Molecular Weights of Polyethyleneimine-Dependent Physicochemical Tuning of Gold Nanoparticles and FRET-Based Turn-On Sensing of Polymyxin B
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Chemicals
2.2. Molecular Weight of Polyethyleneimine-Dependent Synthesis of Gold Nanoparticles
2.3. Physical Characterization of as-Produced Gold Nanoparticles (PEI@AuNPs)
2.4. Experimental Setup for Sensing of Polymyxin B
2.5. Time-Resolved Fluorescence Lifetime Analysis
3. Result and Discussion
3.1. Synthesis and Characterization of as-Produced PEI-Stabilized Gold Nanoparticles
3.2. Effect of Polymyxin B (PMB) on the LSPR Properties of PEI@AuNPs
3.3. PEI@AuNP-Mediated Sensing of Polymyxin B (PMB)
3.4. Lifetime Decay Analysis
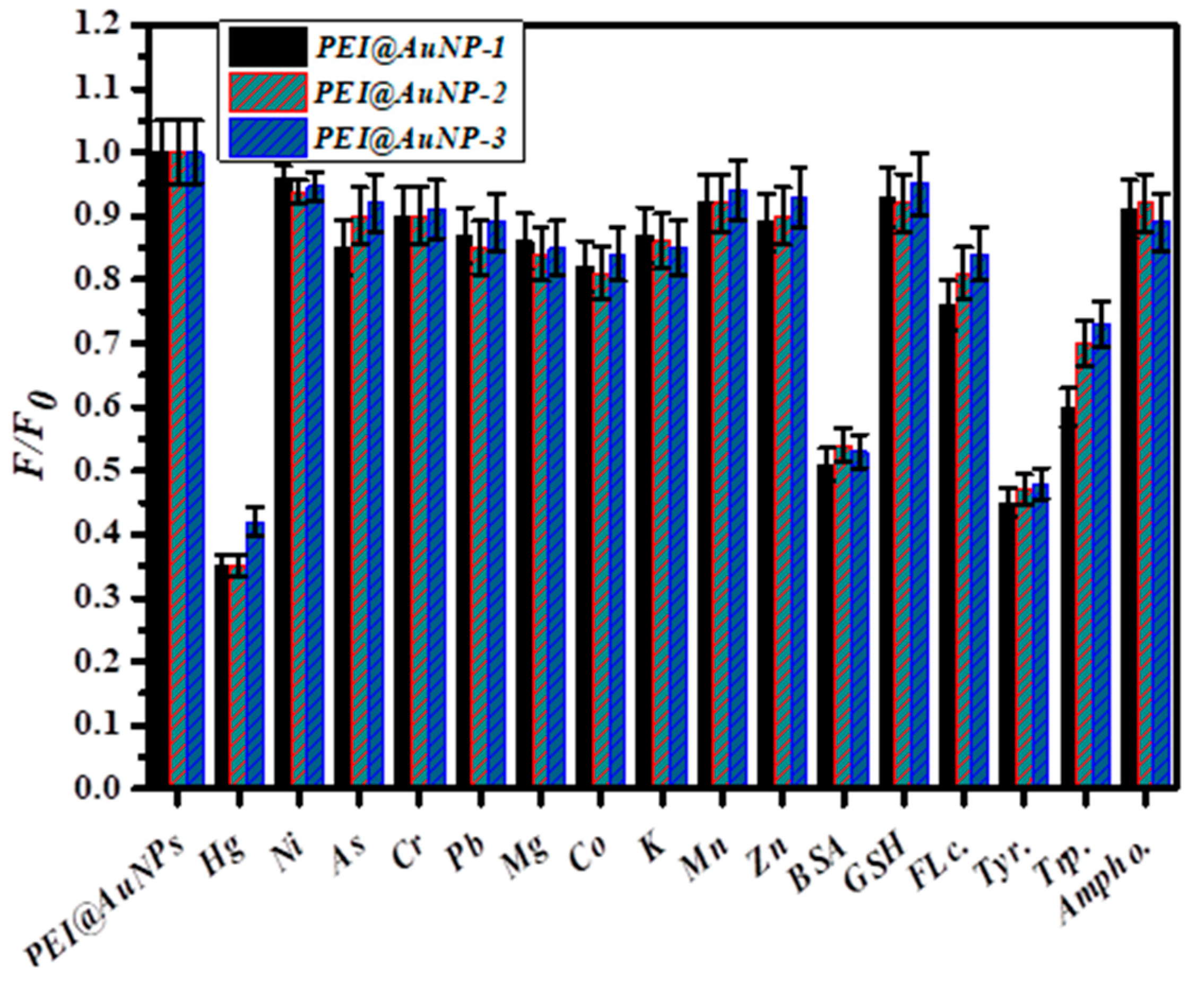
3.5. Selectivity of PEI@AuNPs for PMB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEI-AuNPs | polyethyleneimine-stabilized gold nanoparticles |
| LSPR | local surface plasmon resonance |
| SPR | surface plasmon resonance |
| DLS | dynamic light scattering |
| XRD | X-ray diffraction |
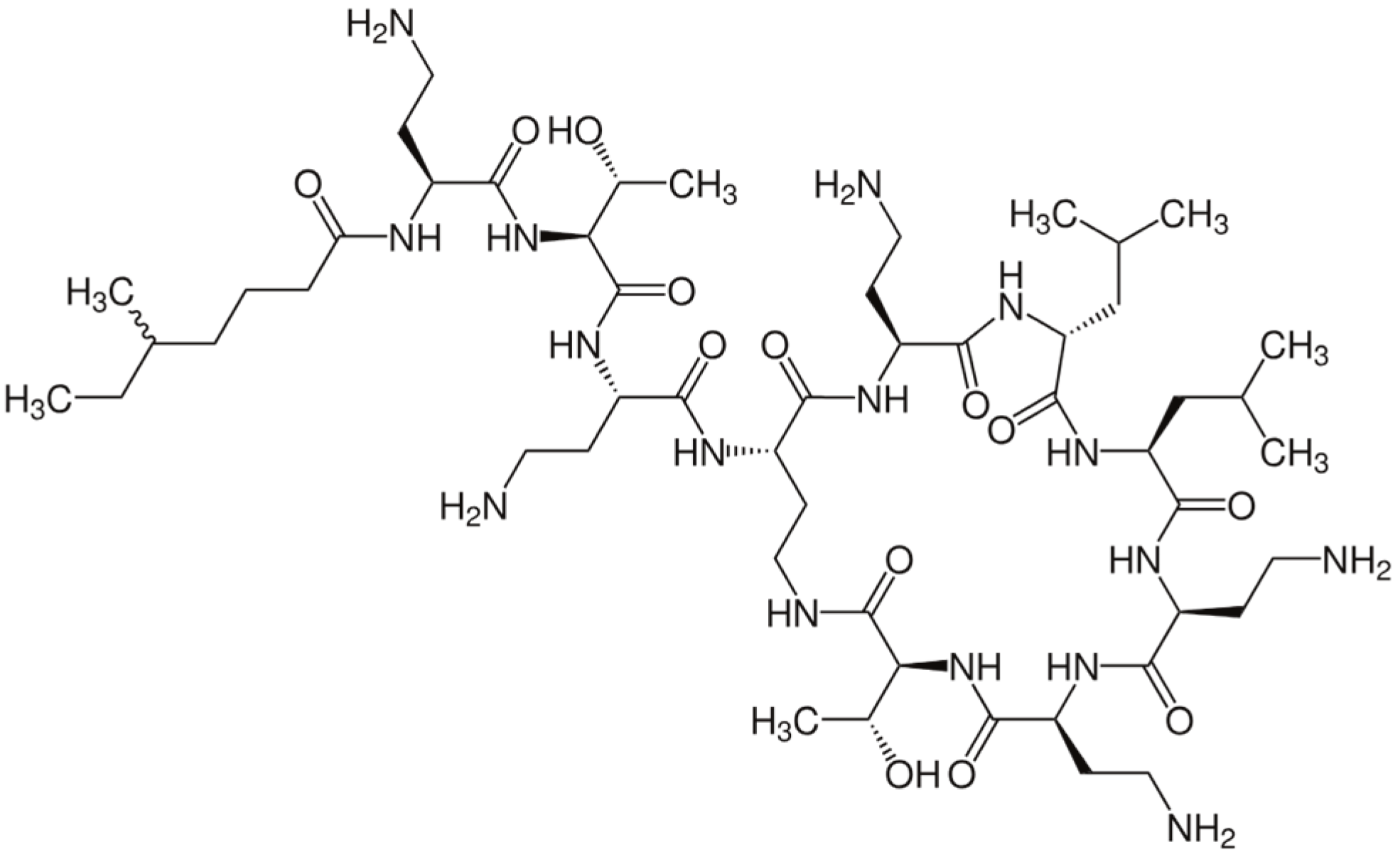
| PMB | polymyxin B |
| BSA | bovine serum albumin |
| LOD | limit of detection |
| FRET | fluorescence resonance energy transfer |
| PEIs | polyethyleneimines |
| TRPL | time-resolved photoluminescence. |
References
- Hancock, R.E.W. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Ismail, B.; Shafei, M.N.; Harun, A.; Ali, S.; Omar, M.; Deris, Z.Z. Predictors of polymyxin B treatment failure in Gram-negative healthcare-associated infections among critically ill patients. J. Microbiol. Immunol. Infect. 2018, 51, 763–769. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Yang, H.; Li, J.; Yu, J.; Yu, Z.; Cao, G.; Wu, X.; Wang, Y.; Wu, H.; et al. Acute toxicity is a dose-limiting factor for intravenous polymyxin B: A safety and pharmacokinetic study in healthy Chinese subjects. J. Infect. 2021, 82, 207–215. [Google Scholar] [CrossRef]
