Efficient Sequential Detection of Two Antibiotics Using a Fiber-Optic Surface Plasmon Resonance Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dopamine Functionalization Modification of Fiber-Optic SPR Sensors
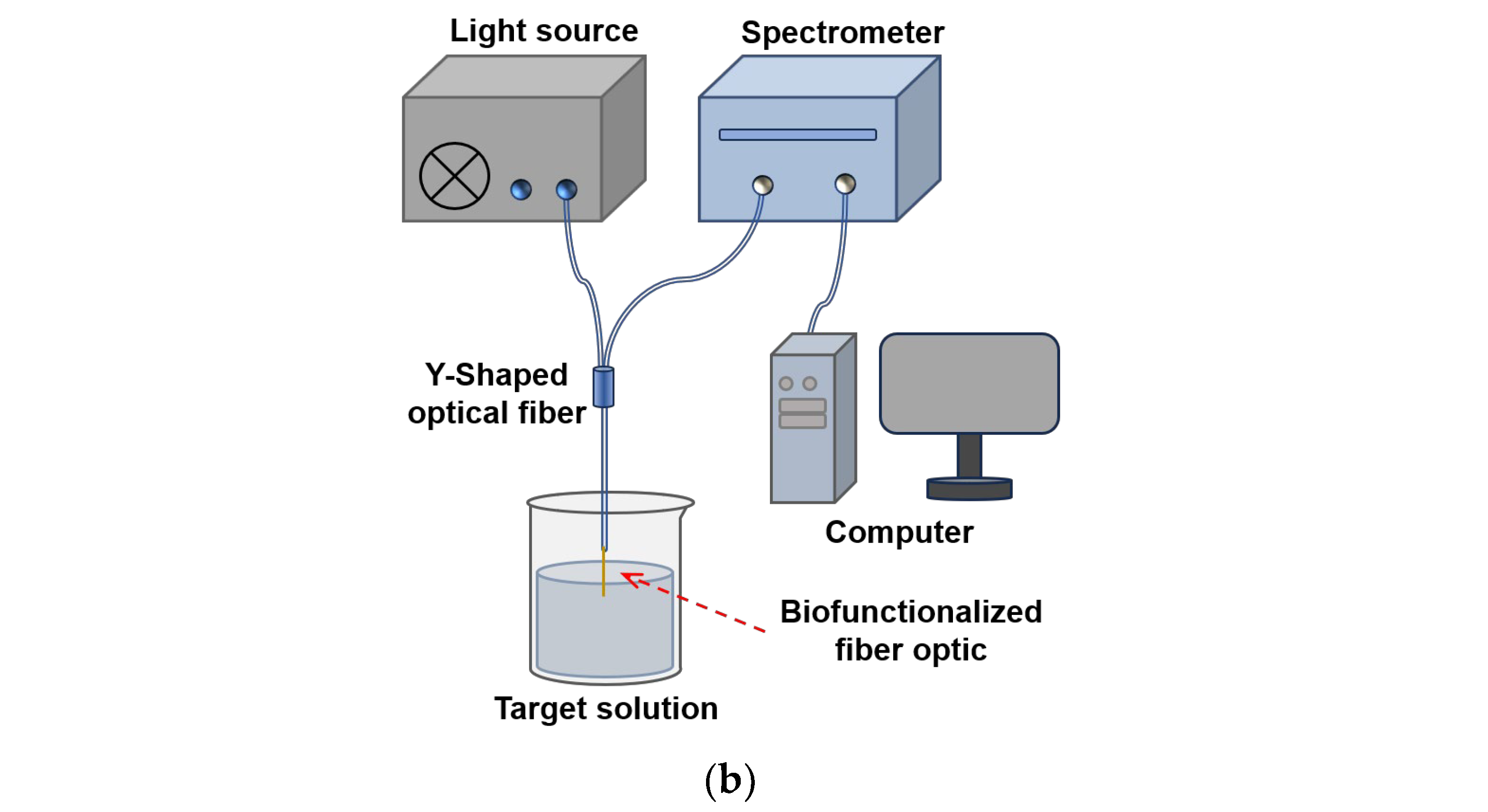
2.3. Biofunctionalization Modification and Detection of Fiber-Optic SPR Sensor
2.4. Milk Sample Additive Recovery Test
- (1)
- Enrofloxacin was added to separate milk samples to reach concentrations of 10, 20, 30, 40, and 50 ng/mL; ciprofloxacin was added to separate milk samples to reach concentrations of 10, 20, 30, 40, and 50 ng/mL;
- (2)
- Each milk samples’ pH was adjusted to 4.6, followed by centrifugation. The supernatant was collected, its pH was adjusted to 7.0, and it was subjected to a second round of centrifugation;
- (3)
- Each supernatant’s upper layer was collected and centrifuged for 15 min using a 3000 molecular weight cutoff ultrafiltration tube. The resultant filtrate was stored at 4 °C for future use.
