Passive Biotelemetric Detection of Tibial Debonding in Wireless Battery-Free Smart Knee Implants
Abstract
1. Introduction
2. Materials and Methods
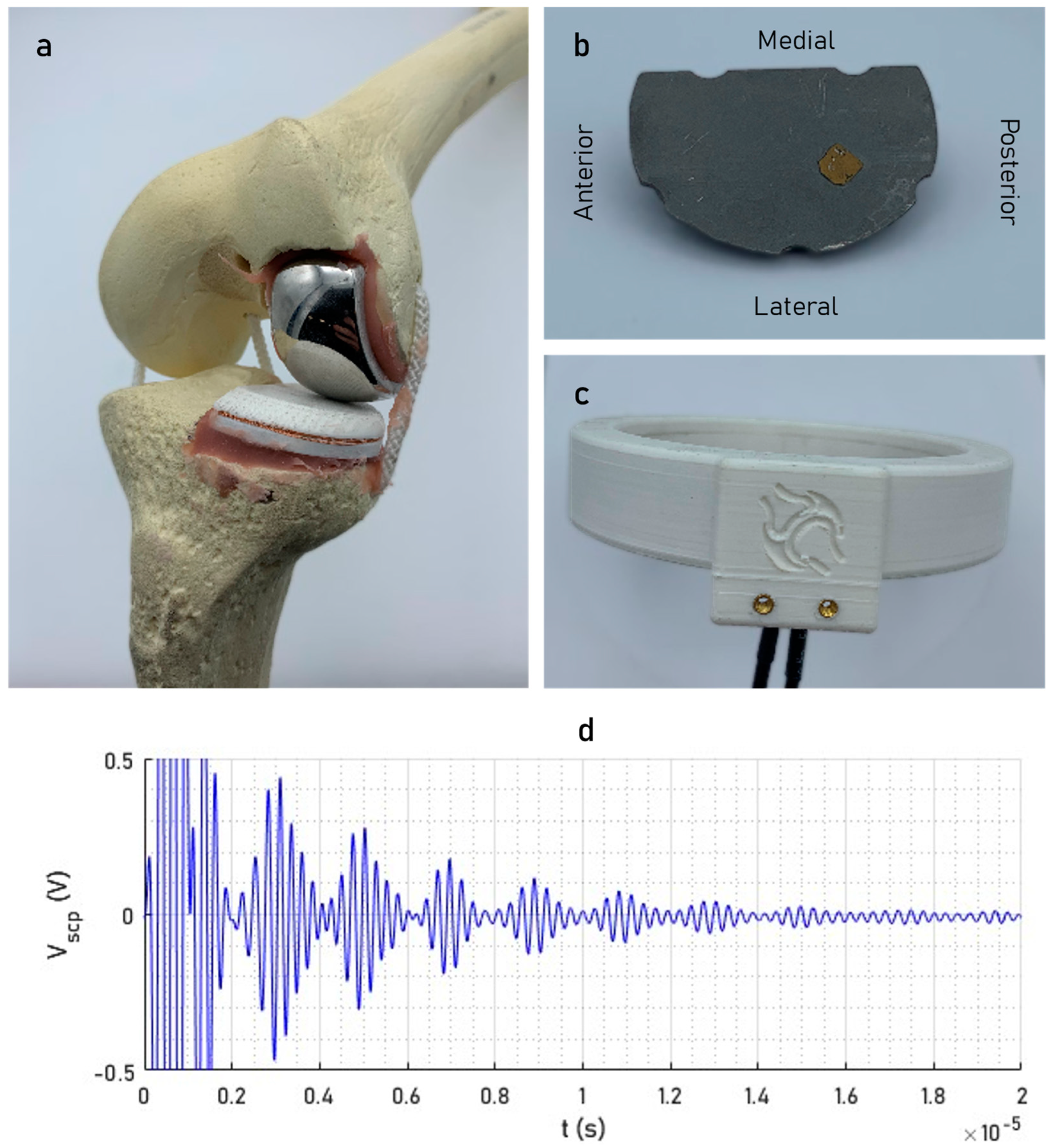
2.1. Smart Implant Design
2.2. Signal Acquisition and Processing
2.3. Simulated Tibial Debonding
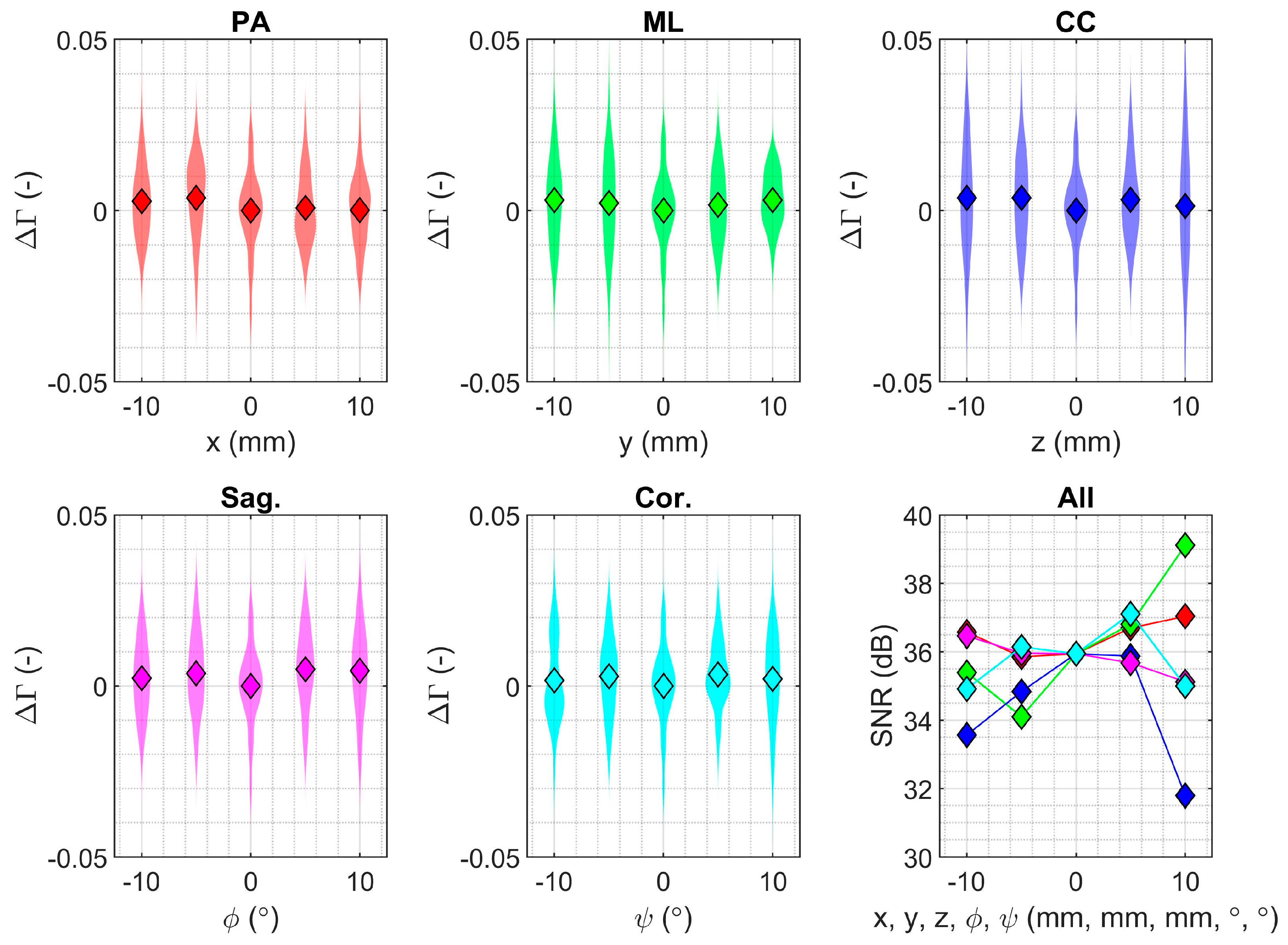
2.4. Measurement Sensitivity
2.5. Statistical Analysis
3. Results
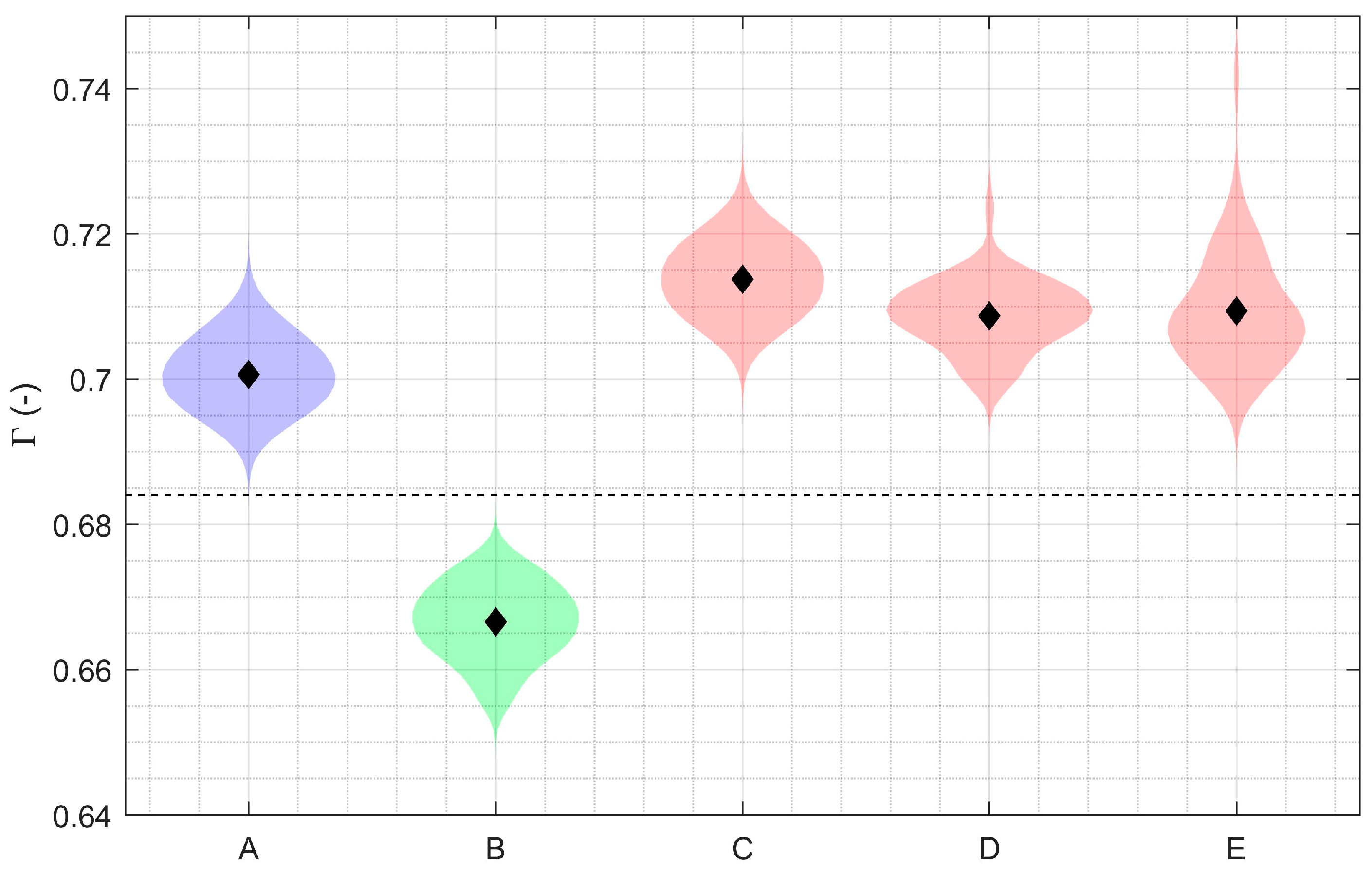
3.1. Debonding Detection with Smaller Partial Knee Replacement
3.2. Debonding Detection with Total Knee Replacement
3.3. Measurement Sensitivity for the Smaller Partial Knee Replacement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef]
- Evans, J.T.; Walker, R.W.; Evans, J.P.; Blom, A.W.; Sayers, A.; Whitehouse, M.R. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 655–663. [Google Scholar] [CrossRef]
- Liddle, A.D.; Judge, A.; Pandit, H.; Murray, D.W. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: A study of data from the National Joint Registry for England and Wales. Lancet 2014, 384, 1437–1445. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Blom, A.; Boulton, C.; Brittain, R.; Clark, E.; Dawson-Bowling, S.; Deere, K.; Esler, C.; Espinoza, O.; Goldberg, A.; et al. The National Joint Registry 18th Annual Report 2021; National Joint Registry: London, UK, 2021. [Google Scholar]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef]
- Sharkey, P.F.; Hozack, W.J.; Rothman, R.H.; Shastri, S.; Jacoby, S.M. Insall Award paper. Why are total knee arthroplasties failing today? Clin. Orthop. Relat. Res. 2002, 404, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Foran, J.R.; Whited, B.W.; Sporer, S.M. Early aseptic loosening with a precoated low-profile tibial component: A case series. J Arthroplasty 2011, 26, 1445–1450. [Google Scholar] [CrossRef]
- Murphy, J.D.; Braunlich, P.R.; Judson Iv, W.R.; Harker, J.N.; Baumann, P.A. Early Aseptic Failure of the Tibial Component-Cement Interface in ATTUNE(R) Total Knee Arthroplasty: A Report of Three Cases. Cureus 2021, 13, e20582. [Google Scholar] [CrossRef]
