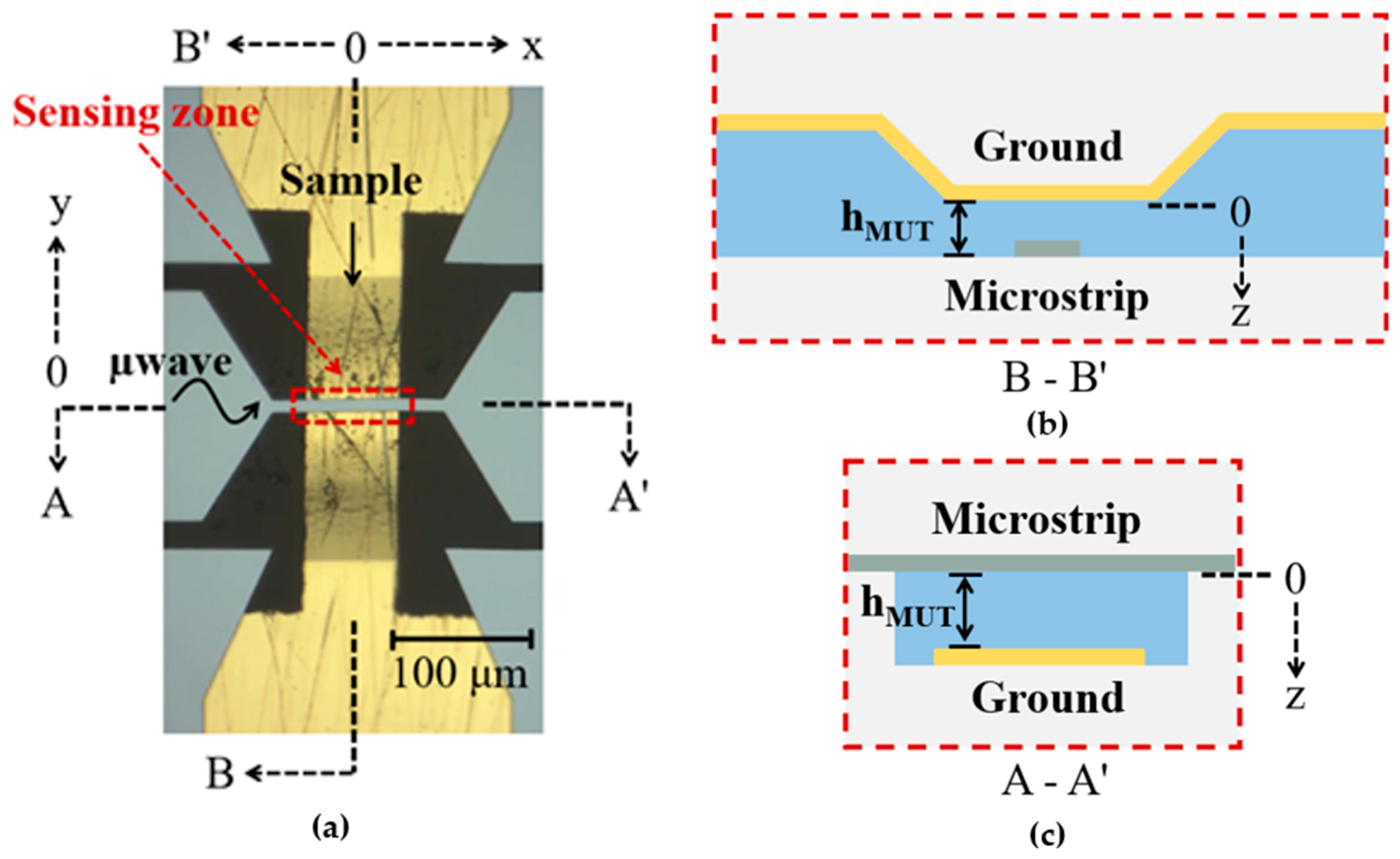
2.2. Microstrip Sensing Devices and Microwave Spectroscopic Flow Cytometer
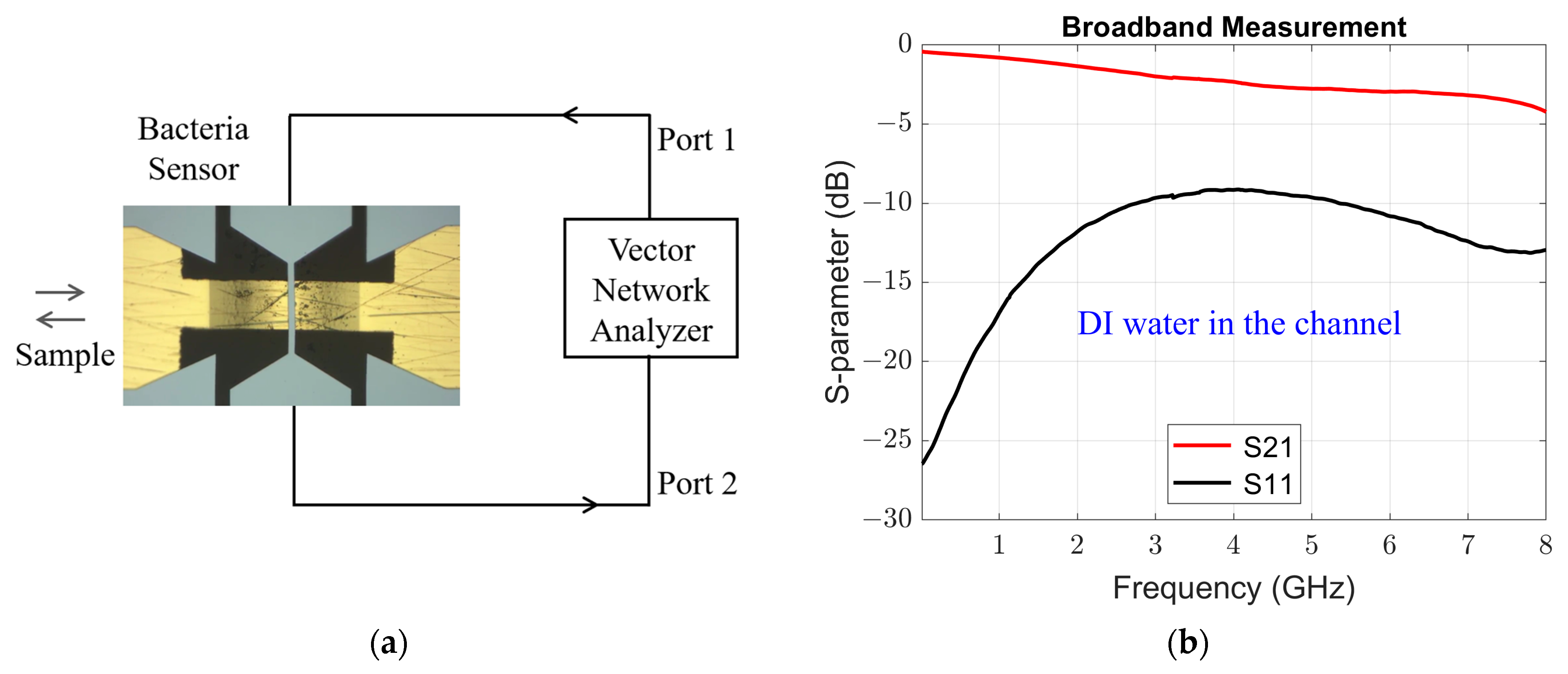
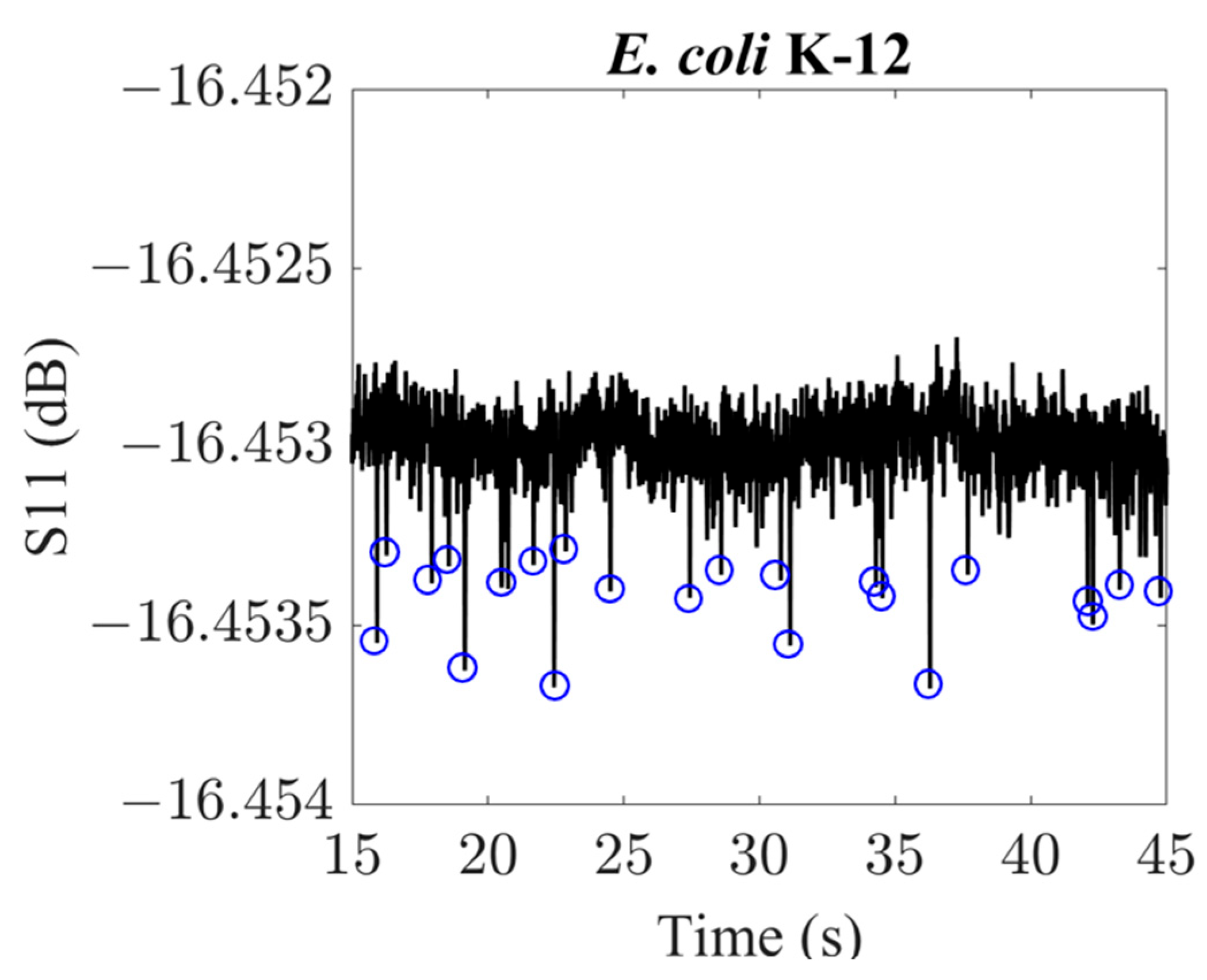
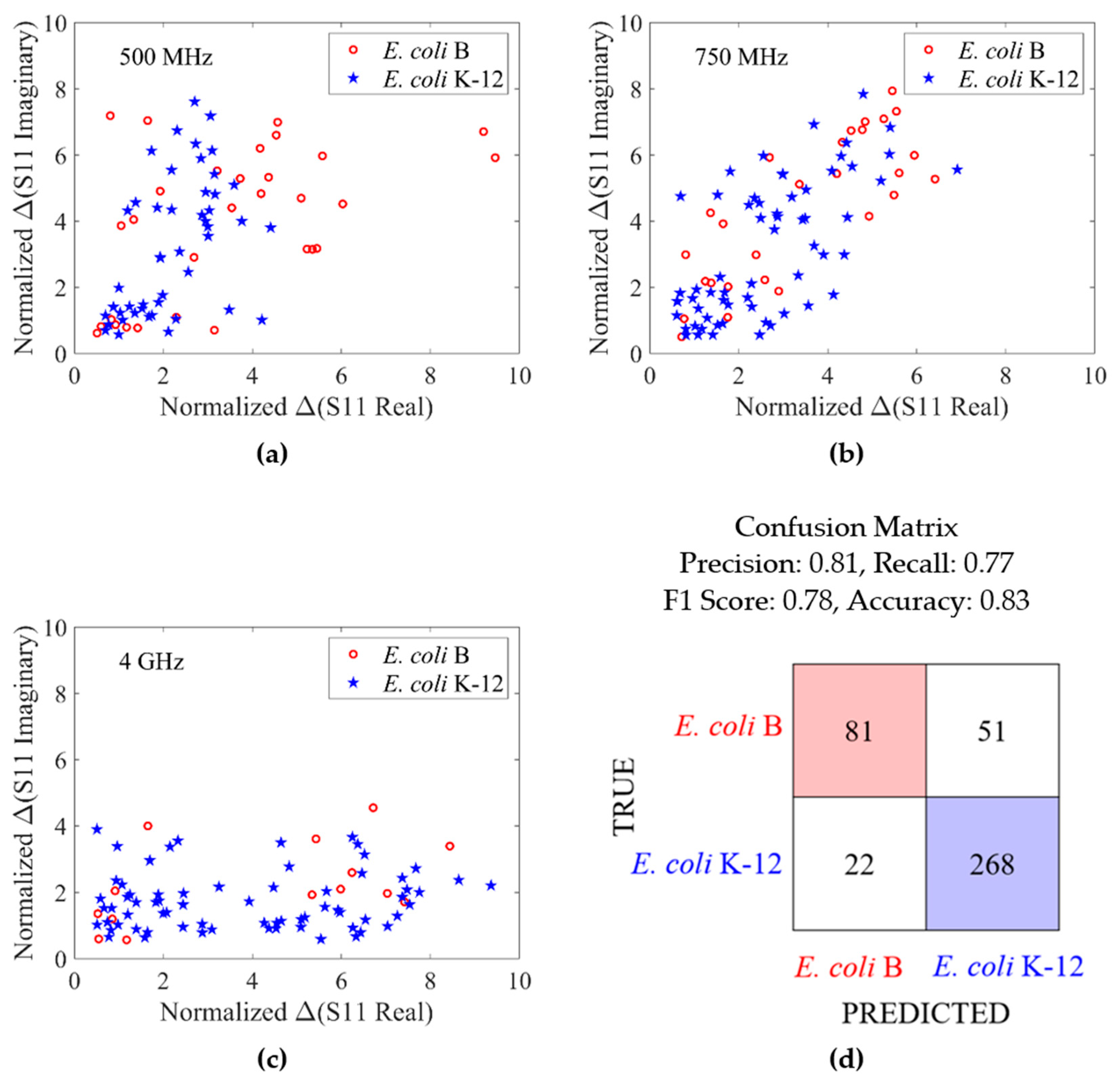
3. Results
4. Discussions and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- CDC—What is Sepsis? Available online: https://www.cdc.gov/sepsis/what-is-sepsis.html (accessed on 13 August 2023).
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef]
- Burden of Foodborne Illness: Findings; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018.
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States-Major Pathogens. Emerg. Infect Dis. 2011, 17, 7. [Google Scholar] [CrossRef]
- Lui, C.; Cady, N.C.; Batt, C.A. Nucleic acid-based detection of bacterial pathogens using integrated microfluidic platform systems. Sensors 2009, 9, 3713–3744. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection O157:H7 Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124, 143–149. [Google Scholar] [CrossRef]
- Nakar, A.; Pistiki, A.; Ryabchykov, O.; Bocklitz, T.; Rösch, P.; Popp, J. Label-free differentiation of clinical E. coli and Klebsiella isolates with Raman spectroscopy. J. Biophotonics 2022, 15, e202200005. [Google Scholar] [CrossRef]
- Wu, W.; Nguyen, B.T.T.; Liu, P.Y.; Cai, G.; Feng, S.; Shi, Y.; Zhang, B.; Hong, Y.; Yu, R.; Zhou, X.; et al. Single Escherichia coli bacteria detection using a chemiluminescence digital microwell array chip. Biosens. Bioelectron. 2022, 215, 114594. [Google Scholar] [CrossRef]
