The Use of Virtual Tissue Constructs That Include Morphological Variability to Assess the Potential of Electrical Impedance Spectroscopy to Differentiate between Thyroid and Parathyroid Tissues during Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Multiscale Thyroid and Parathyroid Model
2.2. Global Sensitivity Analysis
2.2.1. Model Parameters
2.2.2. Parameter Space Sampling
2.2.3. Sensitivity Assessment
2.2.4. EIS Spectra Parameterisation
3. Model Verification and Differentiation Assessment
4. Results
4.1. EIS Spectra Computation
4.2. Global Sensitivity Macroscale Results
4.2.1. Thyroid Results
4.2.2. Parathyroid Results
4.3. Computed Spectra Verification against Measured Data
4.4. Thyroid and Parathyroid Tissue Differentiation
4.4.1. Qualitative Separation
4.4.2. Manual ROC-Based Classification
4.4.3. Machine Learning Classifier Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EIS | Electrical impedance spectroscopy |
| FE | Finite element |
| ROC | Receiver operating characteristic |
| ECS | Extracellular space |
| LHS | Latin hypercube sampling |
| PRCC | Partial rank correlation coefficient |
| AUC | Area under curve |
| TPR | True positive rate |
| FPR | False positive rate |
| SVM | Support vector machine |
| KNN | K-nearest neighbor |
| RFC | Random forest classifier |
Appendix A. Electrical Material Properties
| Compartment | Electric Conductivity [] | Relative Permittivity [−] |
|---|---|---|
| Cytoplasm | 0.55 | 150 |
| ECS | 1.1 | 72 |
| Cell membrane | 1 × | 8.7 |
| Thyroid colloid | 0.55 | 150 |
| Follicular connective tissue | 0.35 | 1 × |
| Fascia | 0.35 | 1 × |
Appendix B. Global Sensitivity Results for the Micro- and Mesoscale
| Scale | Output Parameter | Significant Input Parameter | PRCC | Correlation Level |
|---|---|---|---|---|
| Microscale | 0.58 | Medium | ||
| −0.86 | High | |||
| −0.95 | High | |||
| −0.58 | Medium | |||
| 0.81 | High | |||
| Mesoscale | 0.40 | Low | ||
| −0.70 | High | |||
| −0.61 | Medium | |||
| 0.72 | High | |||
| −0.60 | Medium | |||
| −0.49 | Medium | |||