- Sisay, M.; Hagos, B.; Edessa, D.; Tadiwos, Y.; Mekuria, A.N. Polymyxin-induced nephrotoxicity and its predictors: A systematic review and meta-analysis of studies conducted using RIFLE criteria of acute kidney injury. Pharmacol. Res. 2021, 163, 105328. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.M. Optical methods of antibiotic residues detections: A comprehensive review. Sens. Actuators B Chem. 2018, 269, 238–256. [Google Scholar] [CrossRef]
- Srisom, P.; Liawruangrath, B.; Liawruangrath, S.; Slater, J.M.; Wangkarn, S. Simultaneous determination of neomycin sulfate and polymyxin B sulfate by capillary electrophoresis with indirect UV detection. J. Pharm. Biomed. Anal. 2007, 43, 1013–1018. [Google Scholar] [CrossRef]
- Cheah, S.E.; Bulitta, J.B.; Li, J.; Nation, R.L. Development and validation of a liquid chromatography–mass spectrometry assay for polymyxin B in bacterial growth media. J. Pharm. Biomed. Anal. 2014, 92, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, L.; Liu, S.; Jaber, O.M.; Gao, L.; Chevrette, L.; Reuschel, S. Simultaneous quantitation of polymyxin B1, polymyxin B2 and polymyxin B1-1 in human plasma and treated human urine using solid phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2016, 1012, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.H.; Holloway, I. Thin-layer chromatographic method for the identification of the polymyxins. J. Chromatogr. A 1978, 161, 417–420. [Google Scholar] [CrossRef]