- Thakur, B.; Zhou, G.; Chang, J.; Pu, H.; Jin, B.; Sui, X.; Yuan, X.; Yang, C.-H.; Magruder, M.; Chen, J. Rapid detection of single E. coli bacteria using a graphene-based field-effect transistor device. Biosens. Bioelectron. 2018, 110, 16–22. [Google Scholar] [CrossRef]
- Spencer, D.; Morgan, H. High-Speed Single-Cell Dielectric Spectroscopy. ACS Sens. 2020, 5, 423–430. [Google Scholar] [CrossRef]
- Salahi, A.; Honrado, C.; Rane, A.; Caselli, F.; Swami, N.S. Modified Red Blood Cells as Multimodal Standards for Benchmarking Single-Cell Cytometry and Separation Based on Electrical Physiology. Anal. Chem. 2022, 94, 2865–2872. [Google Scholar] [CrossRef]
- Holmes, D.; Pettigrew, D.; Reccius, C.H.; Gwyer, J.D.; van Berkel, C.; Holloway, J.; Davies, D.E.; Morgan, H. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip 2009, 9, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Honrado, C.; Bisegna, P.; Swami, N.S.; Caselli, F. Single-cell microfluidic impedance cytometry: From raw signals to cell phenotypes using data analytics. Lab Chip 2021, 21, 22–54. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, H. Positional dependence of particles and cells in microfluidic electrical impedance flow cytometry: Origin, challenges and opportunities. Lab Chip 2020, 20, 3665–3689. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Feng, Y.; Zhu, J.; Huang, L.; Wang, W. Floating-Electrode-Enabled Impedance Cytometry for Single-Cell 3D Localization. Anal. Chem. 2023, 95, 6374–6382. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, C.V.; Franco, J.C.; Skands, G.E.; Dimaki, M.; Svendsen, W.E. Investigating the Use of Impedance Flow Cytometry for Classifying the Viability State of E. coli. Sensors 2020, 20, 6339. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, J.A.; Dahal, N.; Divan, R.; Miller, C.S.; Moline, D.; Caldwell, T.P.; Yu, X.; Harcum, S.W.; Wang, P. Microwave sensing of yeast cell species and viability. IEEE Trans. Microw. Theory Techn. 2021, 69, 1875–1886. [Google Scholar] [CrossRef]
- Dahal, N.; Osterberg, J.A.; Braun, B.; Caldwell, T.P.; Divan, R.; Harcum, S.W.; Wang, P. Spectroscopic Analysis of Candida Species, Viability, and Antifungal Drug Effects with a Microwave Flow Cytometer. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 6, 566–573. [Google Scholar] [CrossRef]
- Clausen, C.H.; Dimaki, M.; Bertelsen, C.V.; Skands, G.E.; Rodriguez-Trujillo, R.; Thomsen, J.D.; Svendsen, W.E. Bacteria Detection and Differentiation Using Impedance Flow Cytometry. Sensors 2018, 18, 3496. [Google Scholar] [CrossRef] [PubMed]
- Farasat, M.; Aalaei, E.; Ronizi, S.K.; Bakhshi, A.; Mirhosseini, S.; Zhang, J.; Nguyen, N.-T.; Kashaninejad, N. Signal-Based Methods in Dielectrophoresis for Cell and Particle Separation. Biosensors 2022, 12, 510. [Google Scholar] [CrossRef]
- Graham, K.A.; Mulhall, H.J.; Labeed, F.H.; Lewis, M.P.; Hoettges, K.F.; Kalavrezos, N.; McCaul, J.; Liew, C.; Porter, S.; Fedele, S.; et al. A dielectrophoretic method of discrimination between normal oral epithelium, and oral and oropharyngeal cancer in a clinical setting. Analyst 2015, 140, 5198–5204. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, X.; Li, K.; Yue, Y.; Zhang, Z.; Meng, Y.; Wang, S. Bipolar Electrode-based Sheath-Less Focusing and Continuous Acoustic Sorting of Particles and Cells in an Integrated Microfluidic Device. Anal. Chem. 2024, 96, 3627–3635. [Google Scholar] [CrossRef]
- Olofsson, K.; Hammarström, B.; Wiklund, M. Acoustic separation of living and dead cells using high density medium. Lab Chip 2020, 20, 1981–1990. [Google Scholar] [CrossRef]
- Zhenqui, J.; Shi, H.; Tang, X.; Qin, J. Recent advances in droplet microfluidics for single-cell analysis. Trend Anal. Chem. 2023, 159, 116932. [Google Scholar]
- Afshar, S.; Salimi, E.; Braasch, K.; Butler, M.; Thomson, D.J.; Bridges, G.E. Multi-frequency DEP cytometer employing a microwave sensor for dielectric analysis of single cells. IEEE Trans. Microw. Theory 2016, 64, 991–998. [Google Scholar] [CrossRef]
- Tamra, A.; Dubuc, D.; Rols, M.P.; Grenier, K. Microwave monitoring of single cell monocytes subjected to electroporation. IEEE Trans. Microw. Theory 2017, 65, 3512–3518. [Google Scholar] [CrossRef]
- Li, H.; Ma, X.; Du, X.; Li, L.; Cheng, X.; Hwang, J.C. Correlation between optical fluorescence and microwave transmission during single-cell electroporation. IEEE Trans. Bio-Med. Eng. 2018, 66, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Multari, C.; Palego, C.; Ma, X.; Du, X.; Ning, Y.; Buceta, J.; Hwang, J.C.; Cheng, X. Differentiation of live and heat-killed E. coli by microwave impedance spectroscopy. Sens. Actuators B Chem. 2018, 255, 1614–1622. [Google Scholar] [CrossRef]