| 0.87 | High |
| Scale | Output Parameter | Significant Input Parameter | PRCC | Correlation Level |
|---|---|---|---|---|
| Microscale | 0.60 | Medium | ||
| −0.77 | High | |||
| −0.76 | High | |||
| −0.42 | Low | |||
| 0.37 | Low | |||
| −0.71 | High | |||
| 0.54 | Medium | |||
| −0.35 | Low |
References
- British Association of Endocrine and Thyroid Surgeons. BAETS Sixth National Audit Report; BAETS: London, UK, 2021. [Google Scholar]
- Kim, S.W.; Lee, H.S.; Lee, K.D. Intraoperative Real-time Localization of Parathyroid Gland with Near Infrared Fluorescence Imaging. Gland. Surg. 2017, 6, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Shinden, Y.; Nakajo, A.; Arima, H.; Tanoue, K.; Hirata, M.; Kijima, Y.; Maemura, K.; Natsugoe, S. Intraoperative Identification of the Parathyroid Gland with a Fluorescence Detection System. World J. Surg. 2017, 41, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Siperstein, A.; Berber, E.; Mackey, R.; Alghoul, M.; Wagner, K.; Milas, M. Prospective Evaluation of Sestamibi Scan, Ultrasonography, and Rapid PTH to Predict the Success of Limited Exploration for Sporadic Primary Hyperparathyroidism. Surgery 2004, 136, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Schwan, H.P. Electrical Properties of Tissues and Cell Suspensions: Mechanisms and Models. In Proceedings of the 16th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Baltimore, MD, USA, 3–6 November 1994; Volume 1, pp. A70–A71. [Google Scholar] [CrossRef]
- Zilico Limited. ZedScan™ a New Standard in Colposcopy—Technical Brochure; Zilico Ltd.: Manchester, UK, 2013. [Google Scholar]
- Dean, D.; Thillaiyan, R.; Machado, D.; Sundararajan, R. Electrical Impedance Spectroscopy Study of Biological Tissues. J. Electrost. 2008, 66, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Schoukens, J.; Bragos, R.; Vandersteen, G. Novel Estimation of the Electrical Bioimpedance Using the Local Polynomial Method. Application to In Vivo Real-Time Myocardium Tissue Impedance Characterization During the Cardiac Cycle. IEEE Trans. Biomed. Eng. 2011, 58, 3376–3385. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Vandersteen, G.; Martin, I.; Castillo, D.; Torrego, A.; Riu, P.J.; Schoukens, J.; Bragos, R. In Vivo Electrical Bioimpedance Characterization of Human Lung Tissue During the Bronchoscopy Procedure. A Feasibility Study. Med Eng. Phys. 2013, 35, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.; Birgersson, U.; Berking, C.; Henderson, C.; Trefzer, U.; Kemeny, L.; Sunderkötter, C.; Dirschka, T.; Motley, R.; Frohm-Nilsson, M.; et al. Electrical Impedance Spectroscopy as a Potential Adjunct Diagnostic Tool for Cutaneous Melanoma. Ski. Res. Technol. 2013, 19, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Das, S.; Chatterjee, J. Electrical Bioimpedance Analysis: A New Method in Cervical Cancer Screening. J. Med Eng. 2015, 2015, 636075. [Google Scholar] [CrossRef]
- Murdoch, C.; Brown, B.H.; Hearnden, V.; Speight, P.M.; D’Apice, K.; Hegarty, A.M.; Tidy, J.A.; Healey, T.J.; Highfield, P.E.; Thornhill, M.H. Use of Electrical Impedance Spectroscopy to Detect Malignant and Potentially Malignant Oral Lesions. Int. J. Nanomed. 2014, 9, 4521–4532. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Nissan, A.; Gallimidi, Z.; Lenington, S.; Logan, W.; Zuley, M.; Yeshaya, A.; Shimonov, M.; Melloul, M.; Fields, S.; et al. Electrical Impedance Scanning for the Early Detection of Breast Cancer in Young Women: Preliminary Results of a Multicenter Prospective Clinical Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 2703–2715. [Google Scholar] [CrossRef]
- Mishra, V.; Bouayad, H.; Schned, A.; Heaney, J.; Halter, R.J. Electrical Impedance Spectroscopy for Prostate Cancer Diagnosis. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 3258–3261. [Google Scholar] [CrossRef]
- Baghbani, R.; Shadmehr, M.B.; Ashoorirad, M.; Molaeezadeh, S.F.; Moradi, M.H. Bioimpedance Spectroscopy Measurement and Classification of Lung Tissue to Identify Pulmonary Nodules. IEEE Trans. Instrum. Meas. 2021, 70, 4006407. [Google Scholar] [CrossRef]