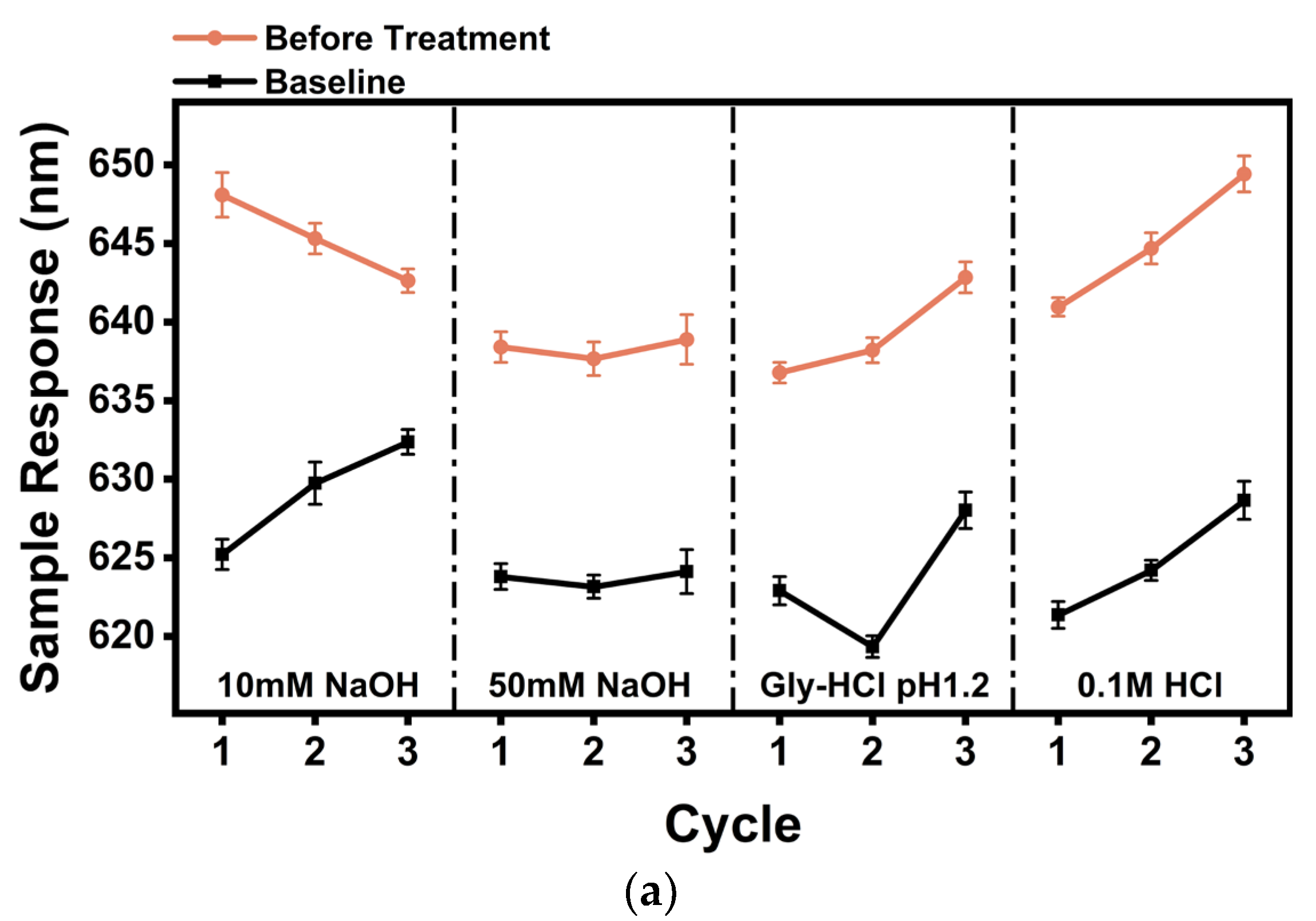
2.5. Screening of Regeneration Fluids
3. Results and Discussion
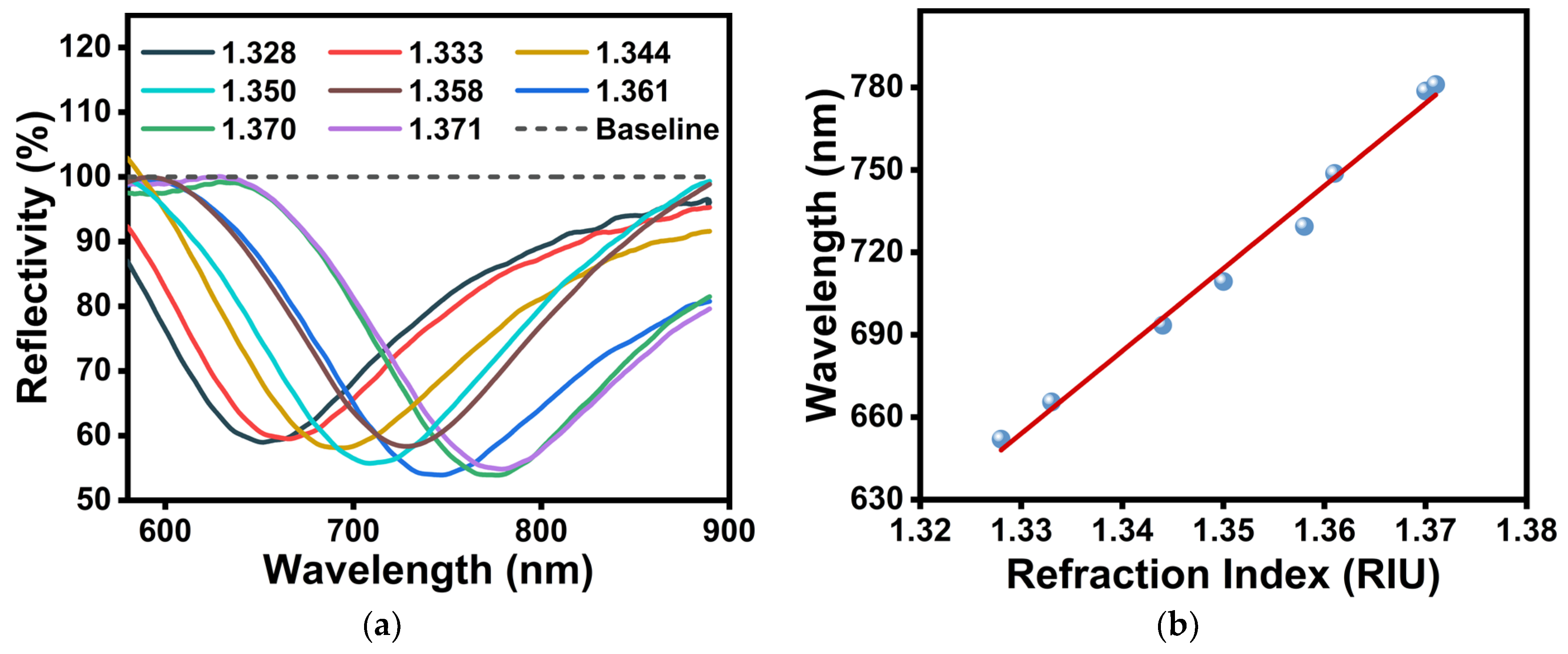
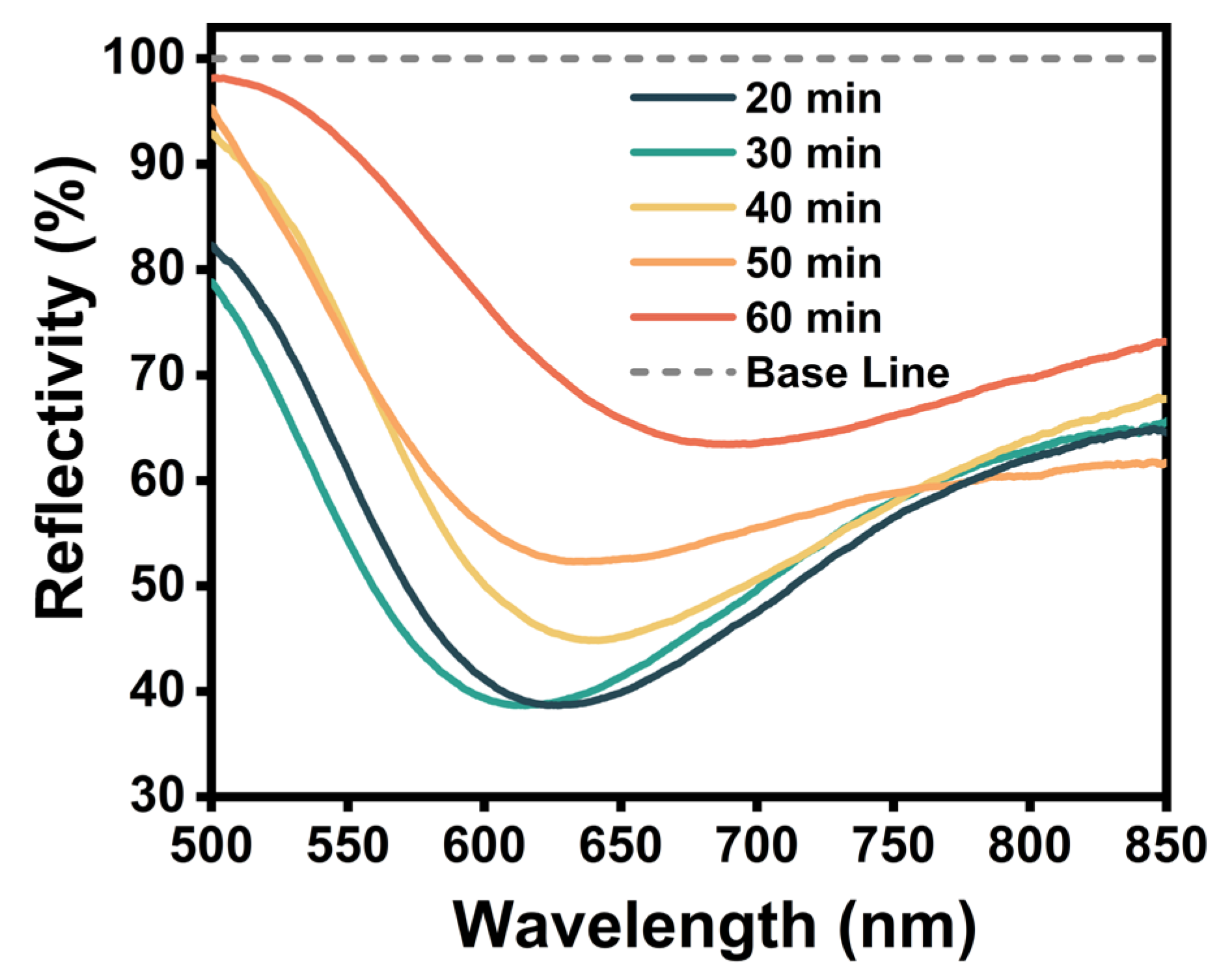
3.1. Characterization of Fiber-Optic SPR Sensors for PDA Functionalization
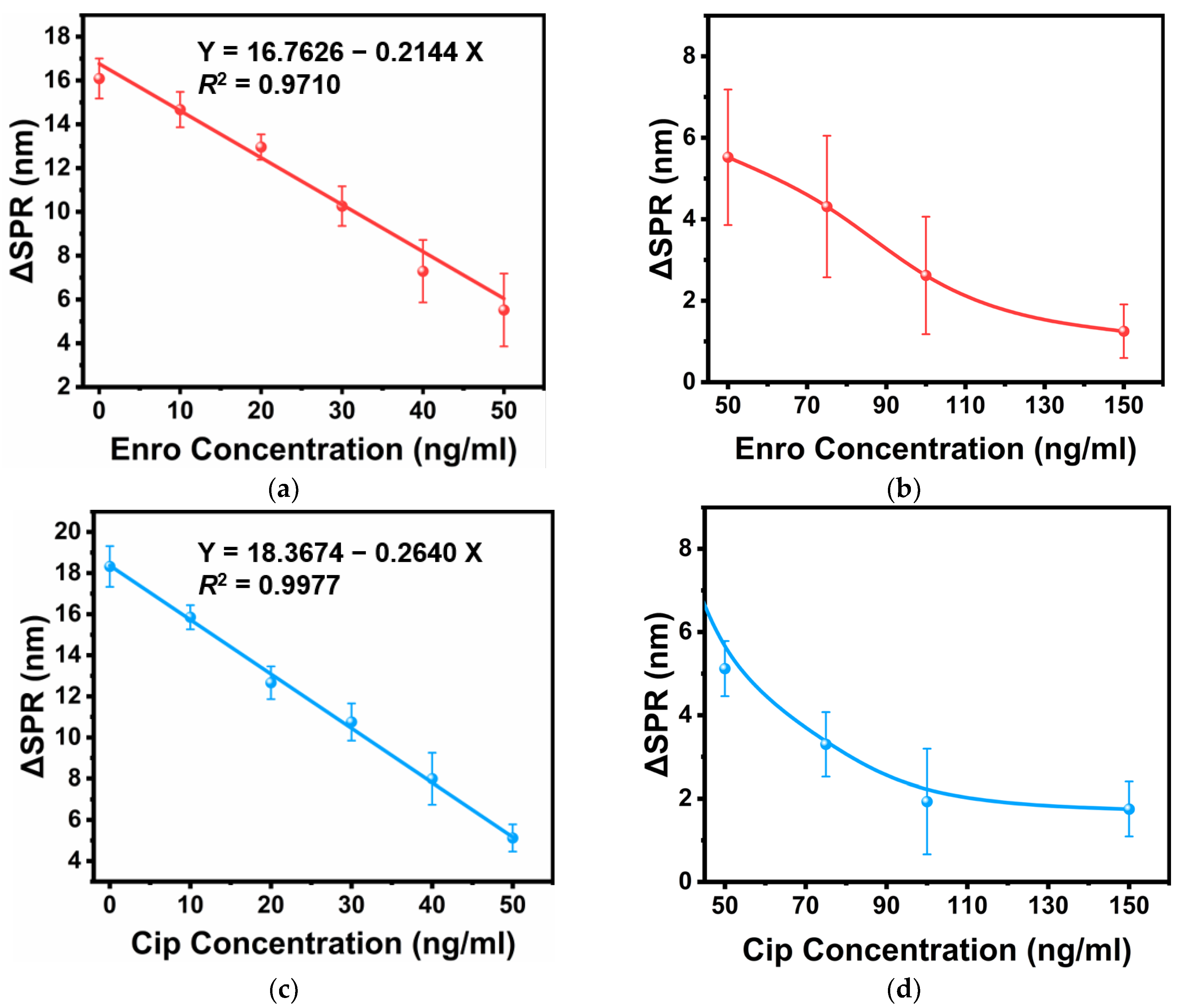
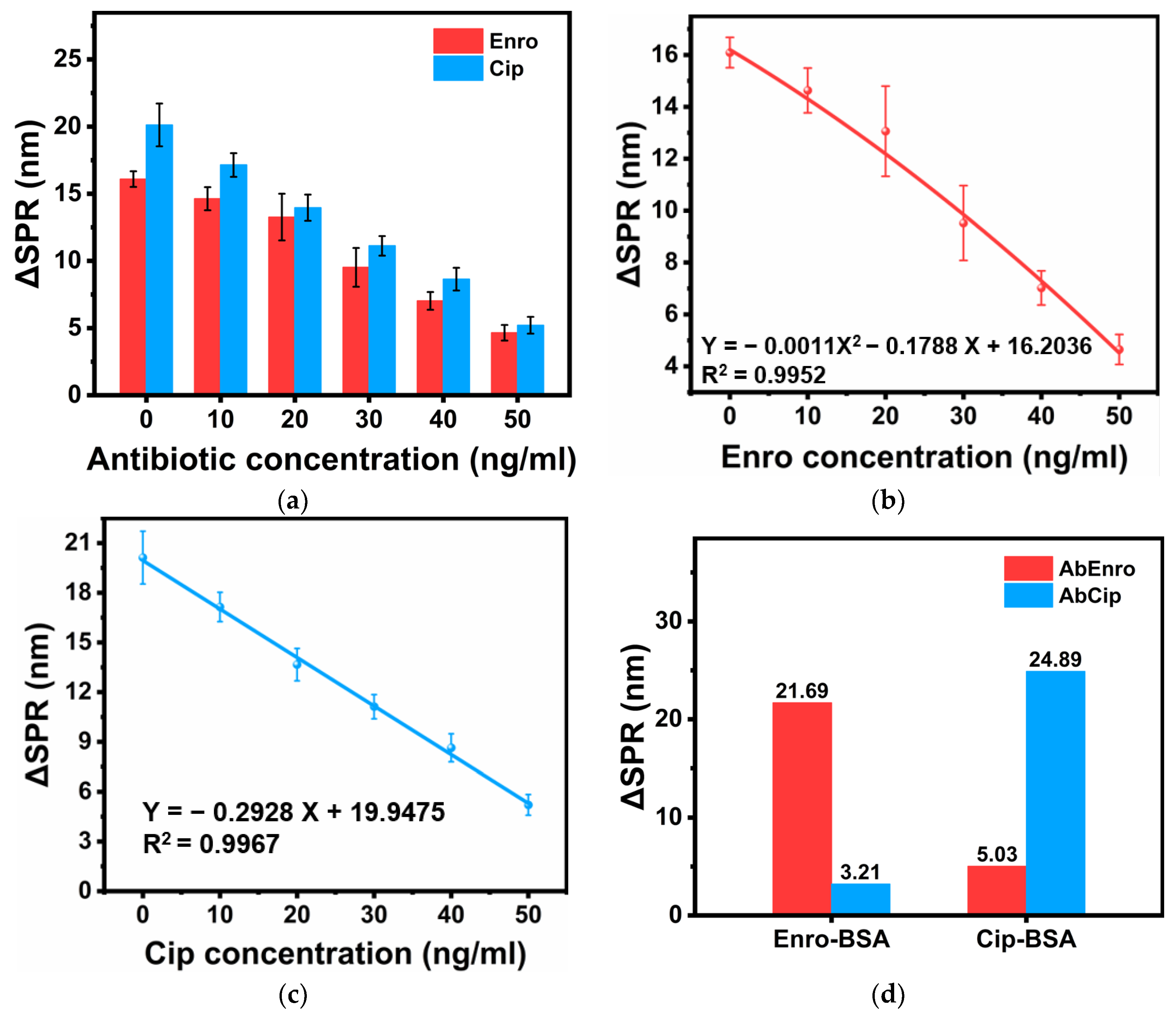
3.2. Individual Detection
3.3. Joint Detection
3.4. Adding Recycling Tests to Practical Systems
3.5. Recycled Liquid Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado, N.; Orozco, J.; Zambrano, S.; Casas-Zapata, J.C.; Marino, D. Veterinary Pharmaceutical as Emerging Contaminants in Wastewater and Surface Water: An Overview. J. Hazard. Mater. 2023, 460, 132431. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zheng, L.; Hao, J.; Huang, L.; Wang, Q.; Yin, Z.; Qi, T.; Jia, L.; Liu, K. Eco-friendly Fabrication of Multifunctional Magnetic Plasmonic Photocatalyst for Adsorption, SERS Monitoring and Photodegradation of Residual Fluoroquinolone Antibiotics in Water. Chemosphere 2023, 331, 138842. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Zong, B.; Fang, X.; Liu, M.; Li, Z.; Mao, S.; Ostrikov, K.K. Discriminative and Quantitative Color-coding Analysis of Fluoroquinolones with Dual-emitting Lanthanide Metal-organic Frameworks. Sens. Actuators B Chem. 2022, 373, 132701. [Google Scholar] [CrossRef]
- Zhou, Z.; Wen, X.; Shi, C.; Wu, L.; Long, Z.; He, J.; Hou, X. Multi-color Fluorescence Sensing Platform for Visual Determination of Norfloxacin Based on a Terbium (III) Functionalized Covalent Organic Framework. Food Chem. 2023, 417, 135883. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, J.; Hu, J.; Li, X.; Li, B. Fluoroquinolone Residues in the Environment Rapidly Induce Heritable Fluoroquinolone Resistance in Escherichia coli. Environ. Sci. Technol. 2023, 57, 4784–4795. [Google Scholar] [CrossRef] [PubMed]
- Cela-Dablanca, R.; Miguez-Gonzalez, A.; Barreiro, A.; Rodriguez-Lopez, L.; Arias-Estevez, M.; Nunez-Delgado, A.; Fernandez-Sanjurjo, M.J.; Castillo-Ramos, V.; Alvarez-Rodriguez, E. Simultaneous Adsorption of Amoxicillin and Ciprofloxacin on Agricultural Soils and By-products Used as Bio-adsorbents: Unraveling the Interactions in Complex Systems. Environ. Res. 2024, 240, 117535. [Google Scholar] [CrossRef]