- Arsoy, D.; Pagnano, M.W.; Lewallen, D.G.; Hanssen, A.D.; Sierra, R.J. Aseptic tibial debonding as a cause of early failure in a modern total knee arthroplasty design. Clin. Orthop. Relat. Res. 2013, 471, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Heinichen, M.; Dietrich, F.; Jakubowitz, E.; Sobau, C.; Heisel, C. Short-keeled cemented tibial components show an increased risk for aseptic loosening. Clin. Orthop. Relat. Res. 2013, 471, 1008–1013. [Google Scholar] [CrossRef]
- Hazelwood, K.J.; O’Rourke, M.; Stamos, V.P.; McMillan, R.D.; Beigler, D.; Robb, W.J., III. Case series report: Early cement-implant interface fixation failure in total knee replacement. Knee 2015, 22, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kopinski, J.E.; Aggarwal, A.; Nunley, R.M.; Barrack, R.L.; Nam, D. Failure at the Tibial Cement-Implant Interface With the Use of High-Viscosity Cement in Total Knee Arthroplasty. J. Arthroplasty 2016, 31, 2579–2582. [Google Scholar] [CrossRef]
- Bonutti, P.M.; Khlopas, A.; Chughtai, M.; Cole, C.; Gwam, C.U.; Harwin, S.F.; Whited, B.; Omiyi, D.E.; Drumm, J.E. Unusually High Rate of Early Failure of Tibial Component in ATTUNE Total Knee Arthroplasty System at Implant-Cement Interface. J. Knee Surg. 2017, 30, 435–439. [Google Scholar] [PubMed]
- Kutzner, I.; Hallan, G.; Hol, P.J.; Furnes, O.; Gothesen, O.; Figved, W.; Ellison, P. Early aseptic loosening of a mobile-bearing total knee replacement. Acta Orthop. 2018, 89, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Cerquiglini, A.; Henckel, J.; Hothi, H.; Allen, P.; Lewis, J.; Eskelinen, A.; Skinner, J.; Hirschmann, M.T.; Hart, A.J. Analysis of the Attune tibial tray backside: A comparative retrieval study. Bone Jt. Res. 2019, 8, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, A.; Engh, C., III; Mehta, M.; Levine, B. Implant Interface Debonding After Total Knee Arthroplasty: A New Cause for Concern? Arthroplast Today 2020, 6, 972–975. [Google Scholar] [CrossRef]