- Yun, J.; Hong, Y.T.; Hong, K.H.; Lee, J.H. Ex Vivo Identification of Thyroid Cancer Tissue Using Electrical Impedance Spectroscopy on a Needle. Sens. Actuators B Chem. 2018, 261, 537–544. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Fields, S.I.; Shriver, C.D.; Lenington, S.; Ginor, R.; Peoples, G.E.; Burch, H.B.; Peretz, T.; Freund, H.R.; Nissan, A. Electrical Impedance Scanning of Thyroid Nodules Before Thyroid Surgery: A Prospective Study. Ann. Surg. Oncol. 2005, 12, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Tublin, M.E.; Klym, A.H.; Gur, D. Classification of Thyroid Nodules Using a Resonance-frequency-based Electrical Impedance Spectroscopy: A Preliminary Assessment. Thyroid. Off. J. Am. Thyroid. Assoc. 2013, 23, 854–862. [Google Scholar] [CrossRef]
- Hillary, S.L.; Brown, B.H.; Brown, N.J.; Balasubramanian, S.P. Use of Electrical Impedance Spectroscopy for Intraoperative Tissue Differentiation During Thyroid and Parathyroid Surgery. World J. Surg. 2019, 44, 479–485. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Wu, J.; Liu, Y.; Wang, P.; Liu, H.; Wang, H.; Wang, T.; Wang, J.; Tang, Y.; et al. Bioelectrical Impedance Spectroscopy Can Assist to Identify the Parathyroid Gland During Thyroid Surgery. Front. Endocrinol. 2022, 13, 963520. [Google Scholar] [CrossRef]
- Matella, M.; Hunter, K.; Balasubramanian, S.; Walker, D.C. Multiscale Model Development for Electrical Properties of Thyroid and Parathyroid Tissues; IEEE Open Journal of Engineering in Medicine and Biology: Piscataway, NJ, USA, 2023; pp. 1–9. [Google Scholar] [CrossRef]
- Saltelli, A.; Aleksankina, K.; Becker, W.; Fennell, P.; Ferretti, F.; Holst, N.; Li, S.; Wu, Q. Why So Many Published Sensitivity Analyses are False: A Systematic Review of Sensitivity Analysis Practices. Environ. Model. Softw. 2019, 114, 29–39. [Google Scholar] [CrossRef]
- Matella, M. Computational Modelling of Electrical Properties of Thyroid and Parathyroid Tissue. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2023. [Google Scholar]
