Optimization of 3D Passive Acoustic Mapping Image Metrics: Impact of Sensor Geometry and Beamforming Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Simulation Model
2.2. Reconstruction Algorithms
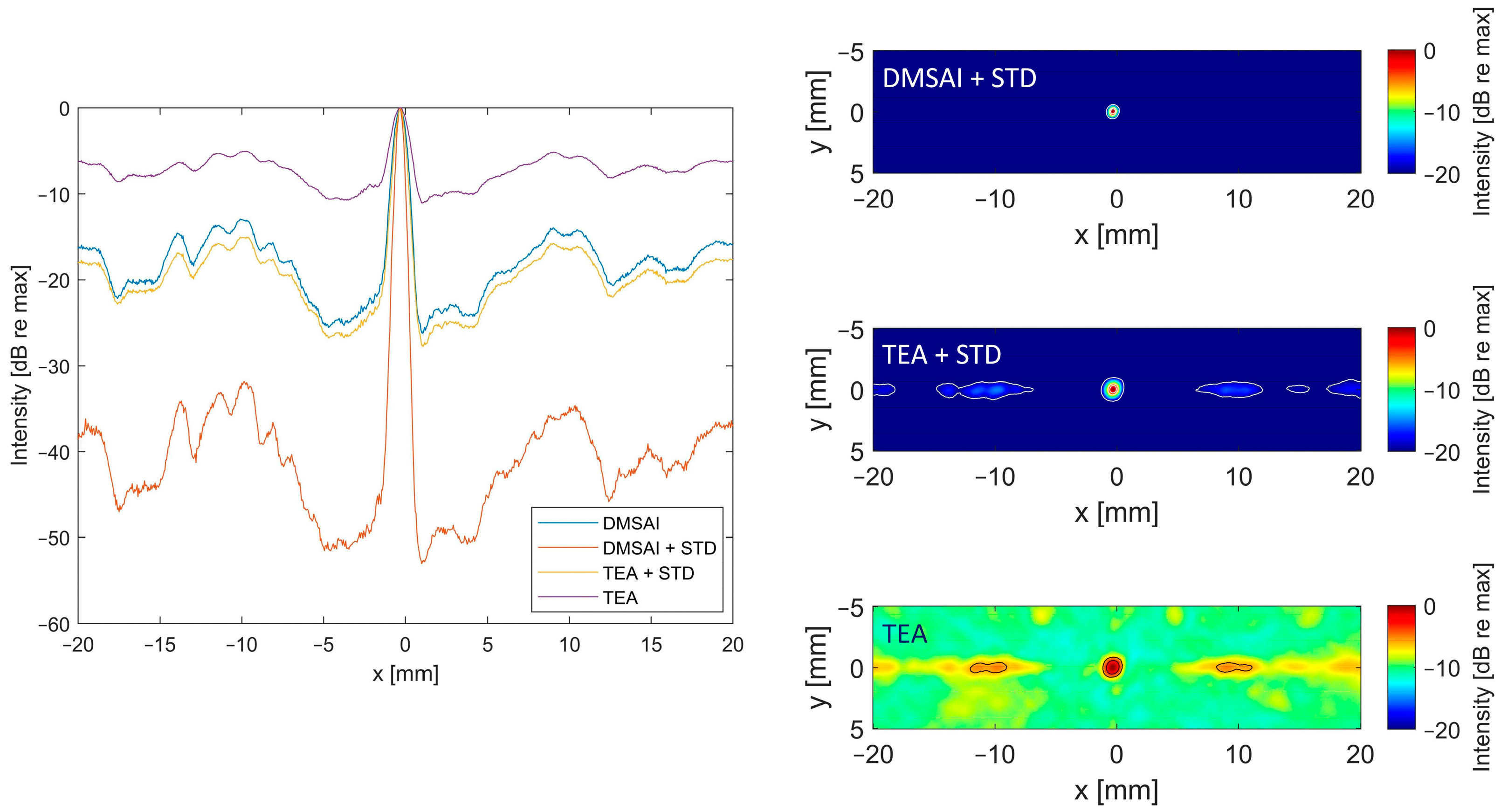
2.2.1. Delay, Multiply, Sum, and Integrate
2.2.2. Time Exposure Acoustics
2.2.3. Standard Deviation-Based Filter
2.3. Performance Metrics
2.4. Experimental Setup
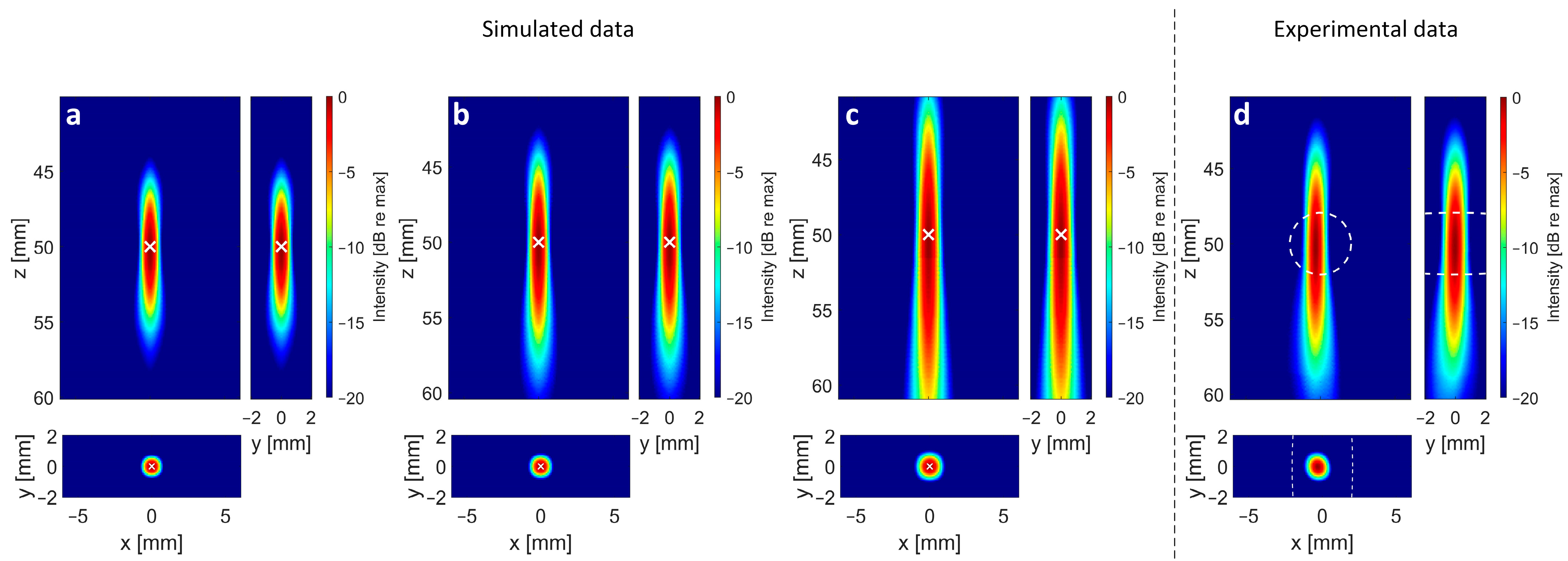
3. Results
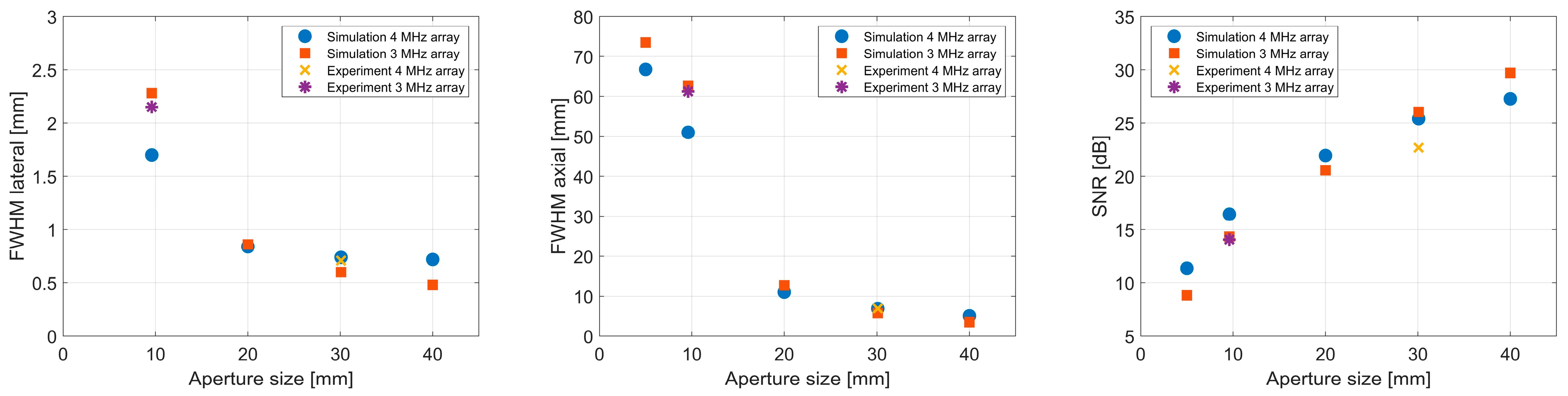
3.1. Impact of Aperture Size
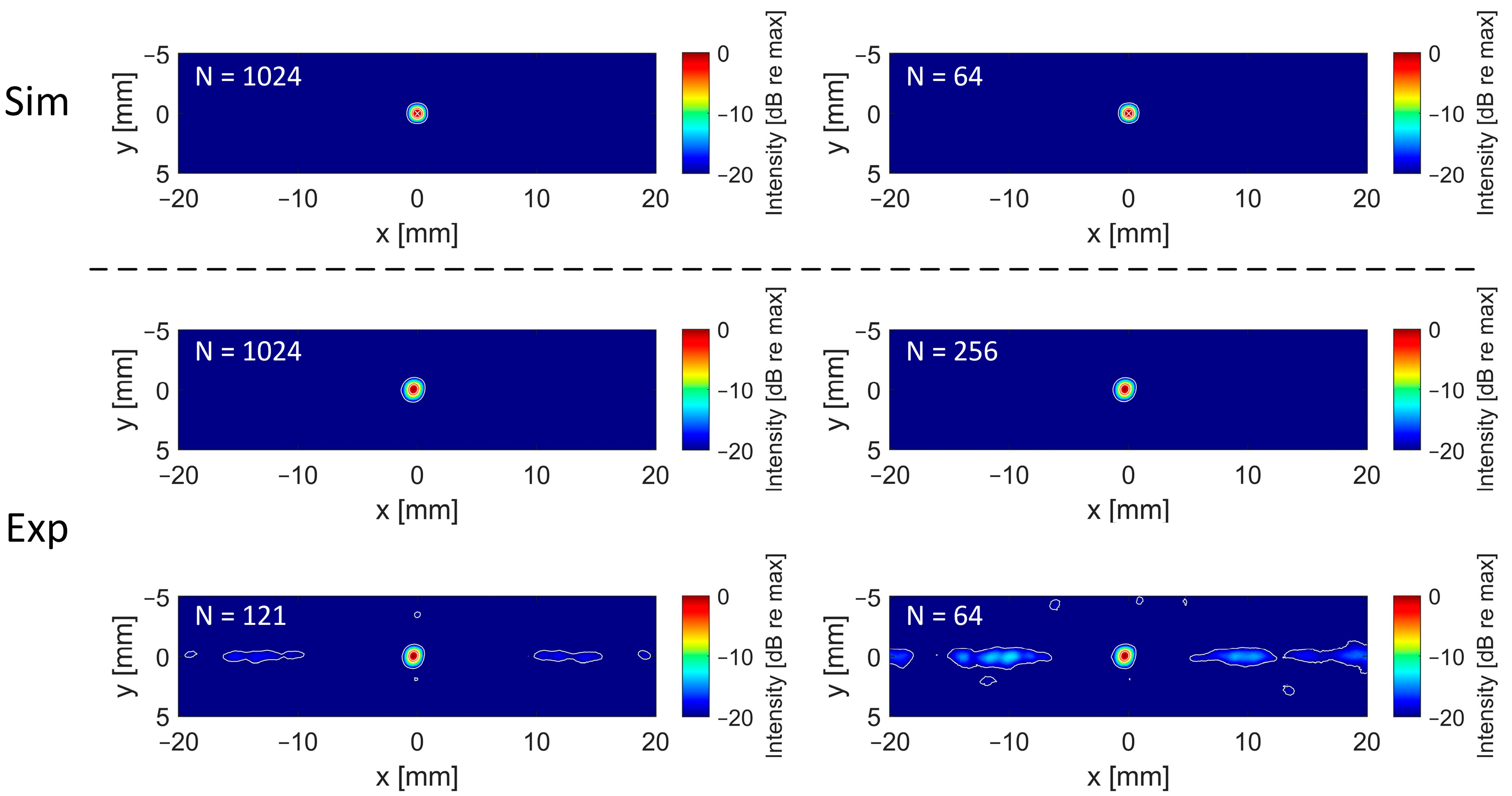
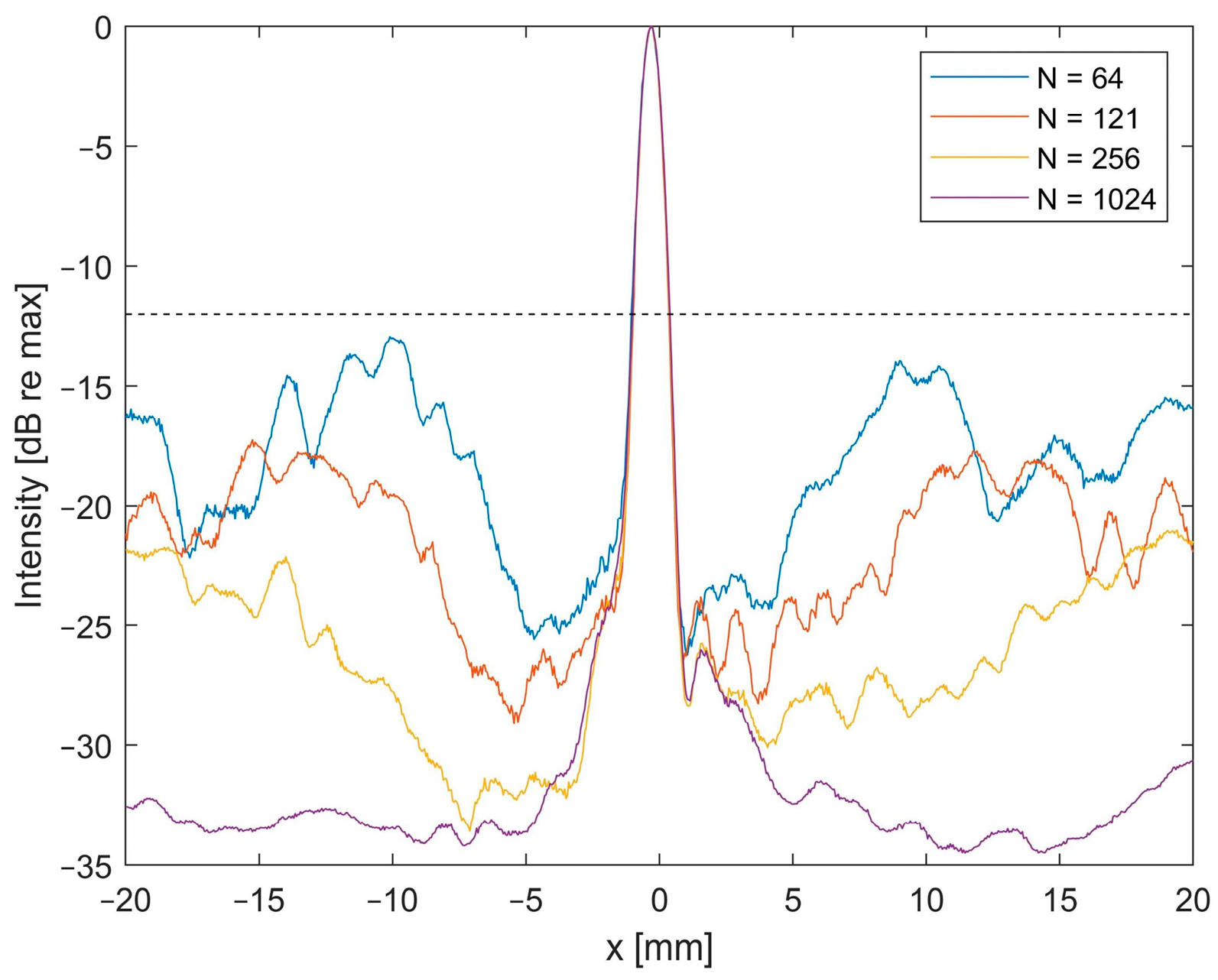
3.2. Impact of Element Count
3.3. Impact of Reconstruction Algorithm and Filter for High Sparsity Schemes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Number of Elements | FWHM Lateral [mm] | FWHM Axial [mm] | SNR [dB] | Localization Error [mm] | |||
|---|---|---|---|---|---|---|---|
| Sim | Exp | Sim | Exp | Sim | Exp | Sim | |
| 63 × 63 | 0.76 | - | 6.06 | - | 24.89 | - | 0.80 |
| 32 × 32 | 0.76 | 0.87 | 5.96 | 6.76 | 25.03 | 18.36 | 0.80 |
| 16 × 16 | 0.76 | 0.87 | 5.86 | 6.50 | 24.99 | 18.43 | 0.70 |
| 11 × 11 | 0.74 | 0.86 | 5.66 | 6.34 | 25.29 | 17.27 | 0.70 |
| 8 × 8 | 0.77 | 0.89 | 5.66 | 6.62 | 24.72 | 16.09 | 1.00 |
| Number of Elements | FWHM Lateral [mm] | FWHM Axial [mm] | SNR [dB] | Localization Error [mm] |
|---|---|---|---|---|
| Sim | Sim | Sim | Sim | |
| 63 × 63 | 0.83 | 5.34 | 24.38 | 0.70 |
| 32 × 32 | 0.83 | 5.40 | 24.45 | 0.70 |
| 16 × 16 | 0.83 | 5.30 | 24.35 | 0.80 |
