Auditory Cue Effects on Gait-Phase-Dependent Electroencephalogram (EEG) Modulations during Overground and Treadmill Walking
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup and Design
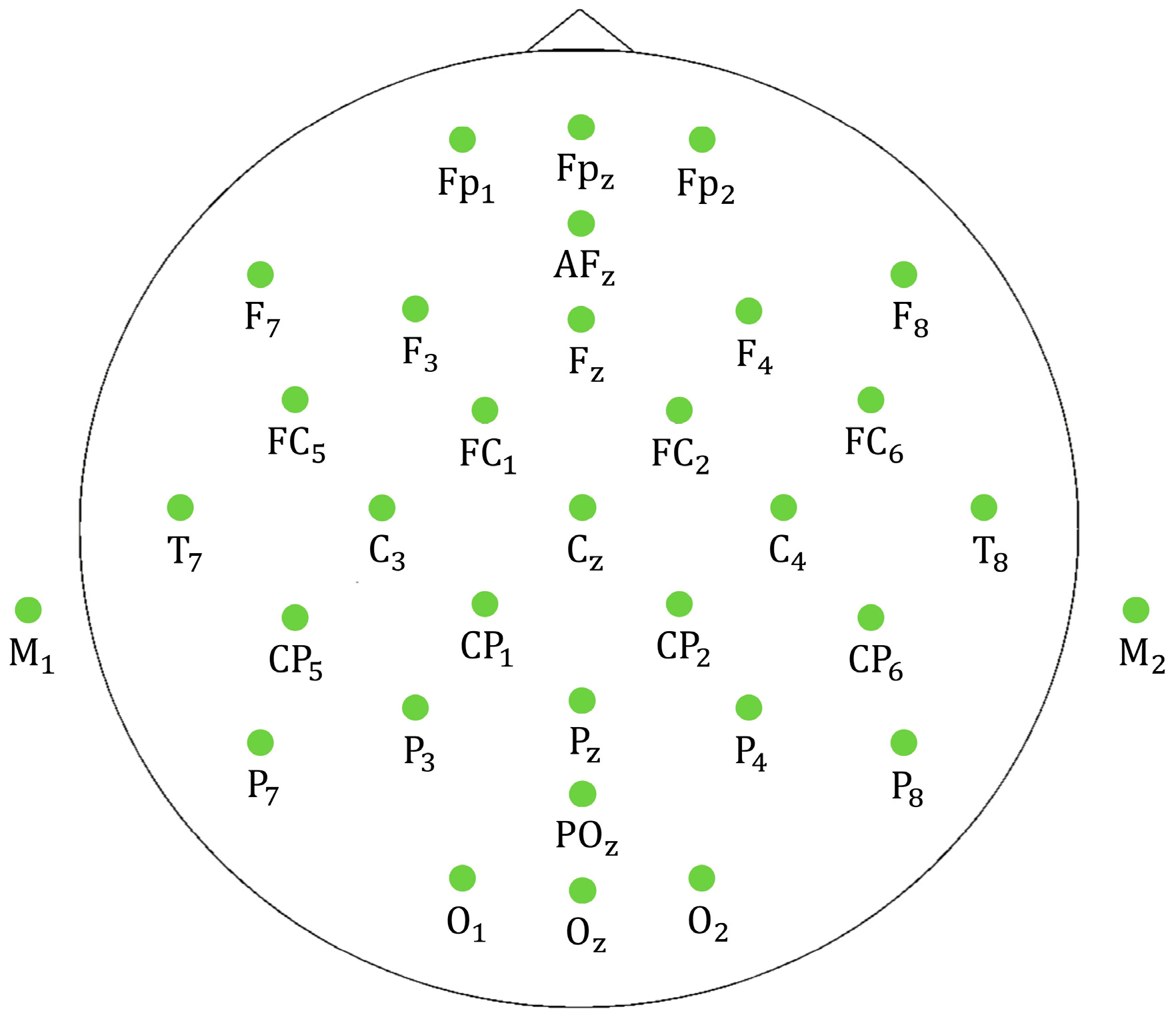
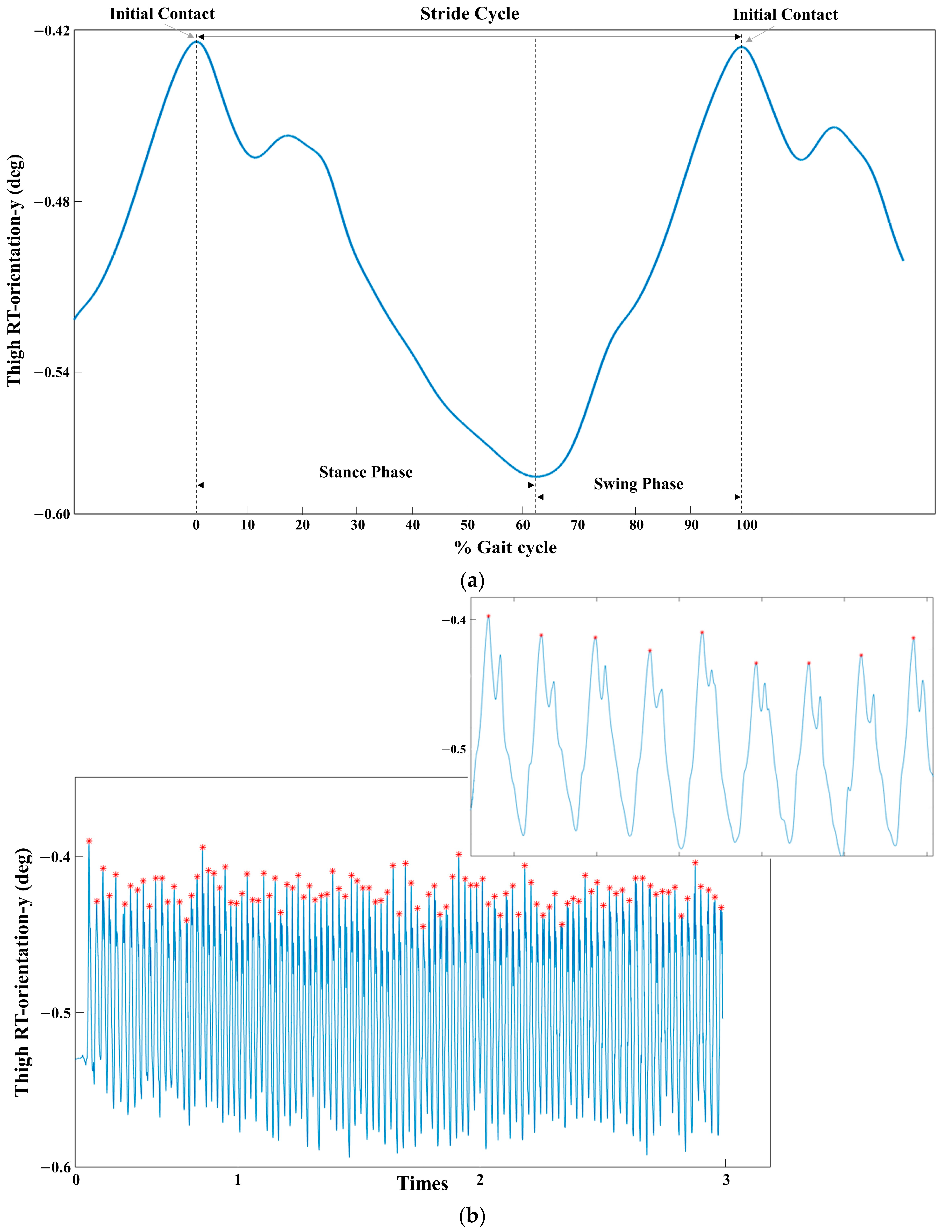
2.3. Data Collection
2.4. EEG Analysis
2.5. Statistical Analysis
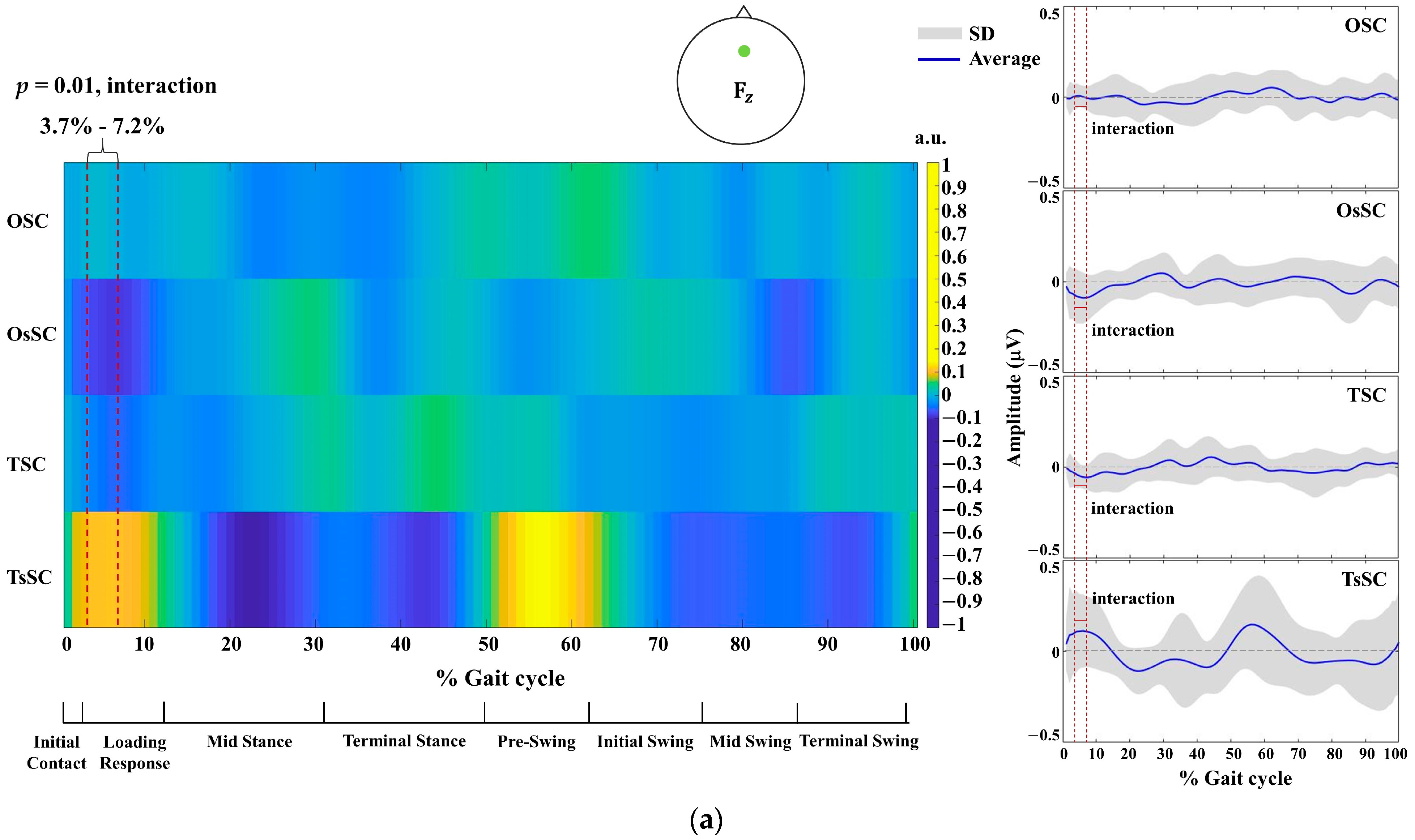
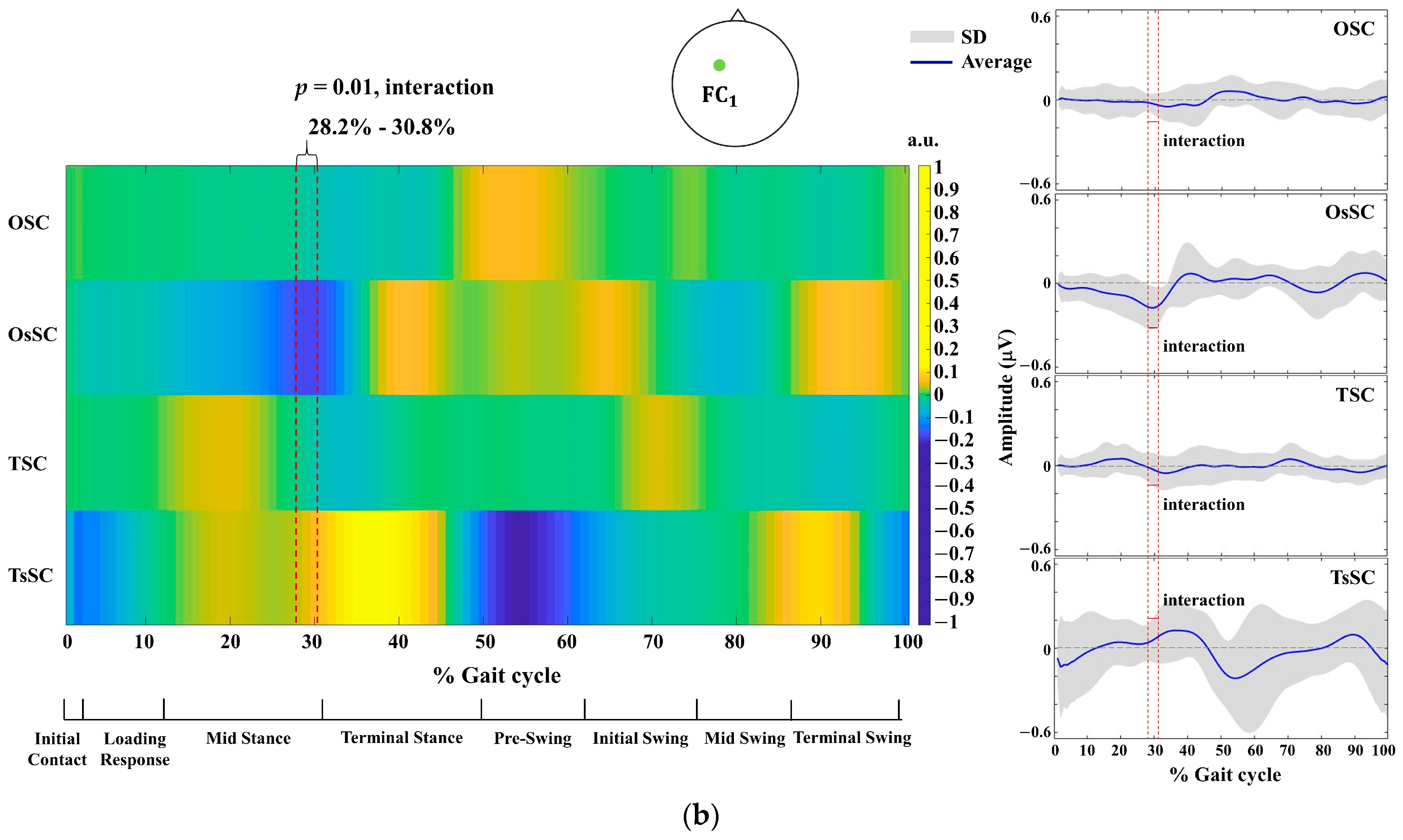
3. Results
4. Discussion
4.1. Between-Group Differences in EEG Activity across the Gait Cycle
4.2. Within-Group Differences in EEG Activity across the Gait Cycle
4.3. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef]
- Leisman, G.; Moustafa, A.A.; Shafir, T. Thinking, Walking, Talking: Integratory Motor and Cognitive Brain Function. Front. Public Health 2016, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Gorassini, M. Spinal and brain control of human walking: Implications for retraining of walking. Neuroscientist 2006, 12, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Drew, T.; Prentice, S.; Schepens, B. Cortical and brainstem control of locomotion. Prog. Brain Res. 2004, 143, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Fortier-Lebel, N.; Nakajima, T.; Yahiaoui, N.; Drew, T. Microstimulation of the Premotor Cortex of the Cat Produces Phase-Dependent Changes in Locomotor Activity. Cereb. Cortex 2021, 31, 5411–5434. [Google Scholar] [CrossRef] [PubMed]
- Nishimaru, H.; Matsumoto, J.; Setogawa, T.; Nishijo, H. Neuronal structures controlling locomotor behavior during active and inactive motor states. Neurosci. Res. 2023, 189, 83–93. [Google Scholar] [CrossRef]
