Abstract
Morphology and structure play a crucial role in influencing the performance of gas sensors. Hollow structures, in particular, not only increase the specific surface area of the material but also enhance the collision frequency of gases within the shell, and have been studied in depth in the field of gas sensing. Taking SnO2 as an illustrative example, a dual-shell structure SnO2 (D-SnO2) was prepared. D-SnO2@Polyaniline (PANI) (DSPx, x represents D-SnO2 molar content) composites were synthesized via the in situ oxidative polymerization method, and simultaneously deposited onto a polyethylene terephthalate (PET) substrate to fabricate an electrode-free, flexible sensor. The impact of the SnO2 content on the sensing performance of the DSPx-based sensor for NH3 detection at room temperature was discussed. The results showed that the response of a 20 mol% D-SnO2@PANI (DSP20) sensor to 100 ppm NH3 at room temperature is 37.92, which is 5.1 times higher than that of a pristine PANI sensor. Moreover, the DSP20 sensor demonstrated a rapid response and recovery rate at the concentration of 10 ppm NH3, with response and recovery times of 182 s and 86 s.
1. Introduction
Conductive polymers [1], owing to their mild synthesis conditions, high conductivity, and specific recognition capabilities towards target gases, have significant appeal in the field of gas sensors [2,3,4]. In particular, PANI is unique among the conductive polymers and is considered to be the most promising material for ammonia detection at room temperature owing to its specific deprotonation/protonation process for NH3 [5,6,7]. NH3, a colorless, unpleasant-smelling, highly toxic gas [8], can cause serious damage to immune systems and cell growth in humans with prolonged exposure [9], and the tolerance limit of humans drops drastically from 8 h to 15 min when the environmental concentration of NH3 rises from 25 ppm to 35 ppm [10,11]. Therefore, there is a big necessity for the capability to detect NH3 in room temperature environments. However, pristine PANI suffers from the drawbacks of low sensitivity and slow response in the field of NH3 sensing, necessitating modifications.
PANI-sensor-enhanced NH3-sensing performance primarily involves strategies such as the formation of p-n heterojunctions by combining PANI with an n-type metal oxide semiconductor (MOS) [12,13], the construction of hollow structures [14,15] and hierarchical structure gas-sensitive materials [16,17]. That is to say, the modulation of surface morphology is particularly essential to enhance the gas-sensitive performance of PANI, which mainly concerns the property enhancement brought by a large specific surface area, multiple active sites and a high porosity with microstructural advantages. For example, Jia et al. fabricated SnO2@PANI sensors based on core-shell nanotube structures via electrostatic spinning and plasma treatment, which exhibited an excellent and stable NH3 response and recovery properties at room temperature [18]. Li et al. successfully synthesized 20 mol% SnO2@Polyaniline (PASn20) sensitive materials with porous spherical structures on flexible substrates of PET by in situ polymerization, benefiting from the microstructure with a large specific surface area, the composites exhibited a 6.2-fold improvement towards room temperature response to NH3 [19]. Hong et al. successfully encapsulated PANI on In2O3 nanofibers with a hollow carbon coating by in situ polymerization and showed a response value of 18.2 for low concentrations of NH3 (1 ppm) at room temperature, which is 5.74 times higher than that of the pure PANI sensor [14]. Overall, PANI can exhibit different NH3-sensitive properties depending on the morphology of the MOS composite. Therefore, it is extremely meaningful to modulate the final growth and polymerization state of PANI according to the morphology of MOS for developing NH3-sensing materials with high sensitivity.
Hollow and hierarchically structured nanomaterials have gradually become a hotspot for the exploration of sensitive materials for gas sensors in recent years by virtue of their special structural advantages [20], such as a large specific surface area and high porosity [21], which are favorable for gas adsorption and diffusion. For example, Chen et al. adjusted the porosity of the prepared PANI composites with a core-shell layered structure by varying the parameters of coaxial electrostatic spinning to achieve gas detection with a high sensitivity and a low detection limit [22]. Gas-sensitive reaction processes primarily involve the activation of oxygen species and the adsorption–desorption processes of the target gas [23]. Specifically, this encompasses the diffusion of gas to the surface of the sensing material, collision and adsorption on the material surface, electron transfer between the adsorbed target gas and adsorbed oxygen, and the subsequent desorption processes [24,25,26,27]. The dual-shell hollow sphere structure possesses unique spatial effects, capable of altering the gas diffusion path and increasing the collision frequency, thereby enhancing the gas-sensing performance [28,29]. The dual-shell hollow sphere structure, characterized by its high specific surface area, large pore volume, and rapid reaction, has attracted attention in catalysis [30], drug delivery [31,32], and other fields [33]. The porous shell facilitates efficient gas transport; however, reports on multi-shell hollow structures for PANI-based sensors are extremely limited.
In this work, we successfully composite PANI with D-SnO2 by in situ chemical oxidative polymerization and prepared a series of room-temperature flexible NH3 sensors based on DSPx. Characterization by SEM, TEM, XRD, and FTIR proved the successful composite of the nanomaterials. Meanwhile, the NH3-sensing performance of the pre-pared DSPx sensors was evaluated at room temperature. The results showed that the DSP20-based sensor exhibited the best sensing performance for 100 ppm NH3 at room temperature, with a response value as high as 37.92, which is 5.1 times higher than that of pure PANI. The sensor also exhibited good response/recovery characteristics, repeatability, and long-term stability. Finally, the improved sensing performance is explained in terms of microstructure and p-n heterojunction.