- Shi, F.; Yao, M.; Huang, Y.; Chen, Z.; Xiao, J.; Zhan, F.; Li, Y.; Lin, L.; Qin, Z. Effects of Antibiotics on Immunity and Apoptosis on Grass Carp Liver and Hepatocytes. J. Environ. Chem. Eng. 2023, 11, 110168. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Chang, J.; Fu, B.; Wang, H. Insight into Advanced Oxidation Processes for the Degradation of Fluoroquinolone Antibiotics: Removal, Mechanism, and Influencing Factors. Sci. Total Environ. 2023, 857, 159172. [Google Scholar] [CrossRef]
- Chen, J.A.; Jin, Y.; Ren, T.; Wang, S.; Wang, X.; Zhang, F.; Tang, Y. A Novel Terbium (III) and Aptamer-based Probe for Label-free Detection of Three Fluoroquinolones in Honey and Water Samples. Food Chem. 2022, 386, 132751. [Google Scholar] [CrossRef]
- Gallegos-Tabanico, A.; Jimenez-Canale, J.; Hernandez-Leon, S.G.; Burgara-Estrella, A.J.; Carmelo Encinas-Encinas, J.; Sarabia-Sainz, J.A. Development of an Electrochemical Sensor Conjugated with Molecularly Imprinted Polymers for the Detection of Enrofloxacine. Chemosensors 2022, 10, 448. [Google Scholar] [CrossRef]
- Asal, M.; Ozen, O.; Sahinler, M.; Polatoglu, I. Recent Developments in Enzyme, DNA and Immuno-Based Biosensors. Sensors 2018, 18, 1924. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, J.-H.; Son, J.; Choi, J.-W. Noble Metal-Assisted Surface Plasmon Resonance Immunosensors. Sensors 2020, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Toan, P.V.; Tin, P.N.; Truong, L.T.N. A Highly Sensitive Impedimetric Sensor Based on a MIP Biomimetic for the Detection of Enrofloxacin. Anal. Methods 2022, 14, 2195–2203. [Google Scholar]
- Wang, P.; Wang, L.; Li, C.; Li, X.; Li, G. Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor. Molecules 2022, 27, 8764. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, X.; Kong, D.; Su, D.; Liu, F.; Sun, P.; Liu, X.; Wang, C.; Jia, X.; Lu, G. Self-assembled Multiprotein Nanostructures with Enhanced Stability and Signal Amplification Capability for Sensitive Fluorogenic Immunoassays. Biosens. Bioelectron. 2022, 206, 114132. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Mao, J.; Zhang, Y.; Wang, S.; Huo, M.; Guo, H. Sensitive Electrochemical Detection of Enrofloxacin in Eggs Based on Carboxylated Multi-walled Carbon Nanotubes-reduced Graphene Oxide Nanocomposites: Molecularly Imprinted Recognition Versus Direct Electrocatalytic Oxidation. Food Chem. 2023, 413, 135579. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo; Darmayanti, R.; Puspitasari, B.; Cheng, T.-M.; Kuo, T.-R. Gold-Based Nanostructures for Antibacterial Application. Int. J. Mol. Sci. 2023, 24, 10006. [Google Scholar] [CrossRef]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbaré, S.; Hsiao, Y.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Photosynthetic Biohybrid Systems. ACS Mater. Lett. 2023, 5, 95–115. [Google Scholar] [CrossRef]