- Vitorio, R.; Stuart, S.; Gobbi, L.T.B.; Rochester, L.; Alcock, L.; Pantall, A. Reduced Gait Variability and Enhanced Brain Activity in Older Adults with Auditory Cues: A Functional Near-Infrared Spectroscopy Study. Neurorehabil. Neural Repair 2018, 32, 976–987. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I. Effects of Rhythmic Auditory Cueing in Gait Rehabilitation for Multiple Sclerosis: A Mini Systematic Review and Meta-Analysis. Front. Neurol. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Almarwani, M.; Van Swearingen, J.M.; Perera, S.; Sparto, P.J.; Brach, J.S. The Effect of Auditory Cueing on the Spatial and Temporal Gait Coordination in Healthy Adults. J. Mot. Behav. 2019, 51, 25–31. [Google Scholar] [CrossRef]
- Lim, S.B.; Yang, C.L.; Peters, S.; Liu-Ambrose, T.; Boyd, L.A.; Eng, J.J. Phase-dependent Brain Activation of the Frontal and Parietal Regions during Walking After Stroke—An fNIRS Study. Front. Neurol. 2022, 13, 904722. [Google Scholar] [CrossRef]
- Schaefer, R.S. Auditory rhythmic cueing in movement rehabilitation: Findings and possible mechanisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130402. [Google Scholar] [CrossRef]
- Travis, F. Temporal and spatial characteristics of meditation EEG. Psychol. Trauma Theory Res. Pract. Policy 2020, 12, 111–115. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Arguissain, F.G.; Andersen, O.K. Cognitive processing for step precision increases beta and gamma band modulation during overground walking. Brain Topogr. 2018, 31, 661–671. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Yanagisawa, T.; Shin, D.; Kambara, H.; Yoshimura, N.; Tanaka, M.; Fukuma, R.; Kishima, H.; Hirata, M.; Koike, Y. Mapping ECoG channel contributions to trajectory and muscle activity prediction in human sensorimotor cortex. Sci. Rep. 2017, 7, 45486. [Google Scholar] [CrossRef]