2. Materials and Methods
2.1. Materials
Sucrose (C12H22O11, 99%, Aladdin, Shanghai, China), sodium hydroxide (NaOH, 95%, Macklin, Shanghai, China), crystalline tin tetrachloride (SnCl4·5H2O, 99.995%, Aladdin), potassium hydroxide (KOH, 90%, Rhawn, Shanghai, China), hydrochloric acid (HCl, 1M), ammonium persulfate (APS, AR, DaMao, Tianji, China), poly ethylene terephthalate, aniline (C4H5N), anhydrous ethanol (EtOH, AR, Chemical Reagent, Tianjin, China), PET (China Resources Chemical Materials Technology Co., Changzhou, China), and ammonia (NH3, 100 ppm and 1%, Liming Gas Co., Ltd., Harbin, China).
2.2. Fabrication of D-SnO2@PANI Composites
2.2.1. Synthesis of D-SnO2 Microspheres
As shown in Figure 1, a simple one-step hydrothermal method was used to synthesize uniformly shaped and sized carbon microspheres [29]. Firstly, carbon microsphere (CMS) templates with uniform size were obtained by dissolving sucrose in deionized water and utilizing the polymerization reaction of a sucrose emulsion under hydrothermal conditions. Then, D-SnO2 microspheres were prepared using CMS as sacrificial templates. The CMSs were dispersed ultrasonically in 100 mL of a 0.05 M NaOH solution and alkaline treated for 3 h at room temperature. Subsequently, the alkali-treated CMS templates and SnCl4·5H2O were sequentially added into 30 mL of anhydrous ethanol, ultrasonically dispersed for 30 min, and aged at room temperature for 4 h. Finally, D-SnO2 microspheres were obtained by alternately washing with deionized aqueous water and anhydrous ethanol, drying, and calcinating at 500 °C for 2 h, with a heating rate of 1 °C/min.
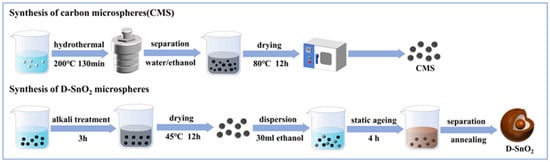
Figure 1.
Schematic diagram of the preparation process of D-SnO2 microspheres.
2.2.2. Synthesis of D-SnO2@PANI Composite
The DSPx binary composite was prepared by an in situ polymerization method [19]. As shown in Figure 2, firstly, 0.2 mmol of APS was added to 1 M HCl and stirred in an ice bath for 30 min to form Solution A. Then, a certain quantity of D-SnO2 powder was added to another portion of HCl and stirred in an ice bath to produce Solution B. Solution A was transferred to Solution B under ice-bath conditions, and then the fixed-size PET sheet (0.8 cm × 1.0 cm) was sequentially added to the solution and purified in aniline liquid by distillation, reacting for 2 h away from light. Finally, the PET sheets containing the DSPx-sensitive material were removed and left to be dried naturally at room temperature within 24 h so as to obtain gas-sensitive elements that can be used for gas-sensing tests. In this experiment, we modulated the molar ratios of the added D-SnO2 and aniline (: × 100% = 0%, 10%, 20%, 30%, and 60%, respectively) to prepare a series of DSPx-sensitive elements and explored the effect of different compounding amounts on gas-sensing performance.
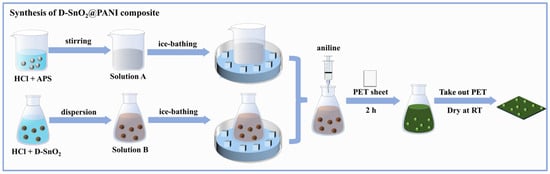
Figure 2.
Schematic diagram of the preparation process of D-SnO2@PANI composite.
2.3. Material Characterization
The crystallographic properties of DSPx were characterized by a Rigaku D/Max-2550 V X-ray powder diffractometer (XRD) irradiated with Cu-Kα rays (λ = 0.15406 nm) in the range of 5–80° at a scanning speed of 10° min−1. Field emission scanning electron microscopy (FESEM, JEOL JSM-7500F) and transmission electron microscopy (TEM, JEOL JEM-2100F) were used to reveal the micro-morphology and compositional structure of the materials at 5 kV. Fourier transform infrared spectroscopy (FT-IR, Spectrum 400) was used to characterize the chemical structure of the materials. The Brunauer–Emmett–Teller (BET) theory and the Barrett–Joyner–Halenda (BJH) model were used to measure the surface area and pore-size distribution of the materials, respectively.