- Burkin, M.A.; Galvidis, I.A.; Surovoy, Y.A.; Plyushchenko, I.V.; Rodin, I.A.; Tsarenko, S.V. Development of ELISA formats for polymyxin B monitoring in serum of critically ill patients. J. Pharm. Biomed. Anal. 2021, 204, 114275. [Google Scholar] [CrossRef]
- Hung, S.H.; Lee, J.Y.; Hu, C.C.; Chiu, T.C. Gold-nanoparticle-based fluorescent “turn-on” sensor for selective and sensitive detection of dimethoate. Food Chem. 2018, 260, 61–65. [Google Scholar] [CrossRef]
- Maiti, P.; Singha, T.; Chakraborty, U.; Roy, S.D.; Karmakar, P.; Dey, B.; Hussain, S.A.; Paul, S.; Paul, P.K. Selective and sensitive detection of L-Cysteine via fluorometric assay using gold nanoparticles and Rhodamine B in aqueous medium. Mater. Chem. Phys. 2019, 234, 158–167. [Google Scholar] [CrossRef]
- Tseng, M.H.; Hu, C.C.; Chiu, T.C. A fluorescence turn-on probe for sensing thiodicarb using rhodamine B functionalized gold nanoparticles. Dye. Pigment. 2019, 171, 107674. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Chen, P.; Pan, D.; Fan, C.; Chen, J.; Huang, K.; Wang, D.; Zhang, H.; Li, Y.; Feng, G.; Liang, P.; et al. Gold nanoparticles for high-throughput genotyping of long-range haplotypes. Nat. Nanotechnol. 2011, 6, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y. Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protoc. 2006, 1, 246–252. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Song, S.; Kuang, H.; Xu, C. A Rapid and Semi-Quantitative Gold Nanoparticles Based Strip Sensor for Polymyxin B Sulfate Residues. Nanomaterials 2018, 8, 144. [Google Scholar] [CrossRef]
- Khanal, S.; Taneja, A.; Kumar, H.; Verma, R.; Kuca, K.; Kumar, D. Detection of colistin (Polymyxin B) a restricted drug in chicken eggs using a label-free immunosensor based on Au screen-printed electrodes. Chem. Pap. 2024, 78, 1009–1020. [Google Scholar] [CrossRef]