- Walker, D.C. Modeling the Electrical Properties of Cervical Epithelium. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2001. [Google Scholar]
- Al-Zghoul, M. Macro- and Micro-Morphological Studies on the Parathyroid Glands of Dromedary Camel. Pak. Vet. J. 2017, 37, 59–64. [Google Scholar]
- Ramas, A.; Jakubovic-Cičkisic, A.; Umihanic, S.; Sulejmanovic, M.; Brkic, F. Correlation Between the Parathyroid Glands Size and Parathormones Value in Patients with Hyperparathyroidism. Med. Arch. 2019, 73, 249–252. [Google Scholar] [CrossRef]
- McKay, M.D.; Beckman, R.J.; Conover, W.J. A Comparison of Three Methods for Selecting Values of Input Variables in the Analysis of Output from a Computer Code. Technometrics 1979, 21, 239–245. [Google Scholar] [CrossRef]
- Marino, S.; Hogue, I.B.; Ray, C.J.; Kirschner, D.E. A Methodology for Performing Global Uncertainty and Sensitivity Analysis in Systems Biology. J. Theor. Biol. 2008, 254, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Sobol′, I.M. Global Sensitivity Indices for Nonlinear Mathematical Models and Their Monte Carlo Estimates. Math. Comput. Simul. 2001, 55, 271–280. [Google Scholar] [CrossRef]
- Eng, J. Receiver Operating Characteristic Analysis: A Primer. Acad. Radiol. 2005, 12, 909–916. [Google Scholar] [CrossRef]
- Pandeya, S.R.; Nagy, J.A.; Riveros, D.; Semple, C.; Taylor, R.S.; Hu, A.; Sanchez, B.; Rutkove, S.B. Using machine learning algorithms to enhance the diagnostic performance of electrical impedance myography. Muscle Nerve 2022, 66, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Chang, E.T.; Strong, M.; Clayton, R.H. Bayesian Sensitivity Analysis of a cCrdiac Cell Model using a Gaussian Process Emulator. PLoS ONE 2015, 10, e0137004. [Google Scholar] [CrossRef]