- Shahri, A.A.; Omidvar, A.H.; Rehder, G.P.; Serrano, A.L.C. A Microwave-Based Microfluidic Cell Detecting Biosensor for Biological Quantification Using the Metallic Nanowire-Filled Membrane Technology. Sensors 2022, 22, 3265. [Google Scholar] [CrossRef]
- Cui, Y.; Delaney, W.F.; Darroudi, T.; Wang, P. Microwave measurement of giant unilamellar vesicles in aqueous solution. Sci. Rep. 2018, 8, 497. [Google Scholar] [CrossRef]
- Secme, A.; Tefek, U.; Sari, B.; Pisheh, H.S.; Uslu, H.D.; Çalışkan, Ö.A.; Kucukoglu, B.; Erdogan, R.T.; Alhmoud, H.; Sahin, O.; et al. High-Resolution Dielectric Characterization of Single Cells and Microparticles Using Integrated Microfluidic Microwave Sensors. IEEE Sens. J. 2023, 23, 6517–6529. [Google Scholar] [CrossRef]
- Wang, Z.; Raval, Y.; Tzeng, T.R.; Booth, B.; Flaherty, B.; Peterson, D.; Moore, J.; Rosenmann, D.; Divan, R.; Yu, G.; et al. Time domain detection and differentiation of single particles and cells with a radio frequency interferometer. In Proceedings of the 2016 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), Austin, TX, USA, 24–27 January 2016. [Google Scholar]
- Sun, T.; Green, N.G.; Gawad, S.; Morgan, H. Analytical electric field and sensitivity analysis for two microfluidic impedance cytometer designs. IET Nanobiotechnol. 2007, 1, 69–79. [Google Scholar] [CrossRef]
- Akiba, T.; Sano, S.; Yanase, T.; Ohta, T.; Koyama, M. Optuna: A next-generation hyperparameter optimization framework. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019. [Google Scholar]