2.4. NH3-Sensing Measurement
The gas-sensing measurement platform was designed by exposing the sensing device to different concentrations of NH3 at room temperature and recording the change in material resistance with a Fluke meter. The measurement device is shown in Figure 3. Firstly, we prepared two 1 L stoppered glass bottles and used a vacuum pump with a three-way switch to remove all the gases from the bottles leading to a negative pressure inside the bottles. Then, the knob of the three-way switch was adjusted to fill only one bottle with fresh air, and the other bottle with a mixture of NH3 and fresh air. The filling amount of NH3 was strictly controlled using a microfeeder. Next, the gas-sensitive test element fitted with a PET sheet was placed into the glass bottle filled with fresh air, the other end of the element was connected to a Fluke meter via a connecting cable, and the change in resistance of the sensitive materials was recorded in real time by an electronic computer. Once the resistance of the test element had stabilized, it was quickly transferred to the glass bottle containing test gases, at which point a large change in resistance occurred. When the resistance of the element stabilized in the test bottle, it was returned to the air bottle for the next test. We define the response of the sensor as S = Rg/Ra, where Rg is the resistance in the presence of the test gas and Ra is the resistance in air. The response and recovery time are defined as the time taken to realize a 90% change in total resistance for the sensor before and after exposure to the target gas.
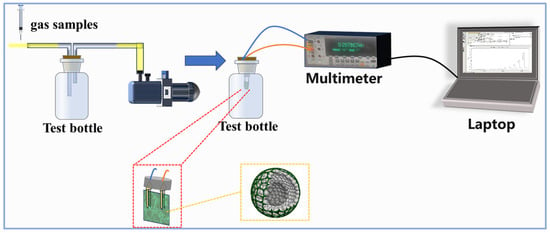
Figure 3.
The diagram of the NH3 measurement device.
3. Results and Discussion
3.1. Structural and Morphological Characteristics
The morphology and microstructure of the prepared D-SnO2 and DSP20 were observed by SEM and TEM as shown in Figure 4. It can be seen that the prepared D-SnO2 exhibits a hierarchical double-shell spherical structure composed of numerous small particles and the diameter is approximately 550 nm (Figure 4a). The emergence of permeable apertures conducive to gas transmission became evident on the surface of the hierarchical microspheres, and was attributed to the sparse arrangement of the diminutive SnO2 particles. In addition, the TEM image in Figure 4c further confirms the microstructure of the double-layer hierarchy of spheres. In Figure 4b,d, the synthesized DSP20 consistently retained the spherical morphology characteristic of D-SnO2. Significantly, polyaniline was intricately grown in situ on the exterior of D-SnO2, creating a conductive network structure. The effective integration of PANI with D-SnO2 is anticipated to facilitate an expedited electron transfer rate, thereby augmenting the gas-sensitive properties of the materials.
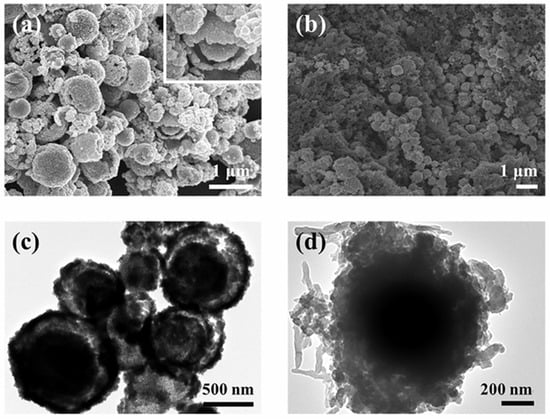
Figure 4.
SEM images of (a) D-SnO2; (b) DSP20; TEM images of (c) D-SnO2; (d) DSP20.
The XRD patterns of the prepared samples were scanned for peaks in the test range of 5–80°, as shown in Figure 5a. In the XRD spectrum of PANI, the appearance of broad diffraction peaks between 10 and 30° can be attributed to the parallel and perpendicular periodicity of the polyaniline chains [34]. The diffraction peaks of D-SnO2 were observed at 2θ = 26.08°, 33.78°, 38.04°, 51.72°, 54.63°, 61.81°, 65.84°, and 71.22°, which correspond to the (110), (101), (200), (211), (220), (310), (301), and (202) crystal planes, respectively. All these peaks can correspond to the standard card of SnO2 (PDF#99-0024). The XRD pattern of DSP20 was similar to that of D-SnO2, indicating that there was no change in the crystalline state of D-SnO2 complexed with polyaniline.
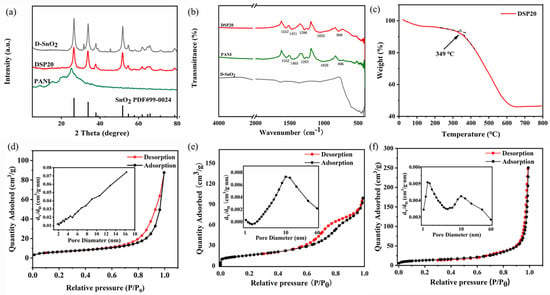
Figure 5.
(a) X−ray diffractograms of D−SnO2, PANI, and in situ−polymerized DSP20 samples; (b) FTIR spectra of D−SnO2, PANI, and DSP20 samples; (c) the thermo−gravimetric analysis curves of DSP20 composites; nitrogen adsorption−desorption isotherms and pore-size distributions of (d) PANI, (e) D−SnO2, and (f) DSP20.