- Karabelas, E.; Longobardi, S.; Fuchsberger, J.; Razeghi, O.; Rodero, C.; Strocchi, M.; Rajani, R.; Haase, G.; Plank, G.; Niederer, S. Global Sensitivity Analysis of Four Chamber Heart Hemodynamics Using Surrogate Models. IEEE Trans. Biomed. Eng. 2022, 69, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Markx, G.H.; Davey, C.L.; Kell, D.B. To What Extent is the Magnitude of the Cole-Cole α of the β-dielectric Dispersion of Cell Suspensions Explicable in Terms of the Cell Size Distribution? Bioelectrochem. Bioenerg. 1991, 25, 195–211. [Google Scholar] [CrossRef]
- Helwan, A.; Idoko, J.B.; Abiyev, R.H. Machine Learning Techniques for Classification of Breast Tissue. Procedia Comput. Sci. 2017, 120, 402–410. [Google Scholar] [CrossRef]
- Tian, D.; Lang, Z.Q.; Zhang, D.; Anumba, D.O. A filter-predictor polynomial feature based machine learning approach to predicting preterm birth from cervical electrical impedance spectroscopy. Biomed. Signal Process. Control 2023, 80, 104345. [Google Scholar] [CrossRef]
- Li, P.; Highfield, P.E.; Lang, Z.Q.; Kell, D. Cervical cancer prognosis and diagnosis using electrical impedance spectroscopy. J. Electr. Bioimpedance 2021, 12, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, C.; Haemmerich, D. Review of Temperature Dependence of Thermal Properties, Dielectric Properties, and Perfusion of Biological Tissues at Hyperthermic and Ablation Temperatures. Crit. Rev. Biomed. Eng. 2015, 42, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Krüskemper, H.L.; Reichertz, P. Der Einfluss von Thyrotropem Hormon auf das Elektrogramm der Schilddruese. Acta Endocrinol. 1960, 34, 390–398. [Google Scholar] [CrossRef]
- Morin, M.; Ruzgas, T.; Svedenhag, P.; Anderson, C.D.; Ollmar, S.; Engblom, J.; Björklund, S. Skin Hydration Dynamics Investigated by Electrical Impedance Techniques In Vivo and In Vitro. Sci. Rep. 2020, 10, 17218. [Google Scholar] [CrossRef] [PubMed]
- Matella, M.; Walker, D.C.; Hunter, K. Computational Modelling of Probe Configurations for Electrical Impedance Spectroscopy-based Differentiation of Thyroid and Parathyroid Tissues. In Proceedings of the 2023 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023; pp. 1–6. [Google Scholar] [CrossRef]
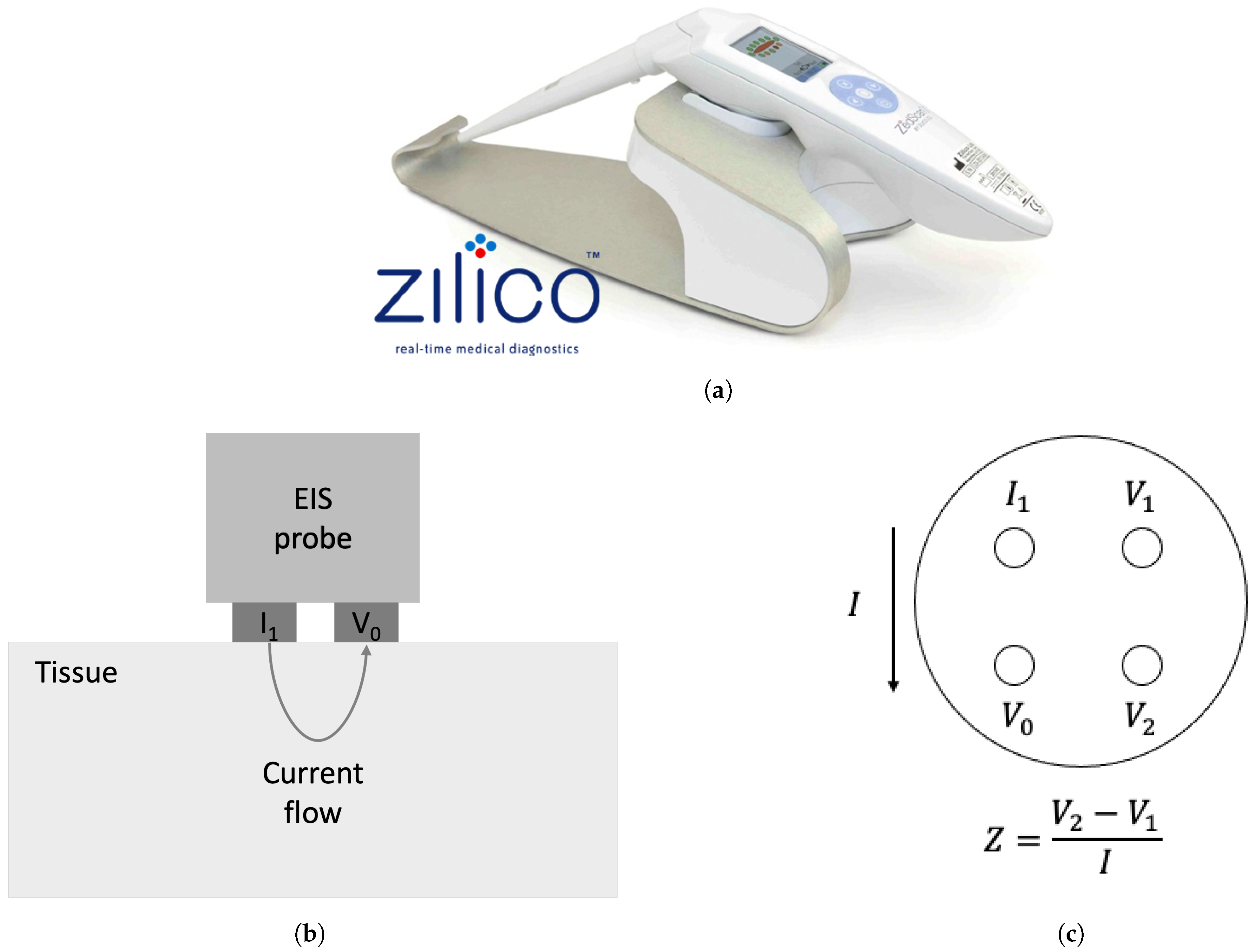
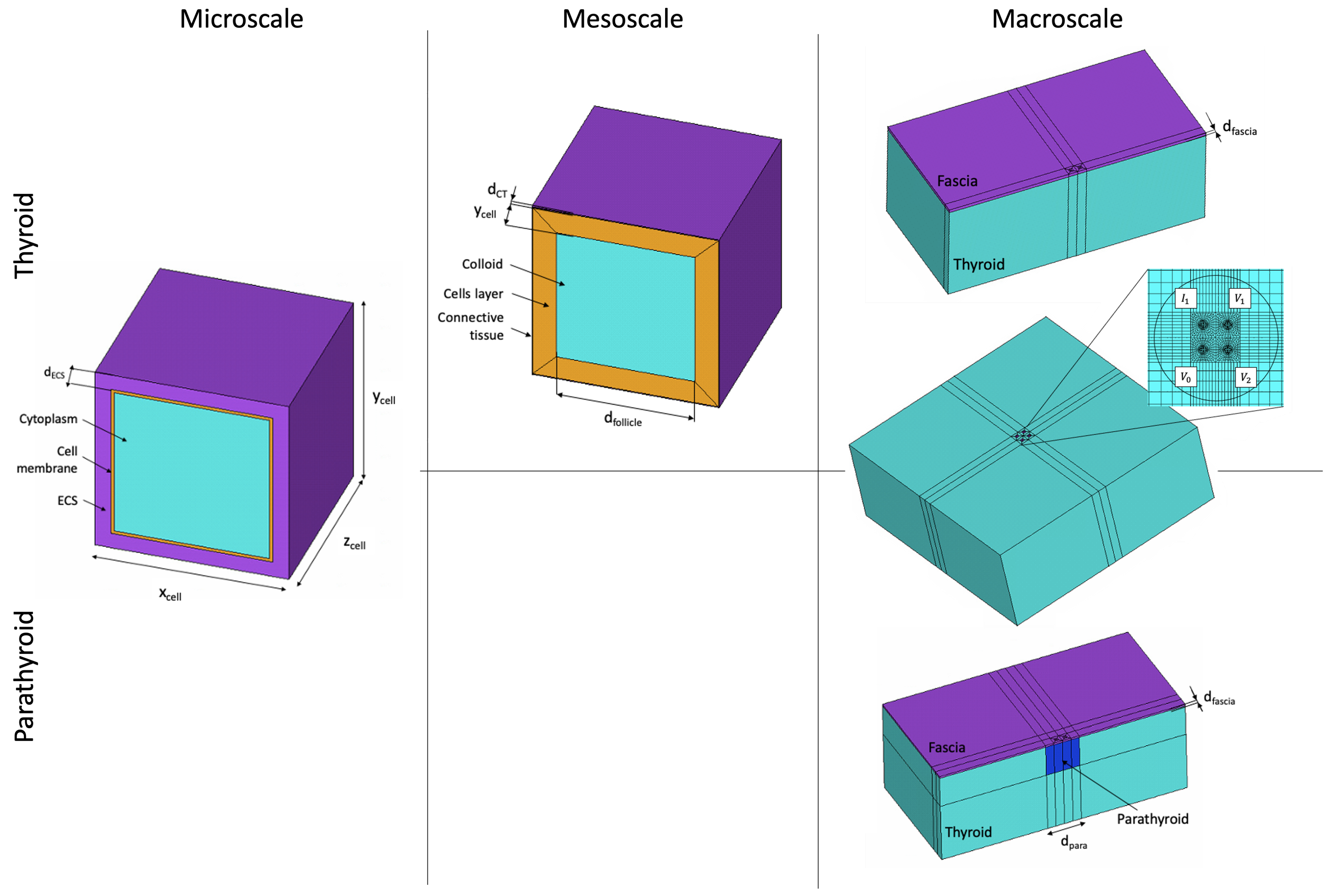