| 11 × 11 | 0.82 | 5.20 | 24.63 | 0.70 |
| 8 × 8 | 0.85 | 4.92 | 23.88 | 0.60 |
| Number of Elements | FWHM Lateral [mm] | FWHM Axial [mm] | SNR [dB] | Localization Error [mm] |
|---|---|---|---|---|
| Sim | Sim | Sim | Sim | |
| 63 × 63 | 0.72 | 5.50 | 26.34 | 0.30 |
| 32 × 32 | 0.72 | 5.42 | 26.27 | 0.20 |
| 16 × 16 | 0.72 | 5.42 | 26.33 | 0.20 |
| 11 × 11 | 0.72 | 5.18 | 26.55 | 0.60 |
| 8 × 8 | 0.73 | 5.22 | 25.99 | 0.60 |
References
- Thies, M.; Oelze, M.L. Real-Time Visualization of a Focused Ultrasound Beam Using Ultrasonic Backscatter. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1213–1223. [Google Scholar] [CrossRef]
- Allen, S.P.; Vlaisavljevich, E.; Shi, J.; Hernandez-Garcia, L.; Cain, C.A.; Xu, Z.; Hall, T.L. The response of MRI contrast parameters in in vitro tissues and tissue mimicking phantoms to fractionation by histotripsy. Phys. Med. Biol. 2017, 62, 7167–7180. [Google Scholar] [CrossRef]
- Thies, M.; Oelze, M.L. Combined Therapy Planning, Real-Time Monitoring, and Low Intensity Focused Ultrasound Treatment Using a Diagnostic Imaging Array. IEEE Trans. Med. Imaging 2022, 41, 1410–1419. [Google Scholar] [CrossRef]
- Ebbini, E.S.; ter Haar, G. Ultrasound-guided therapeutic focused ultrasound: Current status and future directions. Int. J. Hyperth. 2015, 31, 77–89. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, L.; Chien, C.-Y.; Yang, Y.; Gong, Y.; Ye, D.; Pacia, C.P.; Chen, H. 3-D Transcranial Microbubble Cavitation Localization by Four Sensors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3336–3346. [Google Scholar] [CrossRef]