- Presacco, A.; Forrester, L.W.; Contreras-Vidal, J.L. Decoding intra-limb and inter-limb kinematics during treadmill walking from scalp electroencephalographic (EEG) signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 212–219. [Google Scholar] [CrossRef]
- Presacco, A.; Goodman, R.; Forrester, L.; Contreras-Vidal, J.L. Neural decoding of treadmill walking from noninvasive electroencephalographic signals. J. Neurophysiol. 2011, 106, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Kaneko, N.; Ogawa, T.; Kawashima, N.; Watanabe, K.; Nakazawa, K. Cortical correlates of locomotor muscle synergy activation in humans: An electroencephalographic decoding study. iScience 2019, 15, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Bulea, T.C.; Prasad, S.; Kilicarslan, A.; Contreras-Vidal, J.L. Sitting and standing intention can be decoded from scalp EEG recorded prior to movement execution. Front. Neurosci. 2014, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Vidal, J.L.; Bortole, M.; Zhu, F.; Nathan, K.; Venkatakrishnan, A.; Francisco, G.E.; Soto, R.; Pons, J.L. Neural decoding of robot-assisted gait during rehabilitation after stroke. Am. J. Phys. Med. Rehabil. 2018, 97, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Removal of movement artifact from high-density EEG recorded during walking and running. J. Neurophysiol. 2010, 103, 3526–3534. [Google Scholar] [CrossRef]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage 2011, 54, 1289–1296. [Google Scholar] [CrossRef]
- Seeber, M.; Scherer, R.; Wagner, J.; Solis-Escalante, T.; Müller-Putz, G.R. High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. Neuroimage 2015, 112, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Roeder, L.; Boonstra, T.W.; Smith, S.S.; Kerr, G.K. Dynamics of corticospinal motor control during overground and treadmill walking in humans. J. Neurophysiol. 2018, 120, 1017–1031. [Google Scholar] [CrossRef]
