Mid-Infrared Photothermal Spectroscopy for the Detection of Caffeine in Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Calibration Standards
2.2. Solid Phase Extraction
2.3. Gas Chromatography
2.4. FTIR Spectroscopy
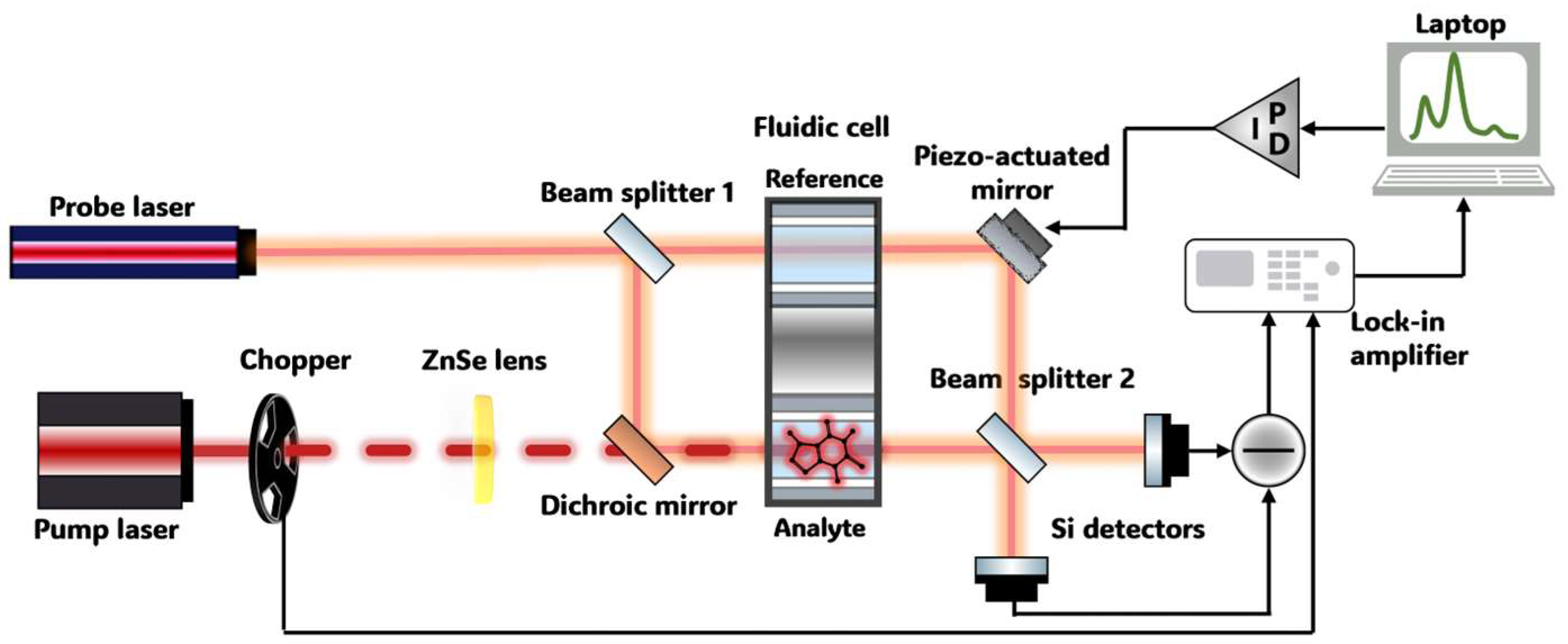
2.5. Photothermal Spectrometer Based on a Mach–Zehnder Interferometer
2.6. PTS Spectrometer Performance Assessment
3. Results and Discussion
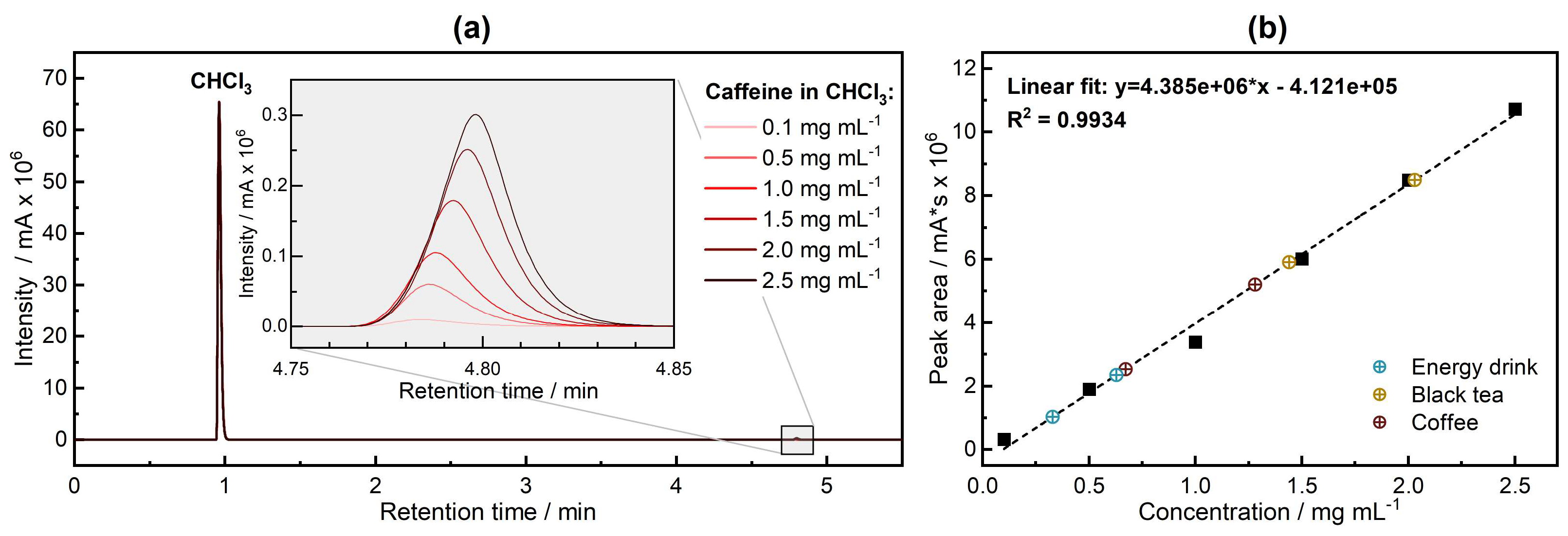
3.1. Quantitative Analysis—Gas Chromatography
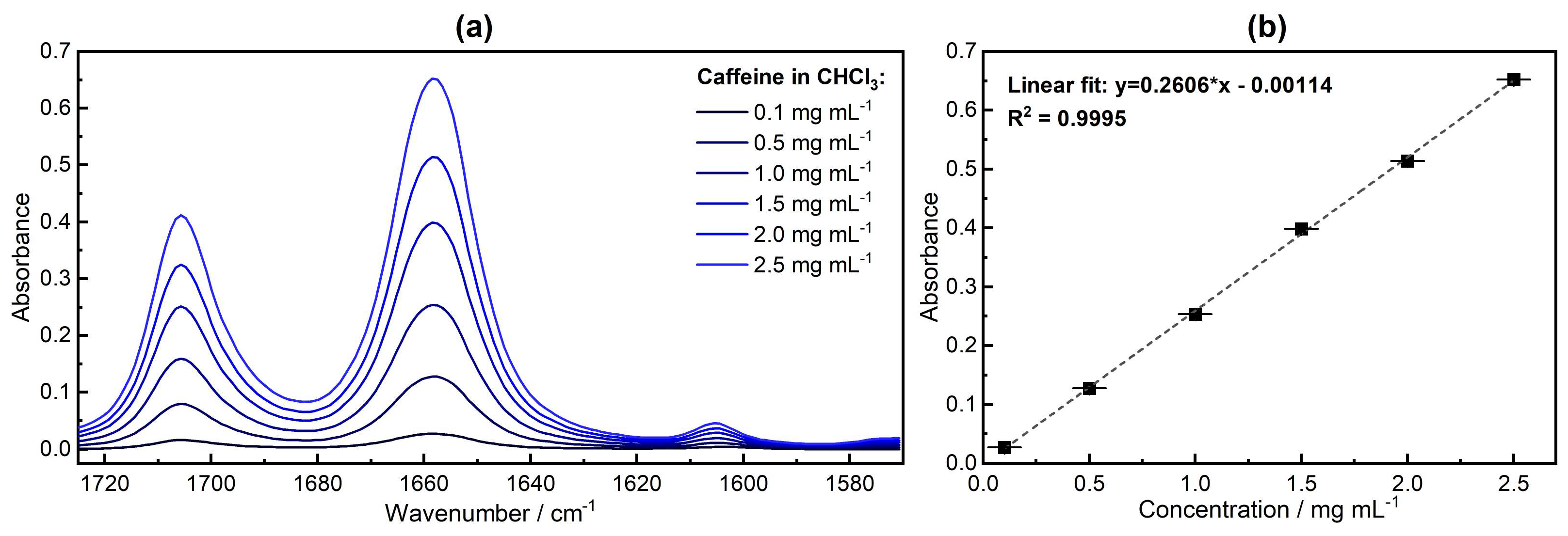
3.2. Qualitative Analysis—FTIR Spectroscopy
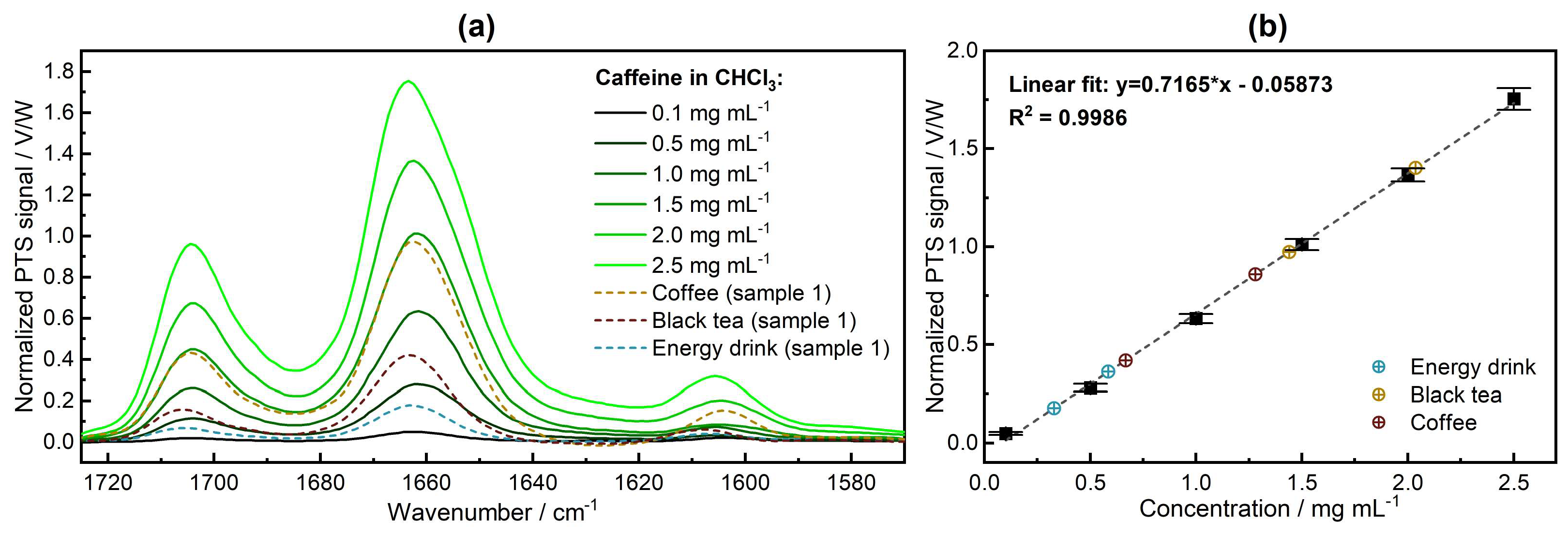
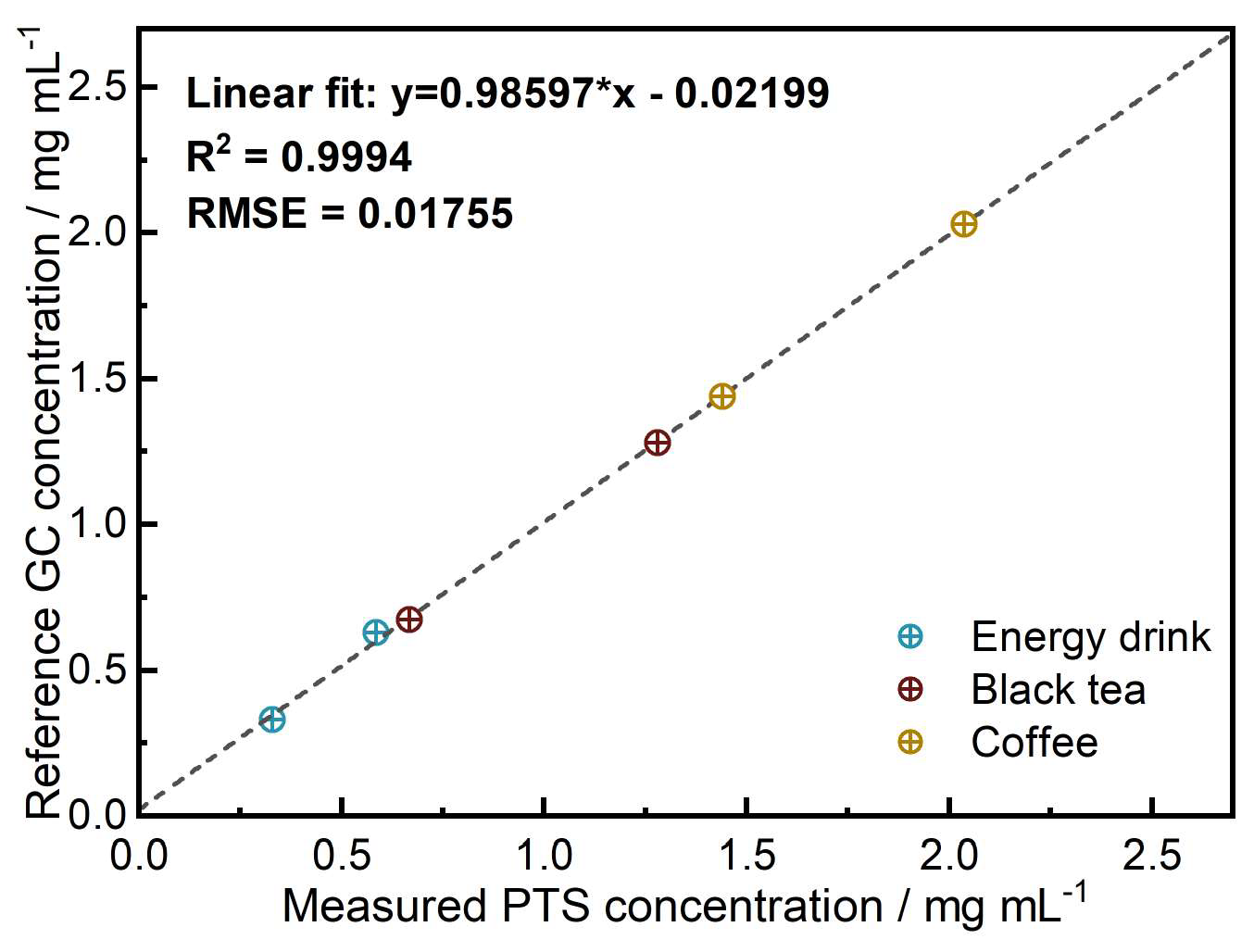
3.3. Qualitative and Quantitative Analysis—PTS Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic Acids, Caffeine Content and Antioxidant Properties of Green Coffee Extracts: Influence of Green Coffee Bean Preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a Factor Influencing the Functioning of the Human Body—Friend or Foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Al-Bratty, M.; Alhazmi, H.A.; Rehman, Z.U.; Javed, S.A.; Ahsan, W.; Najmi, A.; Khuwaja, G.; Makeen, H.A.; Khalid, A. Determination of Caffeine Content in Commercial Energy Beverages Available in Saudi Arabian Market by Gas Chromatography-Mass Spectrometric Analysis. J. Spectrosc. 2020, 2020, 3716343. [Google Scholar] [CrossRef]