The FTIR spectra of PANI, D-SnO2, and DSP20 are shown in Figure 5b. For the PANI spectrum, the main characteristic absorption peaks are located at 1552, 1465, 1283, 1020, and 806 cm−1 [35,36,37]. In this case, the absorption peak centered at 1552 cm−1 was attributed to the C=C stretching vibration of the quinone ring (N=Q=N) and the absorption peak centered at 1465 cm−1 was attributed to the absorption vibration of the benzene structure (N-B-N). The peak located at 1283 cm−1 is attributed to the C-N stretching mode of the benzene unit. The peaks at 1020 and 806 cm−1 indicate the in-plane and out-of-plane C-H bending vibrations of the benzene ring, respectively. In the spectrum of D-SnO2, the two weak peaks shown in the region of 500–750 cm−1 are assigned to the vibrational bands of the Sn-O bonds [38]. The FTIR spectra of DSP20 contained the principal characteristic peaks of PANI. Moreover, under the influence of SnO2, these peaks exhibit a high wavenumber shift, specifically at 1557, 1471, 1286, 1035, and 808 cm−1.
The DSP20 composites were characterized by thermogravimetric analysis and the results are shown in Figure 5c. The DSP20 composites exhibit a small amount of mass loss until the temperature reaches 100 °C, which is caused by the release of water molecules absorbed on the surface of the materials. The weight loss between 100 °C and 349 °C can be attributed to the elimination of dopant HCl in the acid-doped PANI. The major weight loss between 349–648 °C could be ascribed to the decomposition of PANI [39]. The weight of DSP20 composite hardly changed within the temperature range of 648–800 °C, remaining at 46.6%.
N2 adsorption–desorption results of PANI, D-SnO2, and DSP20-sensitive materials are shown in Figure 5d–f. Among them, the specific surface area of the pure PANI is 22.771 m2/g (Figure 5d). BET was used to calculate the specific surface area of the DSP20-sensing materials (46.619 m2/g), and a more concentrated pore size distribution was found at 2 nm and 10 nm (Figure 5f). Compared with pure PANI, the specific surface area of DSP20-sensing materials increased remarkably. This increase can lead to the exposure of multiple active sites and the enhancement of gas adsorption and desorption, thus improving the sensing performance [40]. Considering that the aniline monomer is polymerized on the surface of D-SnO2, it is inevitable that the specific surface area of the DSP20 composites would be slightly smaller than that of D-SnO2 (55.951 m2/g). Notably, the successful composite of PANI and D-SnO2 forms electrically conductive networks that can effectively accelerate the electron transport rate and enhance the gas-sensitive performance.
3.2. Gas-Sensing Properties
The NH3-sensing performances of the pure PANI and DSPx-based sensors were evaluated at room temperature with a relative humidity of about 60%. As observed in Figure 6a, the composite with D-SnO2 significantly influences the NH3-sensing capabilities. The response of the pure PANI-based sensor at a concentration of 100 ppm NH3 is only 7.43, with an increase in the content of D-SnO2, the response exhibits a distinct “volcanic” trend. Specifically, when the molar composite ratio of D-SnO2 to aniline is below 20%, the relative diffusion of electrons and holes between them forms a number of p-n heterojunctions conducive to enhanced NH3 sensing, which results in the gradually enhanced sensing characteristics exhibited by PANI, DSP10, and DSP20-based sensors. However, as the molar composite ratio of the two continues increasing from 20% to 60%, the proportion of D-SnO2 in the composite will increase dramatically and compete for NH3 adsorption [39], resulting in a decrease in the amount of NH3 that should have been adsorbed by the gas-sensitive active sites, leading to a decrease in the performance of the DSP30 and DSP60-based sensors. Particularly noteworthy is the performance of the sensor based on DSP20, demonstrating a prominent maximum response of 37.92 to 100 ppm NH3, which is 5.1 times higher than that of the pure PANI sensor.
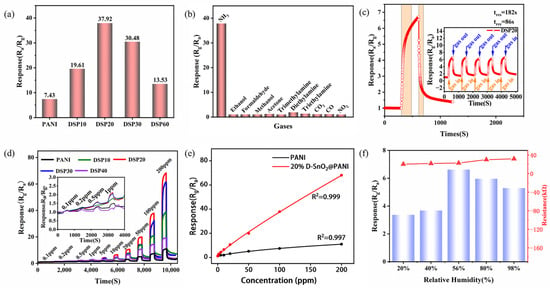
Figure 6.
(a) Effect of different molar addition percentages of D-SnO2 and PANI on the sensing performance of sensors at room temperature and an ammonia concentration of 100 ppm; (b) the selectivity of DSP20-based sensors towards different gases with 100 ppm concentration at room temperature; (c) transient curves of the response and recovery of the DSP20-based sensor to 10 ppm concentration of ammonia at room temperature, and transient curves of the recovery of the response of the DSP20 sensor to 10 ppm NH3 five consecutive times at room temperature in the inset; (d) recovery curves of the dynamic response of the PANI- and DSP-based sensors to 0.1–200 ppm NH3 at room temperature, and the inset shows a localized zoomed-in plot of the dynamic response of all the sensors at concentrations of 0.1–1 ppm NH3; (e) nonlinear fitting curves of the response of the PANI- and DSP20-based sensors to 0.1–200 ppm NH3 at room temperature; (f) the response and fundamental resistance plots of the DSP20-based sensor under 10 ppm NH3 at different relative humidities.