- De Lima Oliveira, E.G.; de Oliveira, M.C.A.; Xing, Y.; Maciel, G.S.; Gomes, A.S.L.; de Oliveira, H.P. Detection of traces of polymyxin B by “turn-on” type fluorescent reporters: The influence of the relative concentration of gold nanoparticles in a complex with rhodamine B. Results Chem. 2022, 4, 100634. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Gupta, M.K.; Pandey, G.; Narayan, R.J.; Pandey, P.C. Molecular weight of polyethylenimine-dependent transfusion and selective antimicrobial activity of functional silver nanoparticles. J. Mater. Res. 2020, 35, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Pandey, G.; Narayan, R.J. Controlled synthesis of polyethyleneimine coated gold nanoparticles: Application in glutathione sensing and nucleotide delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1191–1199. [Google Scholar] [CrossRef]
- Amin, Z.R.; Rahimizadeh, M.; Eshghi, H.; Dehshahri, A.; Ramezani, M. The effect of cationic charge density change on transfection efficiency of polyethylenimine. Iran. J. Basic Med. Sci. 2013, 16, 150–156. [Google Scholar]
- Mulens-Arias, V.; Nicolás-Boluda, A.; Gehanno, A.; Balfourier, A.; Carn, F.; Gazeau, F. Polyethyleneimine-assisted one-pot synthesis of quasi-fractal plasmonic gold nanocomposites as a photothermal theranostic agent. Nanoscale 2019, 11, 3344–3359. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999, 45, 268–275. [Google Scholar] [CrossRef]
- Kretschmer, F.; Mansfeld, U.; Hoeppener, S.; Hager, M.D.; Schubert, U.S. Tunable synthesis of poly (ethylene imine)–gold nanoparticle clusters. Chem. Commun. 2014, 50, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Nanometals: Formation and Color. In Colloidal Synthesis of Plasmonic Nanometals, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2020; pp. 1–13. [Google Scholar]