- Telichko, A.V.; Lee, T.; Hyun, D.; Chowdhury, S.M.; Bachawal, S.; Herickhoff, C.D.; Paulmurugan, R.; Dahl, J.J. Passive Cavitation Mapping by Cavitation Source Localization From Aperture-Domain Signals-Part II: Phantom and In Vivo Experiments. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1198–1212. [Google Scholar] [CrossRef]
- Gyöngy, M.; Coussios, C.-C. Passive spatial mapping of inertial cavitation during HIFU exposure. IEEE Trans. Biomed. Eng. 2010, 57, 48–56. [Google Scholar] [CrossRef]
- Gray, M.D.; Elbes, D.; Paverd, C.; Lyka, E.; Coviello, C.M.; Cleveland, R.O.; Coussios, C.C. Dual-Array Passive Acoustic Mapping for Cavitation Imaging With Enhanced 2-D Resolution. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 647–663. [Google Scholar] [CrossRef]
- Meng, Y.; Hynynen, K.; Lipsman, N. Applications of focused ultrasound in the brain: From thermoablation to drug delivery. Nat. Rev. Neurol. 2021, 17, 7–22. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 2014, 17, 322–329. [Google Scholar] [CrossRef]
- Johnston, K.; Tapia-Siles, C.; Gerold, B.; Postema, M.; Cochran, S.; Cuschieri, A.; Prentice, P. Periodic shock-emission from acoustically driven cavitation clouds: A source of the subharmonic signal. Ultrasonics 2014, 54, 2151–2158. [Google Scholar] [CrossRef]