The selectivity of the DSP20-based sensor was evaluated at room temperature over various gases at a concentration of 100 ppm, and the results are shown in Figure 6b. The response of the DSP20-based sensor for NH3, ethanol, formaldehyde, methanol, acetone, trimethylamine, diethylamine, triethylamine, CO2, CO, and NO2 under the same conditions are 37.92, 1.04, 1.05, 1.03, 1.24, 1.01, 1.91, 1.35, 1.10, 1.14, and 1.01 respectively, which indicates excellent sensing properties for NH3. In Figure 6c, the dynamic response and recovery characteristics of the DSP20-based sensor are demonstrated at a concentration of 10 ppm NH3. According to the definition of tres and trec, the response and recovery time of the prepared DSP20 sensor for 10 ppm NH3 are 182 s and 86 s, respectively. Furthermore, as shown in the inset of Figure 6c, the sensor also exhibits excellent repeatability, as it is capable of exhibiting the same response and recovery characteristics over five consecutive test cycles.
Figure 6d describes the dynamic response of the sensors prepared using pure PANI and DSPx at different concentrations (0.1–200 ppm) of NH3 at room temperature. With the NH3 concentration increasing, the response of all the sensors showed an increasing trend. The gas-sensing performances of the PANI and DSPx-based sensors were varied. Of these, the DSP20-based sensors showed the most remarkable improvement and the response to NH3 concentrations of 0.1 ppm, 0.2 ppm, 0.5 ppm, 1 ppm, 5 ppm, 10 ppm, 20 ppm, 50 ppm, 100 ppm, and 200 ppm were 1.07, 1.25, 1.59, 2.10, 2.89, 4.35, 6.13, 6.53, 10.61, 19.96, 37.92, and 68.04, respectively. The fitting curves depicting the variation with NH3 concentration for pure PANI and DSP20-based sensors are illustrated in Figure 6e. The fitting equation based on the DSP20 sensor is given as y = 1.06602 + 0.63772 × |x − 0.1|^0.87739, and adheres to the typical model for composite gas sensing, and the corresponding R2 value is 0.999. According to this equation, the calculated theoretical detection limit is 268 ppb (response value of 1.2).
We also investigated the sensing performance of DSP20-based sensors for 10 pm NH3 at different humidity levels, and the results are shown in Figure 6f. As the relative humidity (RH) increases from 20% to 56%, the response of the sensor gradually increased and reached a maximum value of 6.62 at 56% RH. This phenomenon can be attributed to the in-doping effect of water on the acidified PANI. After that, with a further increase in relative humidity, the presence of more water molecules occupies the active sites which are meant to be sensitively reactive to NH3 gas [41], thus leading to the degradation of the sensor performance.
Additionally, the long-term stability of the sensor based on DSP20 was evaluated for its response to 10 ppm NH3 at room temperature over a continuous period of 17 days. As shown in Figure S1, the response of the sensor drops to ~50% of the initial value on the 3rd day and then stabilizes. The factors responsible for this phenomenon are the aging of the PET film and the disappearance of the unstable adsorption sites.
The comparison of the sensing performance of DSP20-based sensors with previously reported devices is provided in Table 1. Notably, the DSP20-based sensors proposed in this research exhibit superior performance in terms of both sensitivity and detection limit, and have favorable application prospects.

Table 1.
Comparison of the sensing properties of DSP20-based sensor in this study with previously reported devices.
3.3. Gas-Sensing Mechanism
Polyaniline undergoes acid doping under acidic conditions, forming polarons and transforming into the conductive emeraldine-salt state of polyaniline. Specifically, the acid produces protons and enters the chain of PANI to combine with the N atom on the imine group (=N-), causing the quinone ring in the structure of PANI to be reduced to a benzene ring and forming a polaron, which makes the less-than-conducting eigenstates of polyaniline electrically conductive and results in an emerald-green imine-salt state. This doping process is reversible; when exposed to an NH3 environment, a de-doping reaction occurs. Hence, this distinctive chemical reaction imparts a specific detection functionality to polyaniline for NH3. The reversible chemisorption process occurring between PANI and NH3 molecules is shown in Equation (1). When the sensor was exposed to NH3, NH3 captures H+ from the -NH- groups, leading to a reduction in the polaron density along the main chain of polyaniline, rendering the PANI in a highly resistive state. The opposite process would occur when the sensor was placed back in air [16].
The dramatic improvement in the sensing performance of the DSP20 compound can be mainly attributed to the following two aspects: firstly, the composite exhibits a dual-shell microstructure, featuring a substantial specific surface area and permeable pores, facilitating enhanced gas diffusion and adsorption [37]. Additionally, when the n-type D-SnO2 surface undergoes in situ polymerization with p-type proton-doped polyaniline (PANI-H+), the holes in PANI-H+ and the electrons in D-SnO2 mutually diffuse. This results in the formation of a p-n heterojunction at the interface, as illustrated in Figure 7. When the sensor is exposed to air, driven by the energy level difference between PANI-H+ and D-SnO2, the holes in PANI-H+ and the electrons in D-SnO2 diffuse in opposite directions, forming a narrow depletion layer. This leads to a re-balancing of the Fermi level, resulting in a lower-resistance state for the sensing material. Upon exposure to NH3, NH3 captures the H+ in PANI-H+, leading to a further increase in the width of the depletion layer, causing a sharp rise in material resistance. Hence, the signal amplification of the p-n heterojunction in this reaction significantly enhances the sensing performance of DSP20.