- Charlé, K.-P.; Schulze, W.; Winter, B. The size-dependent shift of the surface plasmon absorption band of small spherical metal particles. Z. Phys. D At. Mol. Clust. 1989, 12, 471–475. [Google Scholar] [CrossRef]
- Raza, S.; Stenger, N.; Kadkhodazadeh, S.; Fischer, S.V.; Kostesha, N.; Jauho, A.P.; Burrows, A.; Wubs, M.; Mortensen, N.A. Blueshift of the surface Plasmon resonance in silver nanoparticles studied with EELS. Nanophotonics 2013, 2, 131–138. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L. Old polymyxins are back: Is resistance close? Clin. Infect. Dis. 2006, 43, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Yin, J.; Wang, G.; Cheng, D.; Fu, J.; Qiu, J.; Yu, Z. Inactivation of polymyxin by hydrolytic mechanism. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Li, G.; Kong, W.; Zhao, M.; Lu, S.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. A fluorescence resonance energy transfer (FRET) based “Turn-On” nanofluorescence sensor using a nitrogen-doped carbon dot-hexagonal cobalt oxyhydroxide nanosheet architecture and application to α-glucosidase inhibitor screening. Biosens. Bioelectron. 2016, 79, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, F.; Xu, L.; Liu, X.; Ma, P.; Sun, Y.; Wang, X.; Song, D. A fluorescence resonance energy transfer biosensor based on carbon dots and gold nanoparticles for the detection of trypsin. Sens. Actuators B Chem. 2018, 273, 1015–1021. [Google Scholar] [CrossRef]