- Sheen, H.-J.; Panigrahi, B.; Kuo, T.-R.; Hsu, W.-C.; Chung, P.-S.; Xie, Q.-Z.; Lin, C.-Y.; Chang, Y.-S.; Lin, C.-T.; Fan, Y.-J. Electrochemical biosensor with electrokinetics-assisted molecular trapping for enhancing C-reactive protein detection. Biosens. Bioelectron. 2022, 210, 114338. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Chung, P.-F.; Krisnawati, D.I.; Rinawati, F.; Irawan, H.; Kristanto, H.; Kuo, T.-R. Gold Nanorod-Decorated Metallic MoS2 Nanosheets for Synergistic Photothermal and Photodynamic Antibacterial Therapy. Nanomaterials 2021, 11, 3064. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, Y.; He, J.; Occhipinti, L.G.; Wang, Z.; Zhou, X. Two-dimensional Material-enhanced Surface Plasmon Resonance for Antibiotic Sensing. J. Hazard. Mater. 2023, 455, 131644. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Tran, K.T.; Pallesen, J.S.; Solbak, S.M.O.; Qin, Y.; Mukminova, E.; Luchini, M.; Vasilyeva, K.O.; Chichon, D.G.; Goutsiou, G.; et al. Development of Noncovalent Small-Molecule Keap1-Nrf2 Inhibitors by Fragment-Based Drug Discovery. J. Med. Chem. 2022, 65, 14481–14526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Mu, T.; Ma, M.; Sun, H.; Zhao, G. Nutritional Composition, Antioxidant Activity, Volatile Compounds, and Stability Properties of Sweet Potato Residues Fermented with Selected Lactic Acid Bacteria and Bifidobacteria. Food Chem. 2022, 374, 131500. [Google Scholar] [CrossRef] [PubMed]
- Forest-Nault, C.; Gaudreault, J.; Henry, O.; Durocher, Y.; De Crescenzo, G. On the Use of Surface Plasmon Resonance Biosensing to Understand IgG-FcγR Interactions. Int. J. Mol. Sci. 2021, 22, 6616. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, P.; Yuan, H.; Su, R.; Huang, R.; Qi, W.; He, Z. Sequential Sandwich Immunoassay for Simultaneous Detection in Trace Samples Using Single-channel Surface Plasmon Resonance. Analyst 2019, 144, 5700–5705. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Present and Future of Surface Plasmon Resonance Biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Karachaliou, C.