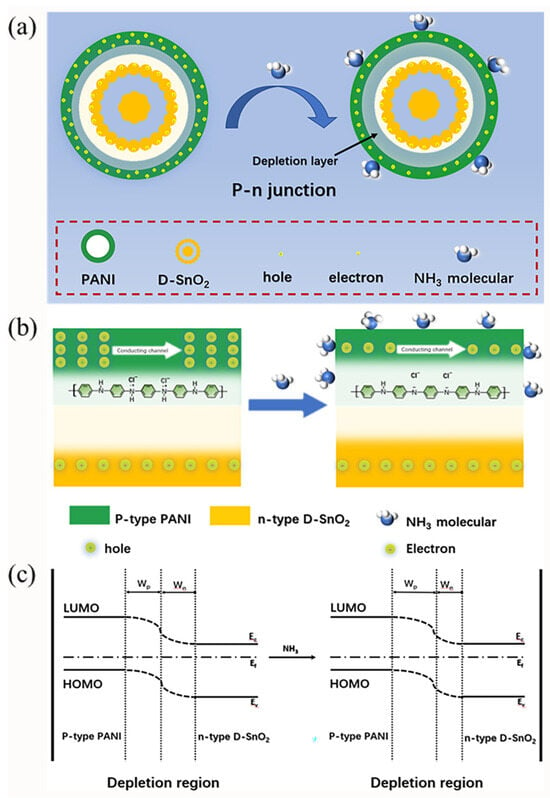
Figure 7.
Sensing mechanism of DSP20 in air and NH3: (a) formulation process of p-n heterojunction; (b) local magnification of heterojunction formation; and (c) schema of energy band structure.
4. Conclusions
In summary, a double-shell SnO2 with a permeable pore structure via a sacrificial template method were prepared, and through the in situ polymerization of PANI simultaneously deposited onto PET substrates to fabricate room-temperature NH3 sensors, namely DSPx sensors. The results show that DSPx-based sensors enhanced the NH3 sensing performance to varying degrees. In particular, the DSP20-based sensor exhibits excellent gas-sensing performance, with a response value of 37.92 to 100 ppm NH3 and excellent selectivity. In addition, it has good response recovery characteristics, repeatability and acceptable long-term stability. This improved performance is attributed to its special microstructure and the p-n heterojunction formed between the D-SnO2 surface and PANI. Therefore, this DSP-based sensitive material is expected to become an ideal material for the highly selective detection of NH3 at room temperature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s24061824/s1, Figure S1: Long-term stability curve of the DSP20-based sensor response to 10 ppm NH3 at room temperature.
Author Contributions
Y.Q.: investigation, writing—original draft, data curation; H.Z.: software, conceptualization; Y.L.: software, validation; Z.D.: visualization, supervision; S.L. (Siqi Li): writing—review & editing; S.L. (Song Liu): writing—review & editing; and W.J.: writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Nature Science Foundation of China (Nos. 62001097 & 22073014), Natural Science Foundation of Heilongjiang Province (LH2020F001 & GZ20220074), and Young Elite Scientists Sponsorship Program by CAST (No. YESS20210262).
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Su, Y.; Li, W.; Cheng, X.; Zhou, Y.; Yang, S.; Zhang, X.; Chen, C.; Yang, T.; Pan, H.; Xie, G.; et al. High-performance piezoelectric composites via β phase programming. Nat. Commun 2022, 13, 4867. [Google Scholar] [CrossRef]
- Xianghong, L.; Wei, Z.; Rahul, K.; Mahesh, K.; Jun, Z. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Kroutil, J.; Laposa, A.; Povolny, V.; Klimsa, L.; Husak, M. Gas sensor with different morphology of pani layer. Sensors 2023, 23, 1106. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Nakamae, T.; Fujisada, R.; Shiba, S. Highly sensitive ammonia gas sensor using micrometer-sized core–shell-type spherical polyaniline particles. Sensors 2021, 21, 7522. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent progress of nanostructured sensing materials from 0D to 3D: Overview of structure–property-application relationship for gas sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent developments in polymer nanocomposite-based electrochemical sensors for detecting environmental pollutants. Ind. Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef]
- Li, S.; Liu, A.; Yang, Z.; Zhao, L.; Wang, J.; Liu, F.; You, R.; He, J.; Wang, C.; Yan, X.; et al. Design and preparation of the WO3 Hollow @ PANI conducting films for room temperature flexible NH3 sensing device. Sens. Actuators B Chem. 2019, 289, 252–259. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Zhou, T.; Liu, L.; Chen, Q.; Gao, B.; Zhang, T. Self-assembly polyaniline films for the high-performance ammonia gas sensor. Sens. Actuators B Chem. 2022, 365, 131928. [Google Scholar] [CrossRef]
- Xiangyu, W.; Yang, C.; Xiaolong, N.; Jinlong, X.; Yuwei, W.; Haoran, S.; Zhuo, L.; Yongming, S.; Changping, L. PSS-doped pani nanoparticle/Ti3C2TX composites for conductometric flexible ammonia gas sensors operated at room temperature. Sens. Actuators B Chem. 2022, 374, 132788. [Google Scholar] [CrossRef]
- Dan, H.; Xiaomei, H.; Xiaoru, L.; Junzhao, Z.; Li, Z.; Xiuli, H.; Weidong, W.; Bingshe, X.; Shengbo, S. Conductometric gas sensor based on p-type gan hexagonal pits /pani for trace-level NH3 detection at room temperature. Sens. Actuators B Chem. 2023, 385, 133688. [Google Scholar] [CrossRef]