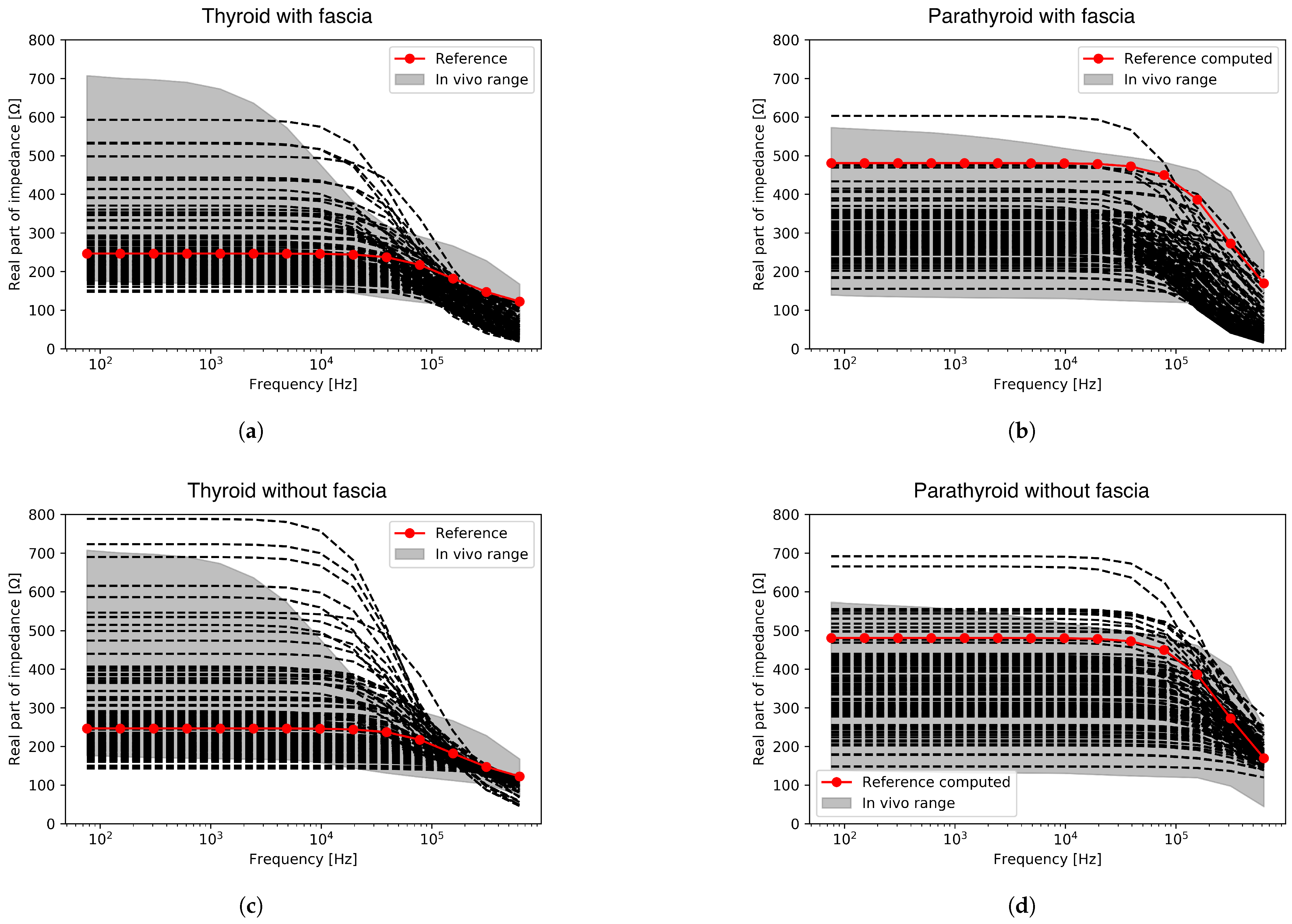
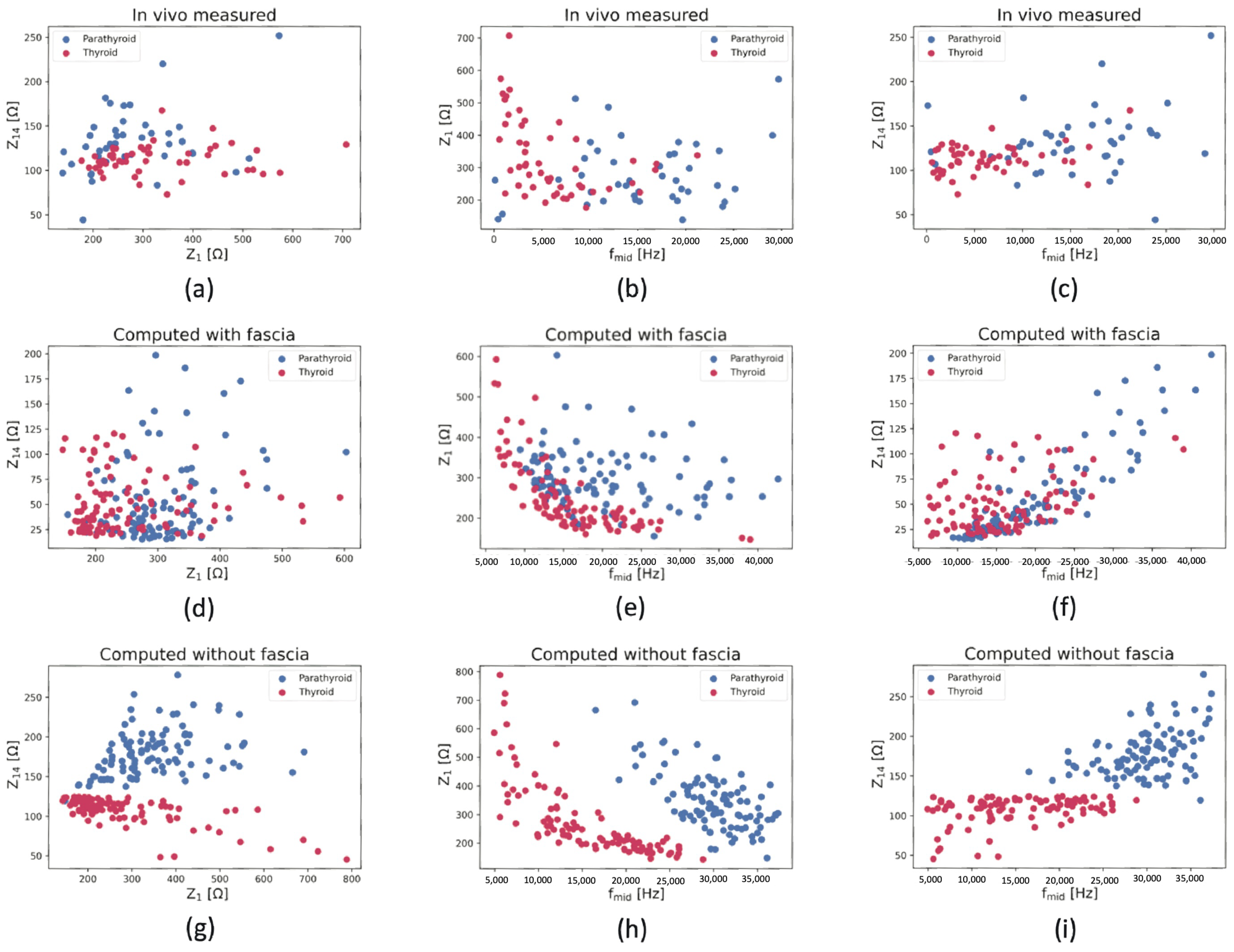

| Parameter | Distribution | Distribution Indices | Value | Reference |
|---|---|---|---|---|
| Microscale | ||||
| [µm] | Normal | Mean Standard deviation | 8.53 µm 1.84 µm | Histology measurements [23] |
| [µm] | Normal | Mean Standard deviation | 8.53 µm 1.84 µm | Histology measurements [23] |
| [µm] | Uniform | Min Max | 0.1 µm 0.5 µm | Initial local sensitivity results and estimated from cervical epithelium measurements [24,25] |
| Mesoscale | ||||
| [µm] | Normal | Mean Standard deviation | 113.77 µm 63.13 µm | Histology measurements [23] |
| [µm] | Uniform | Min Max | 0.8 µm 2.5 µm | Estimated |
| Macroscale | ||||
| [mm] | Uniform | Min Max | 0.025 mm 0.5 mm | Estimated |
| Parameter | Distribution | Distribution Indices | Value | Reference |
|---|---|---|---|---|
| Microscale | ||||
| [µm] | Normal | Mean Standard deviation | 7.59 µm 1.45 µm | Histology measurements [23] |
| [µm] | Normal | Mean Standard deviation | 7.59 µm 1.45 µm | Histology measurements [23] |
| [µm] | Uniform | Min Max | 0.4 µm 0.9 µm | Estimated based on previous simulation results and on cervical epithelium measurements [24,25] |
| Macroscale | ||||
| [mm] | Uniform | Min Max | 0.025 mm 0.500 mm | Estimated |
| [mm] | Uniform | Min Max | 3 mm 8 mm | Estimated based on literature [26] |
| Output Parameter | Significant Input Parameter | PRCC | Correlation Level | |
|---|---|---|---|---|
| Model including fascia | −0.68 | Medium | ||
| −0.61 | Medium | |||
| 0.41 | Medium | |||
| −0.97 | High | |||
| 0.73 | High | |||
| 0.41 | Medium | |||
| Model excluding fascia | 0.39 | Low | ||
| −0.87 | High | |||
| −0.70 | High | |||
| 0.85 | High | |||
| −0.50 | Medium | |||
| 0.87 | High |
| Output Parameter | Significant Input Parameter | PRCC | Correlation Level | |
|---|---|---|---|---|
| Model including fascia | 0.49 | Medium | ||
| −0.70 | High | |||
| −0.88 | High | |||
| 0.42 | Medium | |||
| −0.34 | Low | |||
| −0.77 | High | |||
| Model excluding fascia | 0.58 | Medium | ||