- Salgaonkar, V.A.; Datta, S.; Holland, C.K.; Mast, T.D. Passive cavitation imaging with ultrasound arrays. J. Acoust. Soc. Am. 2009, 126, 3071–3083. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Crake, C.; McDannold, N.; Clement, G.T. Passive Acoustic Mapping with the Angular Spectrum Method. IEEE Trans. Med. Imaging 2017, 36, 983–993. [Google Scholar] [CrossRef]
- Coviello, C.; Kozick, R.; Choi, J.; Gyöngy, M.; Jensen, C.; Smith, P.P.; Coussios, C.-C. Passive acoustic mapping utilizing optimal beamforming in ultrasound therapy monitoring. J. Acoust. Soc. Am. 2015, 137, 2573–2585. [Google Scholar] [CrossRef]
- Lu, S.; Li, R.; Yu, X.; Wang, D.; Wan, M. Delay multiply and sum beamforming method applied to enhance linear-array passive acoustic mapping of ultrasound cavitation. Med. Phys. 2019, 46, 4441–4454. [Google Scholar] [CrossRef]
- Lu, S.; Yu, X.; Chang, N.; Zong, Y.; Zhong, H.; Wan, M. Passive acoustic mapping of cavitation based on frequency sum and robust capon beamformer. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Boulos, P.; Varray, F.; Poizat, A.; Ramalli, A.; Gilles, B.; Bera, J.-C.; Cachard, C. Weighting the Passive Acoustic Mapping Technique with the Phase Coherence Factor for Passive Ultrasound Imaging of Ultrasound-Induced Cavitation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 2301–2310. [Google Scholar] [CrossRef]
- Haworth, K.J.; Bader, K.B.; Rich, K.T.; Holland, C.K.; Mast, T.D. Quantitative Frequency-Domain Passive Cavitation Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 177–191. [Google Scholar] [CrossRef]