- Yi, L.; Weixiong, L.; Ziyang, J.; Xiaolan, L.; Guangzhong, X.; Huiling, T.; Yadong, J.; Yajie, Y.; Yuanjie, S. Ternary ordered assembled piezoelectric composite for self-powered ammonia detection. Nano Energy 2024, 122, 109291. [Google Scholar] [CrossRef]
- Saravanan, K.K.; Karthik, P.S.; Mirtha, P.R.; Balaji, J.; Rajeshkanna, B. A one-pot hydrothermal-induced PANI/SnO2 and PANI/SnO2/rGO ternary composites for high-performance chemiresistive-based H2S and NH3 gas sensors. J. Mater. Sci. Mater. Electron. 2020, 31, 8825–8836. [Google Scholar] [CrossRef]
- Alharthy, R.D.; Saleh, A. A novel trace-level ammonia gas sensing based on flexible PANI-CoFe2O4 nanocomposite film at room temperature. Polymers 2021, 13, 3077. [Google Scholar] [CrossRef]
- Hong, S.-Z.; Huang, Q.-Y.; Wu, T.-M. Facile synthesis of polyaniline/carbon-coated hollow indium oxide nanofiber composite with highly sensitive ammonia gas sensor at the room temperature. Sensors 2022, 22, 1570. [Google Scholar] [CrossRef]
- Xiaohui, D.; Zaihua, D.; Yajie, Z.; Bohao, L.; Xian, L.; Qiuni, Z.; Zhen, Y.; Yadong, J.; Huiling, T. Enhanced NH3 sensing performance of polyaniline via a facile morphology modification strategy. Sens. Actuators B Chem. 2022, 369, 132302. [Google Scholar] [CrossRef]
- Faricha, A.; Yoshida, S.; Chakraborty, P.; Okamoto, K.; Chang, T.-F.M.; Sone, M.; Nakamoto, T. Array of miniaturized amperometric gas sensors using atomic gold decorated Pt/PANI electrodes in room temperature ionic liquid films. Sensors 2023, 23, 4132. [Google Scholar] [CrossRef]
- Li, S.; Diao, Y.; Yang, Z.; He, J.; Wang, J.; Liu, C.; Liu, F.; Lu, H.; Yan, X.; Sun, P.; et al. Enhanced room temperature gas sensor based on au-loaded mesoporous In2O3 @ core-shell nanohybrid assembled on flexible pet substrate for NH3 detection. Sens. Actuators B Chem. 2018, 276, 526–533. [Google Scholar] [CrossRef]
- Jia, A.; Liu, B.; Liu, H.; Li, Q.; Yun, Y. Interface design of SnO2@PANI nanotube with enhanced sensing performance for ammonia detection at room temperature. Front. Chem. 2020, 8, 338. [Google Scholar] [CrossRef]
- Li, S.; Liu, A.; Yang, Z.; He, J.; Wang, J.; Liu, F.; Lu, H.; Yan, X.; Sun, P.; Liang, X.; et al. Room temperature gas sensor based on tin @ polyaniline nanocomposite assembled on flexible substrate: Ppb-level detection of NH3. Sens. Actuators B Chem. 2019, 299, 126970. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, S.; Du, K. Chemiresistive gas sensors based on hollow heterojunction: A review. Adv. Mater. Interfaces 2021, 8, 2002122. [Google Scholar] [CrossRef]
- Yi, S.; Shi, W.; Yang, X.; Yao, Z. Engineering sensitive gas sensor based on mof-derived hollow metal-oxide semiconductor heterostructures. Talanta 2023, 258, 124442. [Google Scholar] [CrossRef]
- Chunxu, C.; Guangzhong, X.; Jing, D.; Weixiong, L.; Yulin, C.; Jing, L.; Qiuping, Z.; Huiling, T.; Yadong, J.; Yuanjie, S. Integrated core-shell structured smart textiles for active NO2 concentration and pressure monitoring. Nano Energy 2023, 116, 108788. [Google Scholar] [CrossRef]
- Zhicheng, C.; Sunghoon, P. Improved SnO2 nanowire acetone sensor with uniform Co3O4 nanoparticle decoration. J. Environ. Chem. Eng. 2023, 11, 111504. [Google Scholar] [CrossRef]
- Shan, W.; Yang, Y.; Xin, L.; Tiantian, W.; Jiaxian, L.; Gaofeng, S.; Guoying, W. Room temperature solid electrolyte ozone sensor based on ag-doped SnO2. Sens. Actuators A Phys. 2023, 365, 114915. [Google Scholar] [CrossRef]
- Songlin, Z.; Xin, L.; Ruibo, X.; Long, P.; Zhenya, L.; Xinhua, H.; Junning, G. Size-dependent response of hydrothermally grown SnO2 for a high-performance NO2 sensor and the impact of oxygen. ACS Sens. 2024, 9, 195–205. [Google Scholar] [CrossRef]
- Jae, Y.D.; Wansik, O.; Ali, M.; Yoon, S.K.; Bi, K.E.; Min, K.H.; Sub, K.S.; Woo, K.H. Enhancement of xylene gas sensing by using Au core structures in regard to Au@SnO2 core-shell nanocomposites. Sens. Actuators B Chem. 2023, 392, 134018. [Google Scholar] [CrossRef]
- Li, S.; Qu, Y.; Lu, X.; Zhang, F.; Liu, S.; Li, B. A gas sensor with enhanced sensing properties towards butyl acetate: Vascular bundle structure Zn2SnO4 derived from maize straw. Chem. Asian J. 2023, 18, e202300505. [Google Scholar] [CrossRef]