- Alsunni, A.A. Energy Drink Consumption: Beneficial and Adverse Health Effects. Int. J. Health Sci. 2015, 9, 459–465. [Google Scholar] [CrossRef]
- Cornelis, M. The Impact of Caffeine and Coffee on Human Health. Nutrients 2019, 11, 416. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Spilling the Beans: How Much Caffeine Is Too Much? 2018. Available online: https://www.fda.gov/consumers/consumer-updates/spilling-beans-how-much-caffeine-too-much (accessed on 9 December 2023).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Ahmad Bhawani, S.; Fong, S.S.; Mohamad Ibrahim, M.N. Spectrophotometric Analysis of Caffeine. Int. J. Anal. Chem. 2015, 2015, 170239. [Google Scholar] [CrossRef]
- Li, H.-B.; Xu, X.-R.; Chen, F. Chapter 1—Determination of Iodine in Seawater: Methods and Applications. In Comprehensive Handbook of Iodine; Analytical Techniques; Academic Press: Cambridge, MA, USA, 2009; pp. 2–13. [Google Scholar] [CrossRef]
- Forgács, E.; Cserháti, T. Gas Chromatography. In Food Authenticity and Traceability; Lees, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 197–217. ISBN 978-1-85573-526-2. [Google Scholar]
- Pasias, I.; Kiriakou, I.; Proestos, C. Development of a Rapid Method for the Determination of Caffeine in Coffee Grains by GC-FID—A Fully Validated Approach. Antioxidants 2017, 6, 67. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Analytical Techniques in the Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2004; ISBN 978-0-470-01114-0. [Google Scholar]
- Chalmers, J.M.; Griffiths, P.R. Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 5. [Google Scholar]
- Dabrowska, A.; Schwaighofer, A.; Lindner, S.; Lendl, B. Mid-IR Refractive Index Sensor for Detecting Proteins Employing an External Cavity Quantum Cascade Laser-Based Mach-Zehnder Interferometer. Opt. Express 2020, 28, 36632. [Google Scholar] [CrossRef]
- Akhgar, C.K.; Ebner, J.; Alcaraz, M.R.; Kopp, J.; Goicoechea, H.; Spadiut, O.; Schwaighofer, A.; Lendl, B. Application of Quantum Cascade Laser-Infrared Spectroscopy and Chemometrics for In-Line Discrimination of Coeluting Proteins from Preparative Size Exclusion Chromatography. Anal. Chem. 2022, 94, 11192–11200. [Google Scholar] [CrossRef]