- Pethig, R.; Schmueser, I. 401Marking 100 years since Rudolf Höber’s discovery of the insulating envelope surrounding cells and of the β-dispersion exhibited by tissue. J. Electr. Bioimpedance 2012, 3, 74–79. [Google Scholar] [CrossRef]
- Prodan, C.; Mayo, F.; Claycomb, J.R.; Miller, J.H., Jr.; Benedik, M.J. Low-frequency, low-field dielectric spectroscopy of living cell suspensions. J. Appl. Phys. 2004, 95, 3754–3756. [Google Scholar] [CrossRef]
- Bothwell, T.P.; Schwann, H.P. Electrical properties of the plasma membrane of erythrocytes at low frequencies. Nature 1956, 178, 265–266. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahal, N.; Peak, C.; Ehrett, C.; Osterberg, J.; Cao, M.; Divan, R.; Wang, P. Microwave Flow Cytometric Detection and Differentiation of Escherichia coli. Sensors 2024, 24, 2870. https://doi.org/10.3390/s24092870
Dahal N, Peak C, Ehrett C, Osterberg J, Cao M, Divan R, Wang P. Microwave Flow Cytometric Detection and Differentiation of Escherichia coli. Sensors. 2024; 24(9):2870. https://doi.org/10.3390/s24092870
Chicago/Turabian StyleDahal, Neelima, Caroline Peak, Carl Ehrett, Jeffrey Osterberg, Min Cao, Ralu Divan, and Pingshan Wang. 2024. "Microwave Flow Cytometric Detection and Differentiation of Escherichia coli" Sensors 24, no. 9: 2870. https://doi.org/10.3390/s24092870
APA StyleDahal, N., Peak, C., Ehrett, C., Osterberg, J., Cao, M., Divan, R., & Wang, P. (2024). Microwave Flow Cytometric Detection and Differentiation of Escherichia coli. Sensors, 24(9), 2870. https://doi.org/10.3390/s24092870