- Keohane, D.; Power, F.; Cullen, E.; O’Neill, A.; Masterson, E. High rate of tibial debonding and failure in a popular knee replacement: A cause for concern. Knee 2020, 27, 459–468. [Google Scholar] [CrossRef]
- Cox, Z.C.; Green, C.C.; Otero, J.E.; Mason, J.B.; Martin, J.R. Varus Collapse in Total Knee Arthroplasty: Does Fixation or Bone Fail First? J. Arthroplasty 2022, 37, 162–167. [Google Scholar] [CrossRef]
- Randall, D.J.; Anderson, M.B.; Gililland, J.M.; Peters, C.L.; Pelt, C.E. A potential need for surgeon consensus: Cementation techniques for total knee arthroplasty in orthopedic implant manufacturers’ guidelines lack consistency. J. Orthop. Surg. (Hong Kong) 2019, 27, 2309499019878258. [Google Scholar] [CrossRef]
- Buller, L.T.; Rao, V.; Chiu, Y.F.; Nam, D.; McLawhorn, A.S. Primary Total Knee Arthroplasty Performed Using High-Viscosity Cement is Associated With Higher Odds of Revision for Aseptic Loosening. J. Arthroplasty 2020, 35, S182–S189. [Google Scholar] [CrossRef]
- Hampton, C.B.; Berliner, Z.P.; Nguyen, J.T.; Mendez, L.; Smith, S.S.; Joseph, A.D.; Padgett, D.E.; Rodriguez, J.A. Aseptic Loosening at the Tibia in Total Knee Arthroplasty: A Function of Cement Mantle Quality? J. Arthroplasty 2020, 35, S190–S196. [Google Scholar] [CrossRef]
- van Tol, A.F.; Tibballs, J.E.; Roar Gjerdet, N.; Ellison, P. Experimental investigation of the effect of surface roughness on bone-cement-implant shear bond strength. J. Mech. Behav. Biomed. Mater. 2013, 28, 254–262. [Google Scholar] [CrossRef] [PubMed]
- van Otten, T.J.M.; van Loon, C.J.M. Early aseptic loosening of the tibial component at the cement-implant interface in total knee arthroplasty: A narrative overview of potentially associated factors. Acta Orthop. Belg. 2022, 88, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, L.; Barnsley, L. Detection of aseptic loosening in total knee replacements: A systematic review and meta-analysis. Skeletal. Radiol. 