-E.; Koukouvinos, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.; Livaniou, E.; Petrou, P. Recent Developments in the Field of Optical Immunosensors Focusing on a Label-Free, White Light Reflectance Spectroscopy-Based Immunosensing Platform. Sensors 2022, 22, 5114. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.C.; Kooh, M.R.R.; Chau, Y.-F.C.; Thotagamuge, R. Susceptible Plasmonic Photonic Crystal Fiber Sensor with Elliptical Air Holes and External-Flat Gold-Coated Surface. Photonics 2022, 9, 916. [Google Scholar] [CrossRef]
- Chao, C.-T.C.; Chen, S.-H.; Huang, H.J.; Kooh, M.R.R.; Lim, C.M.; Thotagamuge, R.; Mahadi, A.H.; Chau, Y.-F.C. Improving Temperature-Sensing Performance of Photonic Crystal Fiber via External Metal-Coated Trapezoidal-Shaped Surface. Crystals 2023, 13, 813. [Google Scholar] [CrossRef]
- Lee, S.; Song, H.; Ahn, H.; Kim, S.; Choi, J.-R.; Kim, K. Fiber-Optic Localized Surface Plasmon Resonance Sensors Based on Nanomaterials. Sensors 2021, 21, 819. [Google Scholar] [CrossRef] [PubMed]
- Merlo, F.; Centenaro, D.; Maraschi, F.; Profumo, A.; Speltini, A. Green and Efficient Determination of Fluoroquinolone Residues in Edible Green Fruits and Leafy Vegetables by Ultrasound-Assisted Extraction Followed by HPLC-MS/MS. Molecules 2022, 27, 6595. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, A.; Rui, G.; Liu, Y.; Ran, D.; Liu, C.; Jing, D.; Anis, M.; Liu, M.; Wang, S.; Jiang, X.; et al. A Mega Study of Antibiotics Contamination in Eastern Aquatic Ecosystems of China: Occurrence, Interphase Transfer Processes, Ecotoxicological Risks, and Source Modeling. J. Hazard. Mater. 2023, 458, 131980. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Y.N.; Pamuk, S.; Gurler, Z. Chloramphenicol and Sulfonamide Residues in Sea Bream (Sparus Aurata) and Sea Bass (Dicentrarchus labrax) Fish from Aquaculture Farm. Environ. Sci. Pollut. Res. 2020, 27, 41248–41252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Wei, H.; Santiago, P.J.; Thrift, W.J.; Ragan, R.; Jiang, S. Sensing Antibiotics in Wastewater Using Surface-Enhanced Raman Scattering. Environ. Sci. Technol. 2023, 57, 4880–4891. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.; Leung, A.H.H.; Lee, H.W.; Wong, W.-t. Rapid Testing Methods for Food Contaminants and Toxicants. J. Integr. Agric. 2015, 14, 2243–2264. [Google Scholar] [CrossRef]