- Mate, K.K. Effects of Visual, Auditory, and Combined Cues on Human Movement and Brain Regions Involved in Perception Action. McGill J. Med. 2022, 20, 38. [Google Scholar] [CrossRef]
- Bulea, T.C.; Kim, J.; Damiano, D.L.; Stanley, C.J.; Park, H.-S. User-driven control increases cortical activity during treadmill walking: An EEG study. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2111–2114. [Google Scholar]
- Michel, C.M. Chapter 12—High-resolution EEG. In Handbook of Clinical Neurology; Levin, K.H., Chauvel, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 185–201. [Google Scholar]
- Knutson, G.A. Anatomic and functional leg-length inequality: A review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: Prevalence, magnitude, effects and clinical significance. Chiropr. Osteopat. 2005, 13, 11. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kaneko, N.; Masugi, Y.; Ogawa, T.; Watanabe, K.; Nakazawa, K. Gait-phase-dependent and gait-phase-independent cortical activity across multiple regions involved in voluntary gait modifications in humans. Eur. J. Neurosci. 2021, 54, 8092–8105. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.E.; Huang, H.J.; Snyder, K.L.; Ferris, D.P. Isolating gait-related movement artifacts in electroencephalography during human walking. J. Neural Eng. 2015, 12, 046022. [Google Scholar] [CrossRef]
- Nathan, K.; Contreras-Vidal, J.L. Negligible motion artifacts in scalp electroencephalography (EEG) during treadmill walking. Front. Hum. Neurosci. 2016, 9, 708. [Google Scholar] [CrossRef]