- Li, M.; Mao, D.; Wan, J.; Wang, F.; Zhai, T.; Wang, D. Hollow multi-shell structured SnO2 with enhanced performance for ultraviolet photodetectors. Inorg. Chem. Front. 2019, 6, 1968–1972. [Google Scholar] [CrossRef]
- Ke, X.; Ziwang, K.; Feiyu, Z.; Yuan, Q.; Siqi, L.; Song, L. Hollow multi-shelled structural SnO2 with multiple spatial confinement for ethanol gas sensing. Mater. Lett. 2023, 338, 134070. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, J.; Wei, W.; Li, S. Recent progress in the syntheses and applications of multishelled hollow nanostructures. Mater. Chem. Front. 2020, 4, 1105–1149. [Google Scholar] [CrossRef]
- Decai, Z.; Yanze, W.; Jing, X.; Chunsheng, G.; Dan, W. Response and regulation of the microenvironment based on hollow structured drug delivery systems. Adv. Funct. Mater. 2023, 33, 2300681. [Google Scholar] [CrossRef]
- Parvin, N.; Nallapureddy, R.R.; Mandal, T.K.; Joo, S.W. Construction of bimetallic hybrid multishell hollow spheres via sequential template approach for less cytotoxic antimicrobial effect. IEEE Trans. NanoBiosci. 2022, 22, 447–452. [Google Scholar] [CrossRef]
- Zhenkai, Z.; Dandan, Z.; Chen, Y.; Zhenyue, L.; Yang, M.; Zhiguo, Y.; Davoud, D.; Xinfang, Z.; Xi-Tao, Y.; Xiaoguang, M. High sensitivity and surface mechanism of mofs-derived metal oxide Co3O4-SnO2 hollow spheres to ethanol. J. Alloys Compd. 2023, 962, 171182. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Li, P.; Zong, X.; Dong, G.; Zhang, Y. Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sens. Actuators B Chem. 2017, 258, 895–905. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, Q.; Jiang, B.; Huang, Y. Design of the novel polyaniline/polysiloxane flexible nanocomposite film and its application in gas sensor. Compos. Part B Eng. 2020, 196, 108131. [Google Scholar] [CrossRef]
- Mohammad, A.H.; Ali, K.A.; Yadollah, M.; Nader, L.M. Novel SnO2/PANI nanocomposites for selective detection of ammonia at room temperature. Appl. Surf. Sci. 2023, 615, 156381. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, H.; Shi, Y.; Yu, X.; Lan, G. Preparation and gas sensing properties of PANI/SnO2 hybrid material. Polymers 2021, 13, 1360. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Li, S.; Lin, P.; Zhao, L.; Wang, C.; Liu, D.; Liu, F.; Sun, P.; Liang, X.; Liu, F.; Yan, X.; et al. The room temperature gas sensor based on @ WO3 nanocomposites and flexible pet substrate for NH3 detection. Sens. Actuators B Chem. 2017, 259, 505–513. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Yang, J.; Han, W.; Ma, J.; Wang, C.; Shimanoe, K.; Zhang, S.; Sun, Y.; Cheng, P.; Wang, Y.; Zhang, H.; et al. Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection. Sens. Actuators B Chem. 2021, 340, 129971. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Guo, C.; Huo, L.; Gao, S.; Zheng, Z.; Cheng, X.; Xu, Y. Template-free synthesis of a wafer-sized polyaniline nanoscale film with high electrical conductivity for trace ammonia gas sensing. J. Mater. Chem. A 2022, 10, 12150–12156. [Google Scholar] [CrossRef]
- Narayan, K.; Shilpa, J.; Rohan, F.; Akshara, S.; Uday, P.; Navinchandra, S.; Dushyant, K. Enhanced sensing performance of an ammonia gas sensor based on Ag-decorated ZnO nanorods/polyaniline nanocomposite. ChemistrySelect 2023, 8, e202204284. [Google Scholar] [CrossRef]
- Pengfei, Z.; Wei, Z.; Wenyi, W.; Peng, D.; Guoqiang, Z.; Kun, D.; Chuntai, L.; Changyu, S. Stretchable strain sensor with high sensitivity, large workable range and excellent breathability for wearable electronic skins. Compos. Sci. Technol. 2022, 229, 109720. [Google Scholar] [CrossRef]
- He, M.; Xie, L.; Luo, G.; Li, Z.; Wright, J.; Zhu, Z. Flexible fabric gas sensors based on PANI/WO3 p−n heterojunction for high performance NH3 detection at room temperature. Sci. China Mater. 2020, 63, 2028–2039. [Google Scholar] [CrossRef]
- Liu, A.; Lv, S.; Jiang, L.; Liu, F.; Zhao, L.; Wang, J.; Hu, X.; Yang, Z.; He, J.; Wang, C.; et al. The gas sensor utilizing polyaniline/ MoS2 nanosheets/ SnO2 nanotubes for the room temperature detection of ammonia. Sens. Actuators B Chem. 2021, 332, 129444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).