- Bae, S.; Liu, K.; Pouliopoulos, A.N.; Konofagou, E.E. Coherence-Factor-based Passive Acoustic Mapping for Real-Time Transcranial Cavitation Monitoring with Improved Axial Resolution. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Gyöngy, M. Passive Cavitation Mapping for Monitoring Ultrasound Therapy. Ph.D. Thesis, University of Oxford, Oxford, UK, 2010. [Google Scholar]
- Schoen, S.; Dash, P.; Arvanitis, C.D. Experimental Demonstration of Trans-Skull Volumetric Passive Acoustic Mapping With the Heterogeneous Angular Spectrum Approach. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 534–542. [Google Scholar] [CrossRef]
- Sivadon, A.; Varray, F.; Nicolas, B.; Bera, J.-C.; Gilles, B. Using Sparse Array for 3D Passive Cavitation Imaging. In Proceedings of the 2020 IEEE International Ultrasonics Symposium (IUS), Las Vegas, NV, USA, 7–11 September 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Acconcia, C.N.; Jones, R.M.; Hynynen, K. Receiver array design for sonothrombolysis treatment monitoring in deep vein thrombosis. Phys. Med. Biol. 2018, 63, 235017. [Google Scholar] [CrossRef]
- Liu, H.-L.; Tsai, C.-H.; Jan, C.-K.; Chang, H.-Y.; Huang, S.-M.; Li, M.-L.; Qiu, W.; Zheng, H. Design and Implementation of a Transmit/Receive Ultrasound Phased Array for Brain Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1756–1767. [Google Scholar] [CrossRef]
- Jones, R.M.; O’Reilly, M.A.; Hynynen, K. Transcranial passive acoustic mapping with hemispherical sparse arrays using CT-based skull-specific aberration corrections: A simulation study. Phys. Med. Biol. 2013, 58, 4981–5005. [Google Scholar] [CrossRef]