2019, 48, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B.P.; Sculco, P.K.; Fehring, K.A.; Trousdale, R.T.; Taunton, M.J. A Novel Percentage-Based System for Determining Aseptic Loosening of Total Knee Arthroplasty Tibial Components. J. Arthroplasty 2017, 32, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Mandalia, V.; Eyres, K.; Schranz, P.; Toms, A.D. Evaluation of patients with a painful total knee replacement. J. Bone Jt. Surg. Br. 2008, 90, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Schiffner, E.; Latz, D.; Karbowski, A.; Grassmann, J.P.; Thelen, S.; Windolf, J.; Jungbluth, P.; Schneppendahl, J. Loosening of total knee arthroplasty—Always aseptic? J. Clin. Orthop. Trauma 2020, 11 (Suppl. 2), S234–S238. [Google Scholar] [CrossRef] [PubMed]
- Claassen, L.; Ettinger, M.; Plaass, C.; Daniilidis, K.; Calliess, T.; Ezechieli, M. Diagnostic value of bone scintigraphy for aseptic loosening after total knee arthroplasty. Technol. Health Care 2014, 22, 767–773. [Google Scholar] [CrossRef]
- Kitchener, M.I.; Coats, E.; Keene, G.; Paterson, R. Assessment of radionuclide arthrography in the evaluation of loosening of knee prostheses. Knee 2006, 13, 220–225. [Google Scholar] [CrossRef]
- Delank, K.S.; Schmidt, M.; Michael, J.W.; Dietlein, M.; Schicha, H.; Eysel, P. The implications of 18F-FDG PET for the diagnosis of endoprosthetic loosening and infection in hip and knee arthroplasty: Results from a prospective, blinded study. BMC Musculoskelet. Disord. 2006, 7, 20. [Google Scholar] [CrossRef]
- Bao, B.; Liu, C.S.; Masson, E.C.O.; Abele, J.T. Diagnostic accuracy of SPECT/CT arthrography in patients with suspected aseptic joint prostheses loosening. Eur. J. Hybrid Imaging 2021, 5, 4. [Google Scholar] [CrossRef]
- Peng, Z.; Jia, Y.; Li, J.; Wang, G. Diagnostic Performance of Single-Photon Emission Computed Tomography/Computed Tomography in Aseptic Loosening: A Systematic Review and Meta-Analysis. J. Arthroplasty 2021, 36, 4003–4012.e3. [Google Scholar] [CrossRef]