- Sabatini, A.M. Quaternion-based extended Kalman filter for determining orientation by inertial and magnetic sensing. IEEE Trans. Biomed. Eng. 2006, 53, 1346–1356. [Google Scholar] [CrossRef]
- Frølich, L.; Dowding, I. Removal of muscular artifacts in EEG signals: A comparison of linear decomposition methods. Brain Inform. 2018, 5, 13–22. [Google Scholar] [CrossRef]
- Gorjan, D.; Gramann, K.; De Pauw, K.; Marusic, U. Removal of movement-induced EEG artifacts: Current state of the art and guidelines. J. Neural Eng. 2022, 19, 011004. [Google Scholar] [CrossRef] [PubMed]
- Bist, K. Artifact Removal in EEG Using Fast ICA; Mondragon Unibertsitatea: Mondragón, Spain, 2015. [Google Scholar] [CrossRef]
- Jacquelin Perry, M. Gait Analysis: Normal and Pathological Function; SLACK: Haddonfield, NJ, USA, 2010. [Google Scholar]
- Abhayasinghe, N.; Murray, I. Human gait phagse recognition based on thigh movement computed using IMUs. In Proceedings of the 2014 IEEE Ninth International Conference on Intelligent Sensors, Sensor Networks and Information Processing (ISSNIP), Singapore, 21–24 April 2014; pp. 1–4. [Google Scholar]
- Semaan, M.B.; Wallard, L.; Ruiz, V.; Gillet, C.; Leteneur, S.; Simoneau-Buessinger, E. Is treadmill walking biomechanically comparable to overground walking? A systematic review. Gait Posture 2022, 92, 249–257. [Google Scholar] [CrossRef]
- do Nascimento, O.F.; Nielsen, K.D.; Voigt, M. Influence of directional orientations during gait initiation and stepping on movement-related cortical potentials. Behav. Brain Res. 2005, 161, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.P.; Nakagome, S.; He, Y.; Contreras-Vidal, J.L. Real-time EEG-based brain-computer interface to a virtual avatar enhances cortical involvement in human treadmill walking. Sci. Rep. 2017, 7, 8895. [Google Scholar] [CrossRef]