- Lindner, S.; Hayden, J.; Schwaighofer, A.; Wolflehner, T.; Kristament, C.; González-Cabrera, M.; Zlabinger, S.; Lendl, B. External Cavity Quantum Cascade Laser-Based Mid-Infrared Dispersion Spectroscopy for Qualitative and Quantitative Analysis of Liquid-Phase Samples. Appl. Spectrosc. 2020, 74, 452–459. [Google Scholar] [CrossRef]
- Patimisco, P.; Sampaolo, A.; Dong, L.; Tittel, F.K.; Spagnolo, V. Recent Advances in Quartz Enhanced Photoacoustic Sensing. Appl. Phys. Rev. 2018, 5, 011106. [Google Scholar] [CrossRef]
- Krzempek, K. A Review of Photothermal Detection Techniques for Gas Sensing Applications. Appl. Sci. 2019, 9, 2826. [Google Scholar] [CrossRef]
- Bialkowski, S.E. Photothermal Spectroscopy Methods for Chemical Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 978-0-471-57467-5. [Google Scholar]
- Pinto, D.; Waclawek, J.P.; Lindner, S.; Moser, H.; Ricchiuti, G.; Lendl, B. Wavelength Modulated Diode Probe Laser for an Interferometric Cavity-Assisted Photothermal Spectroscopy Gas Sensor. Sens. Actuators B Chem. 2023, 377, 133061. [Google Scholar] [CrossRef]
- Vitiello, M.S.; Scalari, G.; Williams, B.; De Natale, P. Quantum Cascade Lasers: 20 Years of Challenges. Opt. Express 2015, 23, 5167. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, D.L.; Davis, C.C. Trace Detection of Hydrazines by Optical Homodyne Interferometry. Appl. Opt. 1991, 30, 756. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, O.; Bauer, A.; Küderle, A.; Pleitez, M.A.; Mäntele, W. Depth-Selective Photothermal IR Spectroscopy of Skin: Potential Application for Non-Invasive Glucose Measurement. Analyst 2017, 142, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, A.J. Real-Time Detection of Ambient Aerosols Using Photothermal Interferometry: Folded Jamin Interferometer. Rev. Sci. Instrum. 2006, 77, 064903. [Google Scholar] [CrossRef]
- Capeloto, O.A.; Lukasievicz, G.V.B.; Zanuto, V.S.; Herculano, L.S.; Souza Filho, N.E.; Novatski, A.; Malacarne, L.C.; Bialkowski, S.E.; Baesso, M.L.; Astrath, N.G.C. Pulsed Photothermal Mirror Technique: Characterization of Opaque Materials. Appl. Opt. 2014, 53, 7985. [Google Scholar] [CrossRef]
- Bauer, A.; Hertzberg, O.; Küderle, A.; Strobel, D.; Pleitez, M.A.; Mäntele, W. IR-Spectroscopy of Skin in Vivo: Optimal Skin Sites and Properties for Non-Invasive Glucose Measurement by Photoacoustic and Photothermal Spectroscopy. J. Biophotonics 2018, 11, e201600261. [Google Scholar] [CrossRef] [PubMed]
- Farahi, R.H.; Passian, A.; Tetard, L.; Thundat, T. Pump–Probe Photothermal Spectroscopy Using Quantum Cascade Lasers. J. Phys. D Appl. Phys. 2012, 45, 125101. [Google Scholar] [CrossRef]