- Jones, R.M.; Deng, L.; Leung, K.; McMahon, D.; O’Reilly, M.A.; Hynynen, K. Three-dimensional transcranial microbubble imaging for guiding volumetric ultrasound-mediated blood-brain barrier opening. Theranostics 2018, 8, 2909–2926. [Google Scholar] [CrossRef]
- Fournelle, M.; Bost, W. Wave front analysis for enhanced time-domain beamforming of point-like targets in optoacoustic imaging using a linear array. Photoacoustics 2019, 14, 67–76. [Google Scholar] [CrossRef]
- Vokurka, K. Cavitation noise modeling and analyzing. In Proceedings of the Forum Acousticum 2002, Seville, Spain, 16–20 September 2002. [Google Scholar]
- Risser, C.; Hewener, H.; Fournelle, M.; Fonfara, H.; Barry-Hummel, S.; Weber, S.; Speicher, D.; Tretbar, S. Real-Time Volumetric Ultrasound Research Platform with 1024 Parallel Transmit and Receive Channels. Appl. Sci. 2021, 11, 5795. [Google Scholar] [CrossRef]
- Yoon, H.; Song, T.-K. Sparse Rectangular and Spiral Array Designs for 3D Medical Ultrasound Imaging. Sensors 2020, 20, 173. [Google Scholar] [CrossRef]
- Fournelle, M.; Degel, C.; Jakob, A.; Nooijens, S.; Weber, S.; D’hooge, J.; Tretbar, S. Development and characterization of a sparse ellipsoidal 256 element array for volumetric ultrasound imaging. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–3. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Phase offset ϕkn | 1 µs ± 14 ns |
| Time constant θkn | 2 ns ± 0.5 ns |
| Peak acoustic pressure pkn | 3 MPa ± 10 kPa |
| Speed of sound c | 1480 m/s |