- Pan, M.; Li, S.; Wang, J.; Sheng, W.; Wang, S. Development and Validation of a Reproducible and Label-Free Surface Plasmon Resonance Immunosensor for Enrofloxacin Detection in Animal-Derived Foods. Sensors 2017, 17, 1984. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Xia, Y.; Su, R.; Huang, R.; Ding, L.; Wang, L.; Qi, W.; He, Z. Design of Elution Strategy for Simultaneous Detection of Chloramphenicol and Gentamicin in Complex Samples Using Surface Plasmon Resonance. Biosens. Bioelectron. 2017, 92, 266–272. [Google Scholar] [CrossRef]
- Drake, A.W.; Klakamp, S.L. A Strategic and Systematic Approach for the Determination of Biosensor Regeneration Conditions. J. Immunol. Methods 2011, 371, 165–169. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Su, R.; Liu, B.; Huang, R.; Qi, W.; He, Z. A Polydopamine-modified Optical Fiber SPR Biosensor Using Electroless-plated Gold Films for Immunoassays. Biosens. Bioelectron. 2015, 74, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, L.; Wang, A.; Huang, R.; Ding, L.; Su, R.; Qi, W.; He, Z. Bioinspired Fabrication of Optical Fiber SPR Sensors for Immunoassays Using Polydopamine-accelerated Electroless Plating. J. Mater. Chem. C 2016, 4, 7554–7562. [Google Scholar] [CrossRef]
- Liu, L.; Deng, S.; Zheng, J.; Yuan, L.; Deng, H.; Teng, C. An Enhanced Plastic Optical Fiber-Based Surface Plasmon Resonance Sensor with a Double-Sided Polished Structure. Sensors 2021, 21, 1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Yin, Z.; Ullah, S.; Cui, X.; Li, G.; Li, K.; Wang, C.; Liu, Y. Dual-Core Photonic Crystal Fiber Surface Plasmon Resonance Sensor with High Sensitivity and Narrow FWHM. Plasmonics 2023, 19, 495–504. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Gao, X.; Liu, X.; Gao, J.; Ma, T.; Li, J. A NIR-responsive Up-conversion Nanoparticle Probe of the NaYF4: Er,Yb type and Coated with a Molecularly Imprinted Polymer for Fluorometric Determination of Enrofloxacin. Microchim. Acta 2017, 184, 3469–3475. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; Zambonin, C. Determination of Ciprofloxacin, Enrofloxacin, and Marbofloxacin in Bovine Urine, Serum, and Milk by Microextraction by a Packed Sorbent Coupled to Ultra-High Performance Liquid Chromatography. Anal. Lett. 2019, 52, 790–802. [Google Scholar] [CrossRef]
- Yan, H.; Tian, M.; Row, K.H. Determination of Enrofloxacin and Ciprofloxacin in Milk Using Molecularly Imprinted Solid-phase Extraction. J. Sep. Sci. 2008, 31, 3015–3020. [Google Scholar] [CrossRef]
- Lei, X.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Xu, C. Immunochromatographic Assays for Ultrasensitive and High Specific Determination of Enrofloxacin in Milk, Eggs, Honey, and Chicken Meat. J. Dairy Sci. 2022, 105, 1999–2010. [Google Scholar] [CrossRef]