- Ricchiuti, G.; Dabrowska, A.; Pinto, D.; Ramer, G.; Lendl, B. Dual-Beam Photothermal Spectroscopy Employing a Mach–Zehnder Interferometer and an External Cavity Quantum Cascade Laser for Detection of Water Traces in Organic Solvents. Anal. Chem. 2022, 94, 16353–16360. [Google Scholar] [CrossRef] [PubMed]
- Ricchiuti, G.; Dabrowska, A.; Pinto, D.; Ramer, G.; Lendl, B. Mid-IR Photothermal Sensing of Liquids for Trace Analysis. In Photonic Instrumentation Engineering X; Soskind, Y., Busse, L.E., Eds.; SPIE: San Francisco, CA, USA, 2023; Volume 12428, p. 32. [Google Scholar]
- Nyoro, F.D.; Sim, S.; Kimura, J.A.L. Estimation of Caffeine Content in Coffee of Malaysian Market Using Fourier Transform Infrared (FTIR) Spectroscopy. Trends Undergrad. Res. 2018, 1, a16–a22. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Singh, V.B. Ab Initio and DFT Studies of the Structure and Vibrational Spectra of Anhydrous Caffeine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 45–50. [Google Scholar] [CrossRef]
- ISO 20481:2008; Coffee and Coffee Products—Determination of the Caffeine Content Using High Performance Liquid Chromatography (HPLC)—Reference Method. ISO: Geneva, Switzerland, 2008.