- Zhang, E.; Ju, P.; Guo, P.; Hou, X.; Hou, X.; Lv, H.; Wang, J.J.; Zhang, Y. A FRET-based fluorescent and colorimetric probe for the specific detection of picric acid. RSC Adv. 2018, 8, 31658–31665. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, S.; Kong, L.; Li, D.; He, Y. Sensitive detection of polymyxin B sulfate at the nanogram level using enhanced resonance scattering signals and decreased fluorescence signals with thioglycolic acid capped CdTe/CdS quantum dots as probe. Sens. Actuators B Chem. 2013, 188, 555–563. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Wu, Y.; Jiang, Y.; Wang, Q.; Lin, X.; Yao, Z. An efficient approach for rapid detection of polymyxin B based on the optically active supramolecular aggregates of water-soluble perylene diimide. Sens. Actuators B Chem. 2020, 321, 128594. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiao, D.; Yan, X.; Zhang, J.; Cheng, Y. Light-assisted noncompetitive fluorescence immunoassay for photosensitive antibiotic detection. Chem. Select. 2024, 9, e202303987. [Google Scholar] [CrossRef]
- Vaishakh, M.; Nampoori VP, N. Thermooptic techniques: A tool for interdisciplinary studies. In Photoacoustic and Photothermal Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 185–216. [Google Scholar]
- Raut, S.; Rich, R.; Fudala, R.; Butler, S.; Kokate, R.; Gryczynski, Z.; Luchowski, R.; Gryczynski, I. Resonance energy transfer between fluorescent BSA-protected Au nanoclusters and organic fluorophores. Nanoscale 2014, 6, 385–391. [Google Scholar] [CrossRef]
- Kim, K.M.; Nam, Y.S.; Lee, Y.; Lee, K.B. A highly sensitive and selective colorimetric Hg2+ ion probe using gold nanoparticles functionalized with polyethyleneimine. J. Anal. Methods Chem. 2018, 2018, 1206913. [Google Scholar] [CrossRef]