- Arami, A.; Delaloye, J.R.; Rouhani, H.; Jolles, B.M.; Aminian, K. Knee Implant Loosening Detection: A Vibration Analysis Investigation. Ann. Biomed Eng. 2018, 46, 97–107. [Google Scholar] [CrossRef]
- Khokle, R.P.; Franco, F.; de Freitas, S.C.; Esselle, K.P.; Heimlich, M.C.; Bokor, D.J. Eddy Current–Tunneling Magneto-Resistive Sensor for Micromotion Detection of a Tibial Orthopaedic Implant. IEEE Sens. J. 2019, 19, 1285–1292. [Google Scholar] [CrossRef]
- Mohammadbagherpoor, H.; Ierymenko, P.; Craver, M.H.; Carlson, J.; Dausch, D.; Grant, E.; Lucey, J.D. An Implantable Wireless Inductive Sensor System Designed to Monitor Prosthesis Motion in Total Joint Replacement Surgery. IEEE Trans. Biomed. Eng. 2020, 67, 1718–1726. [Google Scholar] [CrossRef]
- Hall, T.A.G.; Cegla, F.; van Arkel, R.J. Simple Smart Implants: Simultaneous Monitoring of Loosening and Temperature in Orthopaedics With an Embedded Ultrasound Transducer. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Watts, C.D.; Levy, D.L.; Miner, T.M.; Springer, B.D.; Kim, R.H. Tibial Tray Thickness Significantly Increases Medial Tibial Bone Resorption in Cobalt-Chromium Total Knee Arthroplasty Implants. J. Arthroplasty 2017, 32, 79–82. [Google Scholar] [CrossRef]
- Grob, A.; Freislederer, F.; Marzel, A.; Audige, L.; Schwyzer, H.K.; Scheibel, M. Glenoid Component Loosening in Anatomic Total Shoulder Arthroplasty: Association between Radiological Predictors and Clinical Parameters-An Observational Study. J. Clin. Med. 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Zmistowski, B.; Carpenter, D.P.; Chalmers, P.N.; Smith, M.J.; Keener, J.D. Symptomatic aseptic loosening of a short humeral stem following anatomic total shoulder arthroplasty. J. Shoulder Elbow Surg. 2021, 30, 2738–2744. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.Y.; Taylor, J.T.; Bowen, C.R.; Gheduzzi, S.; Miles, A.W. Sensing methodology for stability evaluation of total hip and knee arthroplasty. Sens. Actuat. A-Phys. 2010, 157, 150–160. [Google Scholar] [CrossRef]