- Teitti, S.; Määttä, S.; Säisänen, L.; Könönen, M.; Vanninen, R.; Hannula, H.; Mervaala, E.; Karhu, J. Non-primary motor areas in the human frontal lobe are connected directly to hand muscles. Neuroimage 2008, 40, 1243–1250. [Google Scholar] [CrossRef]
- Praamstra, P.; Oostenveld, R. Attention and movement-related motor cortex activation: A high-density EEG study of spatial stimulus–response compatibility. Cogn. Brain Res. 2003, 16, 309–322. [Google Scholar] [CrossRef]
- Tamás, G.; Chirumamilla, V.C.; Anwar, A.R.; Raethjen, J.; Deuschl, G.; Groppa, S.; Muthuraman, M. Primary sensorimotor cortex drives the common cortical network for gamma synchronization in voluntary hand movements. Front. Hum. Neurosci. 2018, 12, 130. [Google Scholar] [CrossRef]
- Rosso, M.; Leman, M.; Moumdjian, L. Neural entrainment meets behavior: The stability index as a neural outcome measure of auditory-motor coupling. Front. Human. Neurosci. 2021, 15, 668918. [Google Scholar] [CrossRef] [PubMed]
- Nenna, F.; Do, C.-T.; Protzak, J.; Gramann, K. Alteration of brain dynamics during natural dual-task walking. bioRxiv 2020. [Google Scholar] [CrossRef]
- Morand, S.M.; Harvey, M.; Grosbras, M.-H. Parieto-occipital cortex shows early target selection to faces in a reflexive orienting task. Cereb. Cortex 2014, 24, 898–907. [Google Scholar] [CrossRef]
- Pasalar, S.; Ro, T.; Beauchamp, M.S. TMS of posterior parietal cortex disrupts visual tactile multisensory integration. Eur. J. Neurosci. 2010, 31, 1783–1790. [Google Scholar] [CrossRef]
- Vandenberghe, R.; Gillebert, C.R. Parcellation of parietal cortex: Convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behav. Brain Res. 2009, 199, 171–182. [Google Scholar] [CrossRef]