- Tao, F.F. Design of an in-house ambient pressure AP-XPS using a bench-top X-ray source and the surface chemistry of ceria under reaction conditions. Chem. Commun. 2012, 48, 3812–3814. [Google Scholar] [CrossRef]
- Gao, D.; Tian, Y.; Bi, S.; Chen, Y.; Yu, A.; Zhang, H. Studies on the interaction of colloidal gold and serum albumins by spectral methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 1203–1208. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Ficarra, S.; Tellone, E.; Leuzzi, U.; Magazù, S.; Galtieri, A.; Bellocco, E. Anti-aggregation properties of trehalose on heat-induced secondary structure and conformation changes of bovine serum albumin. Biophys. Chem. 2010, 147, 146–152. [Google Scholar] [CrossRef] [PubMed]
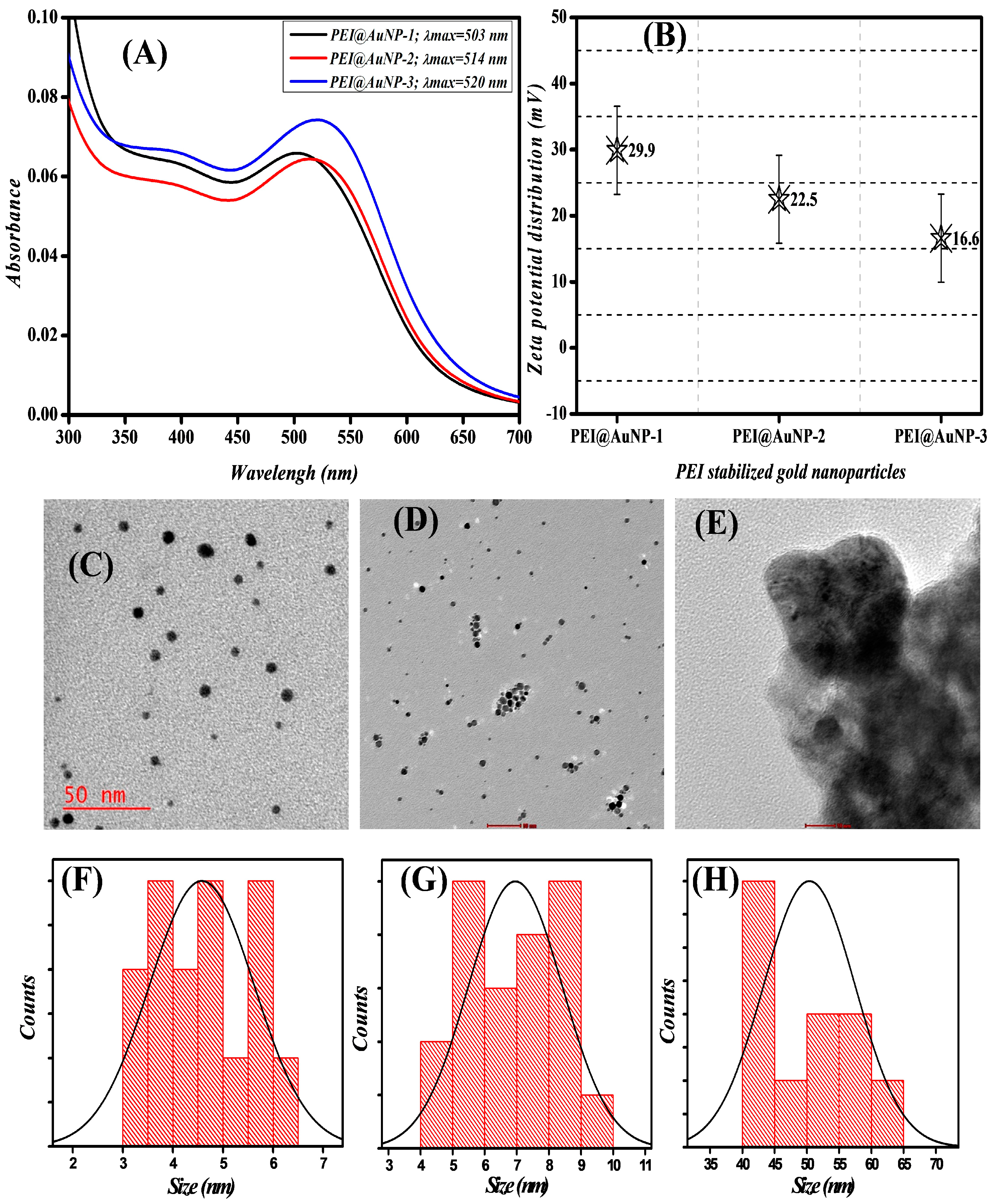
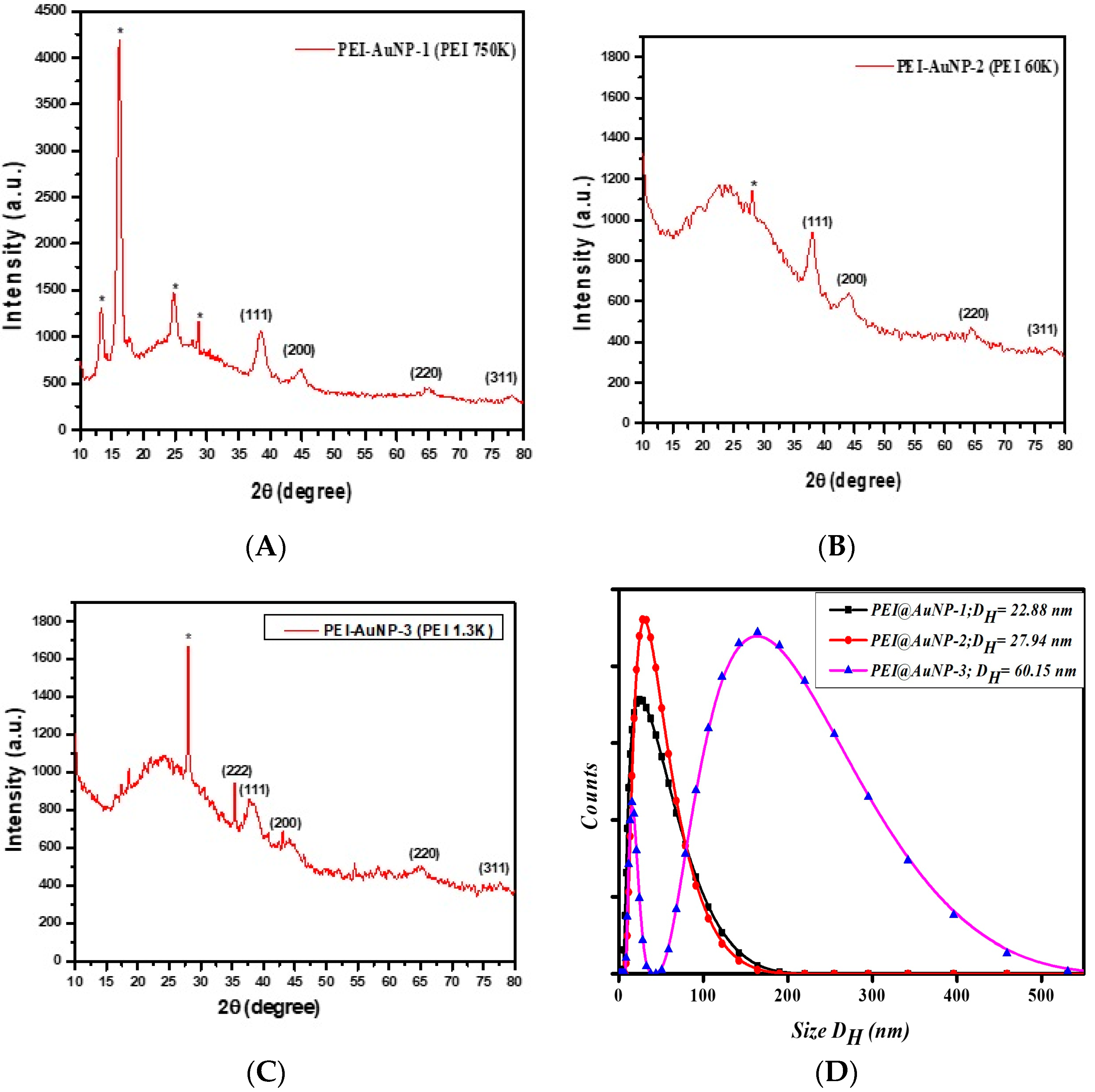

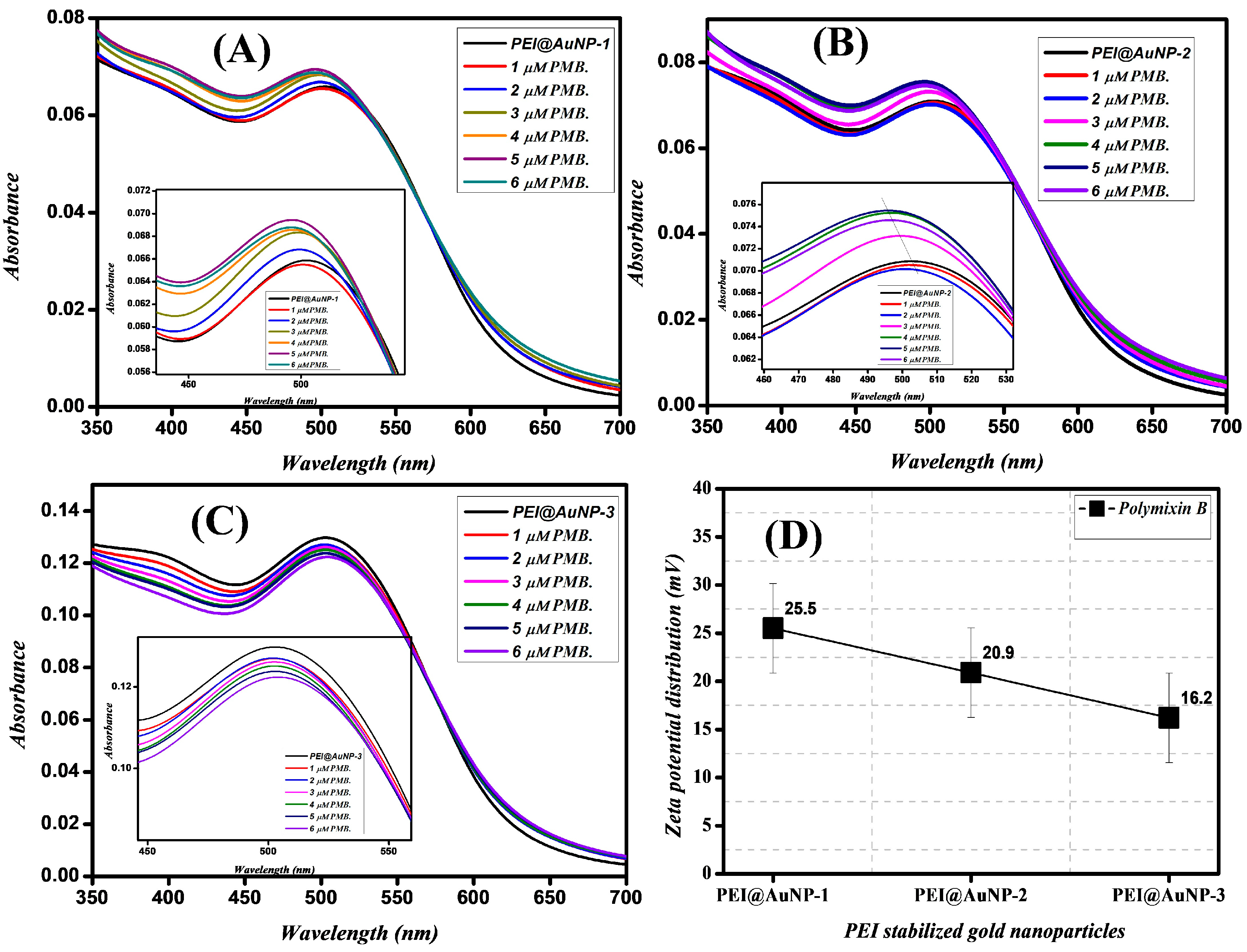


| Gold Nanoparticles | LSPR Band (nm) | Shape/Size (nm) | Zeta Potential (mV) | Hydrodynamic Radii (DLS) (d = nm) | hkl Planes |
|---|---|---|---|---|---|
| PEI@AuNP-1 (PEI; 750 k) | 503 | Spherical/4.5 | 29.9 | 22.55 | (111), (200), (220), and (311) |
| PEI@AuNP-2 (PEI; 60 k) | 514 | Spherical/7.0 | 22.5 | 27.4 | (111), (200), (220), and (311) |
| PEI@AuNP-3 (PEI; 1.3 k) | 520 | Spherical/52.5 | 16.6 | 60.15 | (111), (200), (220), and (311) |
| Probes | Method | Linear Range | Limit of Detection (LOD) | References |
|---|---|---|---|---|
| Thioglycolic acid-capped CdTe/CdS QDs | Fluorescence and RRS spectroscopy | 0.09~5.25 µg·mL−1 and 0.02~6.0 µg·mL−1 | 27.5 ng mL−1 and 6.36 ng mL−1 | [36] |
| Water-soluble perylene diimide (PDI) derivative | Fluorometric | 1 to 2000 nmol/L | 18.5 nM | [37] |
| Antibodies | ELISA-based method | 5.0–192 ng/mL | 30.6 and 1.8 ng/mL | [10] |