- Iadanza, S.; Mendoza-Castro, J.H.; Oliveira, T.; Butler, S.M.; Tedesco, A.; Giannino, G.; Lendl, B.; Grande, M.; O’Faolain, L. High-Q Asymmetrically Cladded Silicon Nitride 1D Photonic Crystals Cavities and Hybrid External Cavity Lasers for Sensing in Air and Liquids. Nanophotonics 2022, 11, 4183–4196. [Google Scholar] [CrossRef]
- Bernini, R.; Testa, G.; Zeni, L.; Sarro, P.M. Integrated Optofluidic Mach–Zehnder Interferometer Based on Liquid Core Waveguides. Appl. Phys. Lett. 2008, 93, 011106. [Google Scholar] [CrossRef]
- Liu, Q.; Tu, X.; Kim, K.W.; Kee, J.S.; Shin, Y.; Han, K.; Yoon, Y.-J.; Lo, G.-Q.; Park, M.K. Highly Sensitive Mach–Zehnder Interferometer Biosensor Based on Silicon Nitride Slot Waveguide. Sens. Actuators B Chem. 2013, 188, 681–688. [Google Scholar] [CrossRef]
| Parameter | Description |
|---|---|
| Injection port | EPC (electronic pressure control)-controlled split/splitless inlet operating in split mode |
| Injection volume | 1 µL |
| Carrier gas | Helium |
| Inlet parameters | Heater temperature: 250 °C, gas pressure: 29.4 psi, total flow 129 mL min−1, split ratio: 1:50, split flow: 125 mL min−1 |
| Column flow | 2.5 mL min−1 (constant flow), 52 cm s−1 velocity |
| Detector temperature | 300 °C |
| GC oven program | 120 °C for 1 min hold time, then ramped at a rate of 20 °C min−1 to 240 °C, then ramped at a rate of 30 °C min−1 to 270 °C for 3 min hold time (total time of 11 min) |
| Coffee | Black Tea | Energy Drink | |||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 1 | Sample 2 | Sample 1 | Sample 2 |
| 1.44 | 2.03 | 0.67 | 1.28 | 0.33 | 0.63 |
| Coffee | Black Tea | Energy Drink | |||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 1 | Sample 2 | Sample 1 | Sample 2 |
| 1.44 | 2.04 | 0.67 | 1.28 | 0.33 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricchiuti, G.; Riedlsperger, L.; Dabrowska, A.; Rosenberg, E.; O’Faolain, L.; Lendl, B. Mid-Infrared Photothermal Spectroscopy for the Detection of Caffeine in Beverages. Sensors 2024, 24, 1974. https://doi.org/10.3390/s24061974
Ricchiuti G, Riedlsperger L, Dabrowska A, Rosenberg E, O’Faolain L, Lendl B. Mid-Infrared Photothermal Spectroscopy for the Detection of Caffeine in Beverages. Sensors. 2024; 24(6):1974. https://doi.org/10.3390/s24061974
Chicago/Turabian StyleRicchiuti, Giovanna, Lisa Riedlsperger, Alicja Dabrowska, Erwin Rosenberg, Liam O’Faolain, and Bernhard Lendl. 2024. "Mid-Infrared Photothermal Spectroscopy for the Detection of Caffeine in Beverages" Sensors 24, no. 6: 1974. https://doi.org/10.3390/s24061974
APA StyleRicchiuti, G., Riedlsperger, L., Dabrowska, A., Rosenberg, E., O’Faolain, L., & Lendl, B. (2024). Mid-Infrared Photothermal Spectroscopy for the Detection of Caffeine in Beverages. Sensors, 24(6), 1974. https://doi.org/10.3390/s24061974