- Bradford, J.C.; Lukos, J.R.; Ferris, D.P. Electrocortical activity distinguishes between uphill and level walking in humans. J. Neurophysiol. 2016, 115, 958–966. [Google Scholar] [CrossRef]
- Bulea, T.C.; Kim, J.; Damiano, D.L.; Stanley, C.J.; Park, H.-S. Prefrontal, posterior parietal and sensorimotor network activity underlying speed control during walking. Front. Hum. Neurosci. 2015, 9, 247. [Google Scholar] [CrossRef]
- Gramann, K.; Gwin, J.T.; Bigdely-Shamlo, N.; Ferris, D.P.; Makeig, S. Visual evoked responses during standing and walking. Front. Hum. Neurosci. 2010, 4, 202. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; König, P.; Ferris, D.P. Restricted vision increases sensorimotor cortex involvement in human walking. J. Neurophysiol. 2017, 118, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Sburlea, A.I.; Montesano, L.; de la Cuerda, R.C.; Alguacil Diego, I.M.; Miangolarra-Page, J.C.; Minguez, J. Detecting intention to walk in stroke patients from pre-movement EEG correlates. J. Neuroeng. Rehabil. 2015, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.L.; Kline, J.E.; Huang, H.J.; Ferris, D.P. Independent component analysis of gait-related movement artifact recorded using EEG electrodes during treadmill walking. Front. Hum. Neurosci. 2015, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.; Scherer, R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. Neuroimage 2012, 63, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Weersink, J.B.; Maurits, N.M.; de Jong, B.M. EEG time-frequency analysis provides arguments for arm swing support in human gait control. Gait Posture 2019, 70, 71–78. [Google Scholar] [CrossRef] [PubMed]
| Subject ID | Group | Speed Condition | Subject ID | Group | Speed Condition | ||
|---|---|---|---|---|---|---|---|
| sSC | SC | sSC | SC | ||||