- Marschner, U.; Grätz, H.; Jettkant, B.; Ruwisch, D.; Woldt, G.; Fischer, W.J.; Clasbrummel, B. Integration of a wireless lock-in measurement of hip prosthesis vibrations for loosening detection. Sens. Actuat. A-Phys. 2009, 156, 145–154. [Google Scholar] [CrossRef]
- Ponder, R.I.; Meneghini, R.M.; Anton, S.R. Evaluation of electromechanical impedance based structural health monitoring for detection of loosening in total knee arthroplasty. In Health Monitoring of Structural and Biological Systems XIII 2019; SPIE: Bellingham, WA, USA, 2019; Volume 10972, pp. 476–486. [Google Scholar]
- Puers, R.; Catrysse, M.; Vandevoorde, G.; Collier, R.J.; Louridas, E.; Burny, F.; Donkerwolcke, M.; Moulart, F. A telemetry system for the detection of hip prosthesis loosening by vibration analysis. Sens. Actuat. A-Phys. 2000, 85, 42–47. [Google Scholar] [CrossRef]
- Ruther, C.; Gabler, C.; Ewald, H.; Ellenrieder, M.; Haenle, M.; Lindner, T.; Mittelmeier, W.; Bader, R.; Kluess, D. In Vivo Monitoring of Implant Osseointegration in a Rabbit Model Using Acoustic Sound Analysis. J. Orthop. Res. 2014, 32, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Yokhana, S.S.; Bergum, C.; Ren, W.; Markel, D.C. Isolated Tibial Component Failure in Total Knee Arthroplasty: A Case Series Evaluating Inflammatory Response versus Mechanical Failure. J. Knee Surg. 2019, 32, 659–666. [Google Scholar] [CrossRef]
- Chong, D.Y.; Hansen, U.N.; Amis, A.A. Analysis of bone-prosthesis interface micromotion for cementless tibial prosthesis fixation and the influence of loading conditions. J. Biomech. 2010, 43, 1074–1080. [Google Scholar] [CrossRef]
- Scott, C.E.; Biant, L.C. The role of the design of tibial components and stems in knee replacement. J. Bone Jt. Surg. Br. 2012, 94, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.J. A Review of Approaches for Mitigating Effects from Variable Operational Environments on Piezoelectric Transducers for Long-Term Structural Health Monitoring. Sensors 2023, 23, 7979. [Google Scholar] [CrossRef]