| Positions (x/y/z) for Sparsity Schemes [mm] | Positions (x/y/z) for Aperture Sizes [mm] |
|---|---|
| (0/0/50) | (0/0/50) |
| (0/0/40) | - |
| (0.9/−5.3/50) | - |
| (6/−5/50) | - |
| Aperture Size [mm] | Element Pitch [mm] | Number of Elements |
|---|---|---|
| 29.61 | 0.47 | 63 × 63 |
| 30.08 | 0.94 | 32 × 32 |
| 29.14 | 1.88 | 16 × 16 |
| 29.14 | 2.82 | 11 × 11 |
| 27.26 | 3.76 | 8 × 8 |
| Aperture Size [mm] | Element Pitch [mm] | Number of Elements |
|---|---|---|
| 5.00 | 0.16 | 32 × 32 |
| 9.60 | 0.30 | 32 × 32 |
| 20.00 | 0.63 | 32 × 32 |
| 30.08 | 0.94 | 32 × 32 |
| 40.00 | 1.25 | 32 × 32 |
| Aperture Size [mm] | FWHM Lateral [mm] | FWHM Axial [mm] | SNR [dB] | Localization Error [mm] | |||
|---|---|---|---|---|---|---|---|
| Sim | Exp | Sim | Exp | Sim | Exp | Sim | |
| 5.00 | 4.68 | - | 66.76 | - | 11.37 | - | 48.50 |
| 9.60 | 1.70 | - | 50.98 | - | 16.44 | - | 3.00 |
| 20.00 | 0.84 | - | 11.02 | - | 21.95 | - | 0.90 |
| 30.08 | 0.74 | 0.71 | 6.90 | 6.92 | 25.40 | 22.68 | 0.40 |
| 40.00 | 0.72 | - | 5.08 | - | 27.27 | - | 0.10 |
| Aperture Size [mm] | FWHM Lateral [mm] | FWHM Axial [mm] | SNR [dB] | Localization Error [mm] | |||
|---|---|---|---|---|---|---|---|
| Sim | Exp | Sim | Exp | Sim | Exp | Sim | |
| 5.00 | 5.50 | - | 73.50 | - | 8.84 | - | 32.80 |
| 9.60 | 2.28 | 2.15 | 62.68 | 61.24 | 14.34 | 14.07 | 16.30 |
| 20.00 | 0.86 | - | 12.70 | - | 20.57 | - | 0.90 |
| 30.08 | 0.60 | - | 5.78 | - | 26.04 | - | 0.20 |
| 40.00 | 0.48 | - | 3.52 | - | 29.70 | - | 0.10 |