| −0.78 | High | |||
| −0.85 | High | |||
| −0.65 | Medium | |||
| 0.44 | Medium |
| Dataset | |||
|---|---|---|---|
| t > p | p > t | p > t | |
| in vivo dataset | 0.604 | 0.719 | 0.862 |
| p > t | t > p | p > t | |
| computed dataset —including fascia | 0.721 | 0.527 | 0.644 |
| p > t | p > t | p > t | |
| computed dataset —excluding fascia | 0.732 | 0.998 | 0.971 |
| Classifier | Computed with Fascia | Computed without Fascia | ||
|---|---|---|---|---|
| AUC | Accuracy | AUC | Accuracy | |
| SVM | 0.649 | 0.588 | 0.978 | 0.908 |
| KNN | 0.608 | 0.517 | 0.956 | 0.879 |
| RFC | 0.918 | 0.840 | 1.000 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matella, M.; Hunter, K.; Balasubramanian, S.; Walker, D. The Use of Virtual Tissue Constructs That Include Morphological Variability to Assess the Potential of Electrical Impedance Spectroscopy to Differentiate between Thyroid and Parathyroid Tissues during Surgery. Sensors 2024, 24, 2198. https://doi.org/10.3390/s24072198
Matella M, Hunter K, Balasubramanian S, Walker D. The Use of Virtual Tissue Constructs That Include Morphological Variability to Assess the Potential of Electrical Impedance Spectroscopy to Differentiate between Thyroid and Parathyroid Tissues during Surgery. Sensors. 2024; 24(7):2198. https://doi.org/10.3390/s24072198
Chicago/Turabian StyleMatella, Malwina, Keith Hunter, Saba Balasubramanian, and Dawn Walker. 2024. "The Use of Virtual Tissue Constructs That Include Morphological Variability to Assess the Potential of Electrical Impedance Spectroscopy to Differentiate between Thyroid and Parathyroid Tissues during Surgery" Sensors 24, no. 7: 2198. https://doi.org/10.3390/s24072198
APA StyleMatella, M., Hunter, K., Balasubramanian, S., & Walker, D. (2024). The Use of Virtual Tissue Constructs That Include Morphological Variability to Assess the Potential of Electrical Impedance Spectroscopy to Differentiate between Thyroid and Parathyroid Tissues during Surgery. Sensors, 24(7), 2198. https://doi.org/10.3390/s24072198