- Kim, N.-G.; Kim, M.-A.; Park, Y.-I.; Jung, T.-S.; Son, S.-W.; So, B.; Kang, H.-G. Magnetic Nanoparticle Based Purification and Enzyme-linked Immunosorbent Assay Using Monoclonal Antibody Against Enrofloxacin. J. Vet. Sci. 2015, 16, 431–437. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Qu, K.; Cui, Z.; Wang, J. Novel Electrochemical Sensor Modified with Molecularly Imprinted Polymers for Determination of Enrofloxacin in Marine Environment. Microchim. Acta 2022, 189, 95. [Google Scholar] [CrossRef]
- Moniri, E.; Panahi, H.A.; Alimadadi, A. Polymer-Grafted Nanographite Support Obtained Using Iminodiacetic Acid/Allyl Glycidyl Ether: Characterization and Application in the Extraction and Determination of Enrofloxacin in Biological and Pharmaceutical Samples. Chromatographia 2016, 79, 293–301. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, X. D-Shaped Fiber Surface Plasmon Resonance Refractive Index Sensor Enhanced By MXene (Ti3C2Tx). IEEE Photonics J. 2022, 14, 7151607. [Google Scholar] [CrossRef]
- Jing, J.-Y.; Liu, K.; Wu, Z.; Liu, Y.-M.; Jiang, J.-F.; Xu, T.-H.; Yan, W.-C.; Xiong, Y.-Y.; Zhan, X.-H.; Xiao, L.; et al. Violet phosphorus-enhanced plug-and-play double-lane fiber optic surface plasmon resonance refractometer. Acta Phys. Sin. 2023, 72, 214206. [Google Scholar] [CrossRef]
- Teng, C.; Ying, S.; Min, R.; Deng, S.; Deng, H.; Chen, M.; Chu, X.; Yuan, L.; Cheng, Y.; Xue, M. Side-Polish Plastic Optical Fiber Based SPR Sensor for Refractive Index and Liquid-Level Sensing. Sensors 2022, 22, 6241. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, Y.; Tan, W.; Zhang, Y.; Liu, C.; Ran, Z.; Tang, Y.; Su, Y.; Liu, Z.; Zhang, Y. Fiber cladding SPR sensor based on V-groove structure. Opt. Commun. 2023, 526, 128944. [Google Scholar] [CrossRef]
- Suzuki, H.; Sugimoto, M.; Matsui, Y.; Kondoh, J. Effects of gold film thickness on spectrum profile and sensitivity of a multimode-optical-fiber SPR sensor. Sens. Actuators B-Chem. 2008, 132, 26–33. [Google Scholar] [CrossRef]


| Target Sample | Spiked Concentration (ng/mL) | Wavelength Shift (nm) | Average ± SD (ng/mL) | Recovery (%) |
|---|---|---|---|---|
| Enro | 0 | 16.01 ± 0.17 | 1.076 ± 0.93 | / |
| 10 | 13.89 ± 0.05 | 12.04 ± 0.24 | 120.46 | |
| 20 | 11.57 ± 0.09 | 22.73 ± 0.39 | 113.66 | |
| 30 | 9.29 ± 0.08 | 32.26 ± 0.32 | 107.53 | |