- Hall, T.A.G.; Theodoridis, K.; Kechagias, S.; Kohli, N.; Denonville, C.; Rorvik, P.M.; Cegla, F.; van Arkel, R.J. Electromechanical and biological evaluations of 0.94Bi0.5Na0.5TiO3-0.06BaTiO3 as a lead-free piezoceramic for implantable bioelectronics. Biomater. Adv. 2023, 154, 213590. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Shieh, G. Optimal sample sizes for the design of reliability studies: Power consideration. Behav. Res. Methods 2014, 46, 772–785. [Google Scholar] [CrossRef]



| Homogeneity of Variance | Equality of Mean | |||
|---|---|---|---|---|
| Stat. | Sig. | Stat. | Sig. | |
| PA | F(4,245) = 0.354 | p = 0.841 | F(4,122.413) = 1.187 | p = 0.329 |
| ML | F(4,245) = 3.264 | p = 0.012 | F(4,121.398) = 0.596 | p = 0.666 |
| CC | F(4,245) = 4.563 | p = 0.001 | F(4,121.816) = 0.898 | p = 0.467 |
| Sag. | F(4,245) = 0.501 | p = 0.735 | F(4,122.397) = 1.361 | p = 0.252 |
| Cor. | F(4,245) = 1.469 | p = 0.212 | F(4,122.189) = 0.591 | p = 0.670 |
| Fem. | F(1,98) = 2.219 | p = 0.140 | t(95.974) = 0.599 | p = 0.551 |
| Ana. | F(1,98) = 0.000 | p = 0.999 | t(97.310) = 0.435 | p = 0.664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, T.A.G.; Cegla, F.; van Arkel, R.J. Passive Biotelemetric Detection of Tibial Debonding in Wireless Battery-Free Smart Knee Implants. Sensors 2024, 24, 1696. https://doi.org/10.3390/s24051696
Hall TAG, Cegla F, van Arkel RJ. Passive Biotelemetric Detection of Tibial Debonding in Wireless Battery-Free Smart Knee Implants. Sensors. 2024; 24(5):1696. https://doi.org/10.3390/s24051696
Chicago/Turabian StyleHall, Thomas A. G., Frederic Cegla, and Richard J. van Arkel. 2024. "Passive Biotelemetric Detection of Tibial Debonding in Wireless Battery-Free Smart Knee Implants" Sensors 24, no. 5: 1696. https://doi.org/10.3390/s24051696
APA StyleHall, T. A. G., Cegla, F., & van Arkel, R. J. (2024). Passive Biotelemetric Detection of Tibial Debonding in Wireless Battery-Free Smart Knee Implants. Sensors, 24(5), 1696. https://doi.org/10.3390/s24051696