| Gold nanoparticle-based strip sensor | Indirect competitive enzyme-linked immunosorbent assay | 0–100 ng/mL | 13.13 ng/mL | [17] |
| Screen-printed gold electrodes (Au-SPEs) | Cyclic voltammetry (CV) | NA | 1.539 µg/kg | [18] |
| Citrate-capped gold nanoparticle acceptors (Au NPs) | FRET | NA | 2.82 µM | [19] |
| 2, 7-Dichlorodihydrofluorescein diacetate (DCFH-DA) | Light-assisted noncompetitive immunoassay | 8–20 μg/mL | 70 ng/mL | [38] |
| Polyethyleneimine-stabilized gold nanoparticles (PEI@AuNPs) | Fluorescence (Turn-on) | 1–6 μM | 8.5 nM | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.K.; Gupta, M.K.; Meena, R.; Pandey, P.C.; Narayan, R.J. Molecular Weights of Polyethyleneimine-Dependent Physicochemical Tuning of Gold Nanoparticles and FRET-Based Turn-On Sensing of Polymyxin B. Sensors 2024, 24, 2169. https://doi.org/10.3390/s24072169
Tiwari AK, Gupta MK, Meena R, Pandey PC, Narayan RJ. Molecular Weights of Polyethyleneimine-Dependent Physicochemical Tuning of Gold Nanoparticles and FRET-Based Turn-On Sensing of Polymyxin B. Sensors. 2024; 24(7):2169. https://doi.org/10.3390/s24072169
Chicago/Turabian StyleTiwari, Atul Kumar, Munesh Kumar Gupta, Ramovatar Meena, Prem C. Pandey, and Roger J. Narayan. 2024. "Molecular Weights of Polyethyleneimine-Dependent Physicochemical Tuning of Gold Nanoparticles and FRET-Based Turn-On Sensing of Polymyxin B" Sensors 24, no. 7: 2169. https://doi.org/10.3390/s24072169
APA StyleTiwari, A. K., Gupta, M. K., Meena, R., Pandey, P. C., & Narayan, R. J. (2024). Molecular Weights of Polyethyleneimine-Dependent Physicochemical Tuning of Gold Nanoparticles and FRET-Based Turn-On Sensing of Polymyxin B. Sensors, 24(7), 2169. https://doi.org/10.3390/s24072169