| 40 | 7.62 ± 0.11 | 38.76 ± 0.42 | 96.90 | |
| 50 | 5.02 ± 0.06 | 48.23 ± 0.51 | 96.46 | |
| Cip | 0 | 19.01 ± 0.11 | 3.21 ± 0.37 | / |
| 10 | 16.23 ± 0.17 | 12.69 ± 0.59 | 126.90 | |
| 20 | 12.96 ± 0.13 | 23.87 ± 0.44 | 119.35 | |
| 30 | 10.25 ± 0.16 | 33.13 ± 0.50 | 110.43 | |
| 40 | 7.56 ± 0.18 | 42.30 ± 0.62 | 105.75 | |
| 50 | 5.78 ± 0.14 | 48.37 ± 0.50 | 96.74 |
| Target Analytes | Detection Methods | Detection Range | LOD | Recovery (%) | Refs. |
|---|---|---|---|---|---|
| Enro | Fluorescent probe | 63–60,000 ng/mL | 8 ng/mL | 83.7–87.7 | [45] |
| Enro/Cip | Fluorescent probe | 1–100 ng/mL | 0.061 ng/mL (Enro) 0.053 ng/mL (Cip) | 80.1–102.48 | [9] |
| Enro/Cip | LC | 48 ng/mL (Enro) 2 ng/mL (Cip) | 79 (Enro) 88 (Cip) | [46] | |
| Enro/Cip | Molecularly imprinted polymers | 0.2–5 ng/mL | 16 ng/mL (Enro) 19 ng/mL (Cip) | 82.6–93.5 (Enro) 81.2–94.8 (Cip) | [47] |
| Enro | Colloidal gold-immunochromatographic sensor | 0.25–1 ng/mL | 0.13 ng/mL | 88.9–108.5 | [48] |
| Enro | Eu-fluorescence-immunochromatographic sensor | 0.25–1 ng/mL | 2.5 ng/mL | 88.6–113.6 | [48] |
| Enro | Elisa | 10–30 ng/mL | 0.7 ng/mL | 72.9–113.36 | [49] |
| Enro | Molecularly imprinted polymers | 2.8–28,000 pM | 0.9 pM | 96.4–102 | [50] |
| Enro | Polymer-grafted nanographite | 0.1–60 μg/mL | 8.5 ng/mL | 68.5–81.2 | [51] |
| Enro/Cip | Fiber-optic SPR sensor | 0–50 ng/mL | 0.81 ng/mL (Enro) 1.20 ng/mL (Cip) | 96.46–120.46 (Enro) 96.74–126.9 (Cip) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Yin, H.; Xiao, J.; Cui, M.; Huang, R.; Su, R. Efficient Sequential Detection of Two Antibiotics Using a Fiber-Optic Surface Plasmon Resonance Sensor. Sensors 2024, 24, 2126. https://doi.org/10.3390/s24072126
Zhao Z, Yin H, Xiao J, Cui M, Huang R, Su R. Efficient Sequential Detection of Two Antibiotics Using a Fiber-Optic Surface Plasmon Resonance Sensor. Sensors. 2024; 24(7):2126. https://doi.org/10.3390/s24072126
Chicago/Turabian StyleZhao, Ze, Huiting Yin, Jingzhe Xiao, Mei Cui, Renliang Huang, and Rongxin Su. 2024. "Efficient Sequential Detection of Two Antibiotics Using a Fiber-Optic Surface Plasmon Resonance Sensor" Sensors 24, no. 7: 2126. https://doi.org/10.3390/s24072126
APA StyleZhao, Z., Yin, H., Xiao, J., Cui, M., Huang, R., & Su, R. (2024). Efficient Sequential Detection of Two Antibiotics Using a Fiber-Optic Surface Plasmon Resonance Sensor. Sensors, 24(7), 2126. https://doi.org/10.3390/s24072126