| N1 | T | 105 | 68 | N13 | O | 72 | 69 |
| N2 | T | 119 | 68 | N14 | O | 93 | 68 |
| N3 | T | 129 | 69 | N15 | O | 89 | 67 |
| N4 | T | 113 | 68 | N16 | O | 106 | 69 |
| N5 | T | 76 | 68 | N17 | O | 91 | 69 |
| N6 | T | 103 | 68 | N18 | O | 81 | 69 |
| N7 | T | 110 | 67 | N19 | O | 99 | 68 |
| N8 | T | 124 | 69 | N20 | O | 84 | 67 |
| N9 | T | 134 | 67 | N21 | O | 79 | 67 |
| N10 | T | 119 | 69 | N22 | O | 65 | 68 |
| N11 | T | 137 | 68 | N23 | O | 78 | 68 |
| N12 | T | 131 | 67 | N24 | O | 124 | 68 |
| Average steps (SD) | T | 116.7 (17.0) | 68 (0.7) | Average steps | O | 88.4 (16.0) | 68 (0.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharawadeepimuk, K.; Limroongreungrat, W.; Pilanthananond, M.; Nanbancha, A. Auditory Cue Effects on Gait-Phase-Dependent Electroencephalogram (EEG) Modulations during Overground and Treadmill Walking. Sensors 2024, 24, 1548. https://doi.org/10.3390/s24051548
Tharawadeepimuk K, Limroongreungrat W, Pilanthananond M, Nanbancha A. Auditory Cue Effects on Gait-Phase-Dependent Electroencephalogram (EEG) Modulations during Overground and Treadmill Walking. Sensors. 2024; 24(5):1548. https://doi.org/10.3390/s24051548
Chicago/Turabian StyleTharawadeepimuk, Kittichai, Weerawat Limroongreungrat, Metaneeya Pilanthananond, and Ampika Nanbancha. 2024. "Auditory Cue Effects on Gait-Phase-Dependent Electroencephalogram (EEG) Modulations during Overground and Treadmill Walking" Sensors 24, no. 5: 1548. https://doi.org/10.3390/s24051548
APA StyleTharawadeepimuk, K., Limroongreungrat, W., Pilanthananond, M., & Nanbancha, A. (2024). Auditory Cue Effects on Gait-Phase-Dependent Electroencephalogram (EEG) Modulations during Overground and Treadmill Walking. Sensors, 24(5), 1548. https://doi.org/10.3390/s24051548