| Number of Elements | Pitch [λ] | FWHM Lateral [mm] | FWHM Axial [mm] | SNR [dB] | Localization Error [mm] | Reconstruction Time [s] | |||
|---|---|---|---|---|---|---|---|---|---|
| Sim | Exp | Sim | Exp | Sim | Exp | Sim | |||
| 63 × 63 | 1.27 | 0.74 | - | 6.96 | - | 25.28 | - | 0.40 | 50,708 |
| 32 × 32 | 2.54 | 0.74 | 0.71 | 6.90 | 6.92 | 25.40 | 22.68 | 0.40 | 7989 |
| 16 × 16 | 5.08 | 0.74 | 0.69 | 6.90 | 6.70 | 25.46 | 22.67 | 0.40 | 1913 |
| 11 × 11 | 7.62 | 0.74 | 0.67 | 6.84 | 6.64 | 25.76 | 21.73 | 0.70 | 805 |
| 8 × 8 | 10.16 | 0.71 | 0.71 | 6.70 | 6.52 | 25.25 | 20.27 | 0.40 | 413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Therre, S.; Fournelle, M.; Tretbar, S. Optimization of 3D Passive Acoustic Mapping Image Metrics: Impact of Sensor Geometry and Beamforming Approach. Sensors 2024, 24, 1868. https://doi.org/10.3390/s24061868
Therre S, Fournelle M, Tretbar S. Optimization of 3D Passive Acoustic Mapping Image Metrics: Impact of Sensor Geometry and Beamforming Approach. Sensors. 2024; 24(6):1868. https://doi.org/10.3390/s24061868
Chicago/Turabian StyleTherre, Sarah, Marc Fournelle, and Steffen Tretbar. 2024. "Optimization of 3D Passive Acoustic Mapping Image Metrics: Impact of Sensor Geometry and Beamforming Approach" Sensors 24, no. 6: 1868. https://doi.org/10.3390/s24061868
APA StyleTherre, S., Fournelle, M., & Tretbar, S. (2024). Optimization of 3D Passive Acoustic Mapping Image Metrics: Impact of Sensor Geometry and Beamforming Approach. Sensors, 24(6), 1868. https://doi.org/10.3390/s24061868







