An Ultrasensitive Room-Temperature H2 Sensor Based on a TiO2 Rutile–Anatase Homojunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Sensing Layers
2.1.1. Preparation of Rutile-Phase TiO2 Nanorod Arrays
2.1.2. Preparation of Rutile- and Anatase-Phase TiO2 Homojunctions
2.2. Characterization
3. Results and Discussion
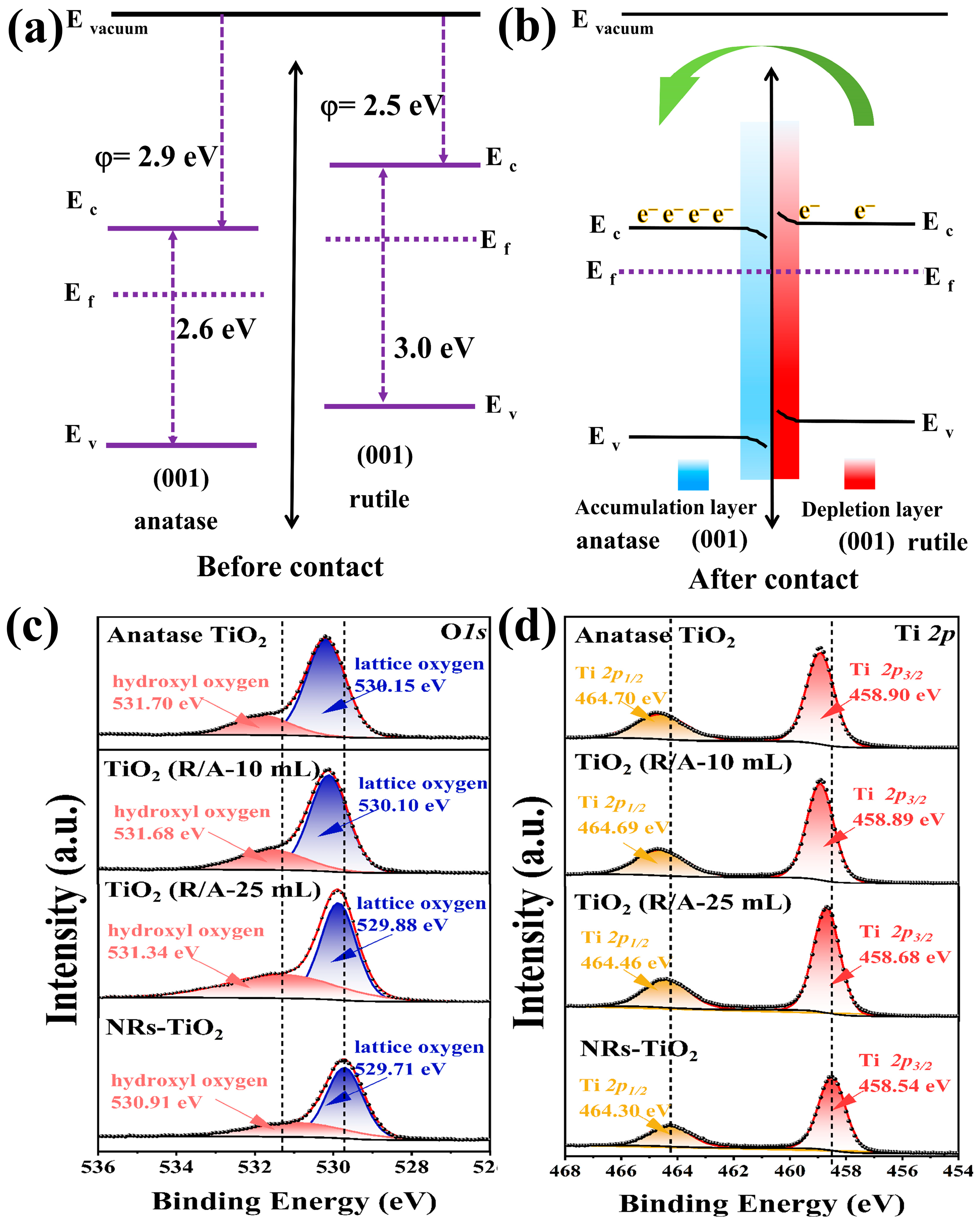
3.1. Characterization of the TiO2(R/A-X) Samples
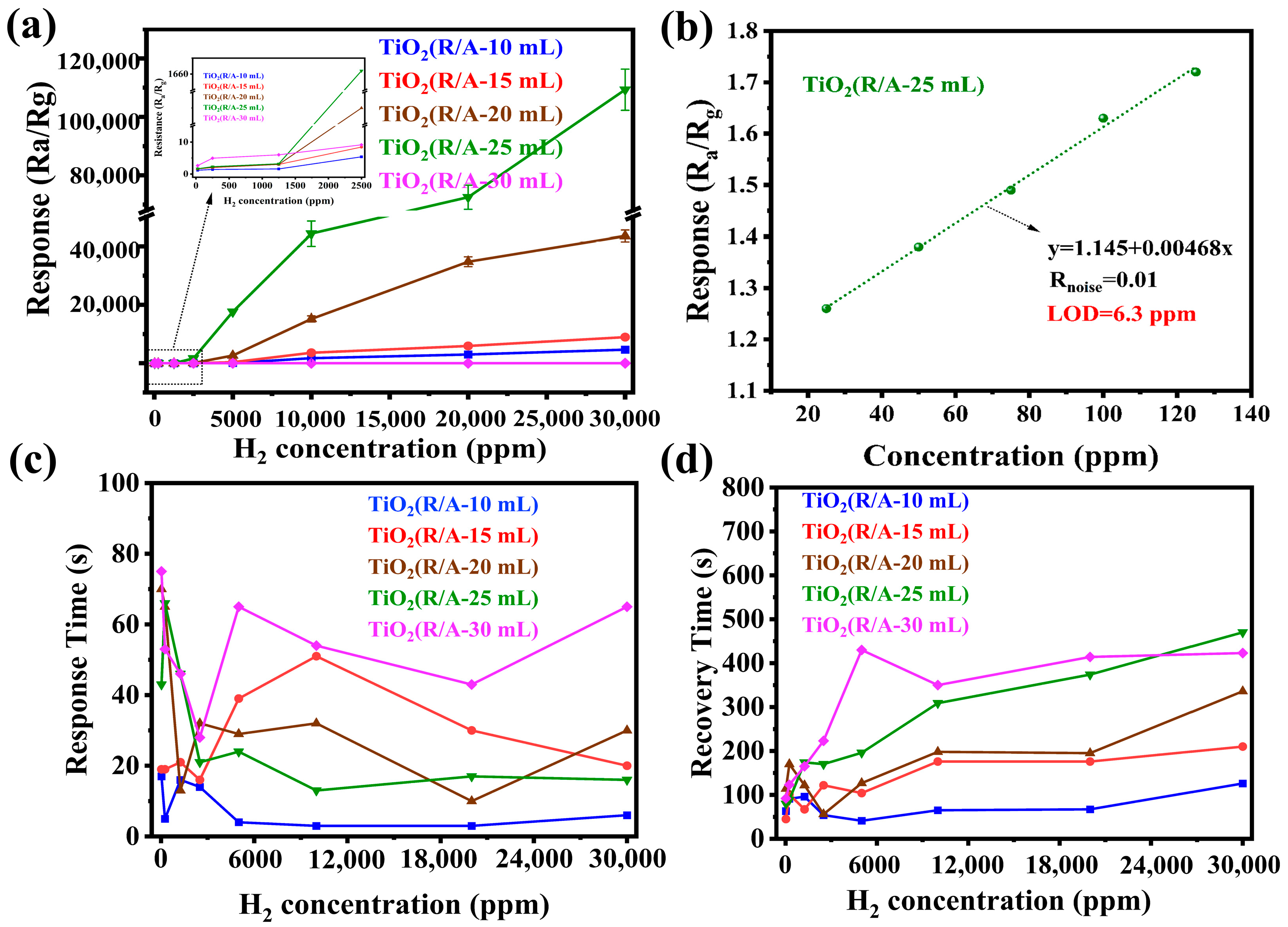
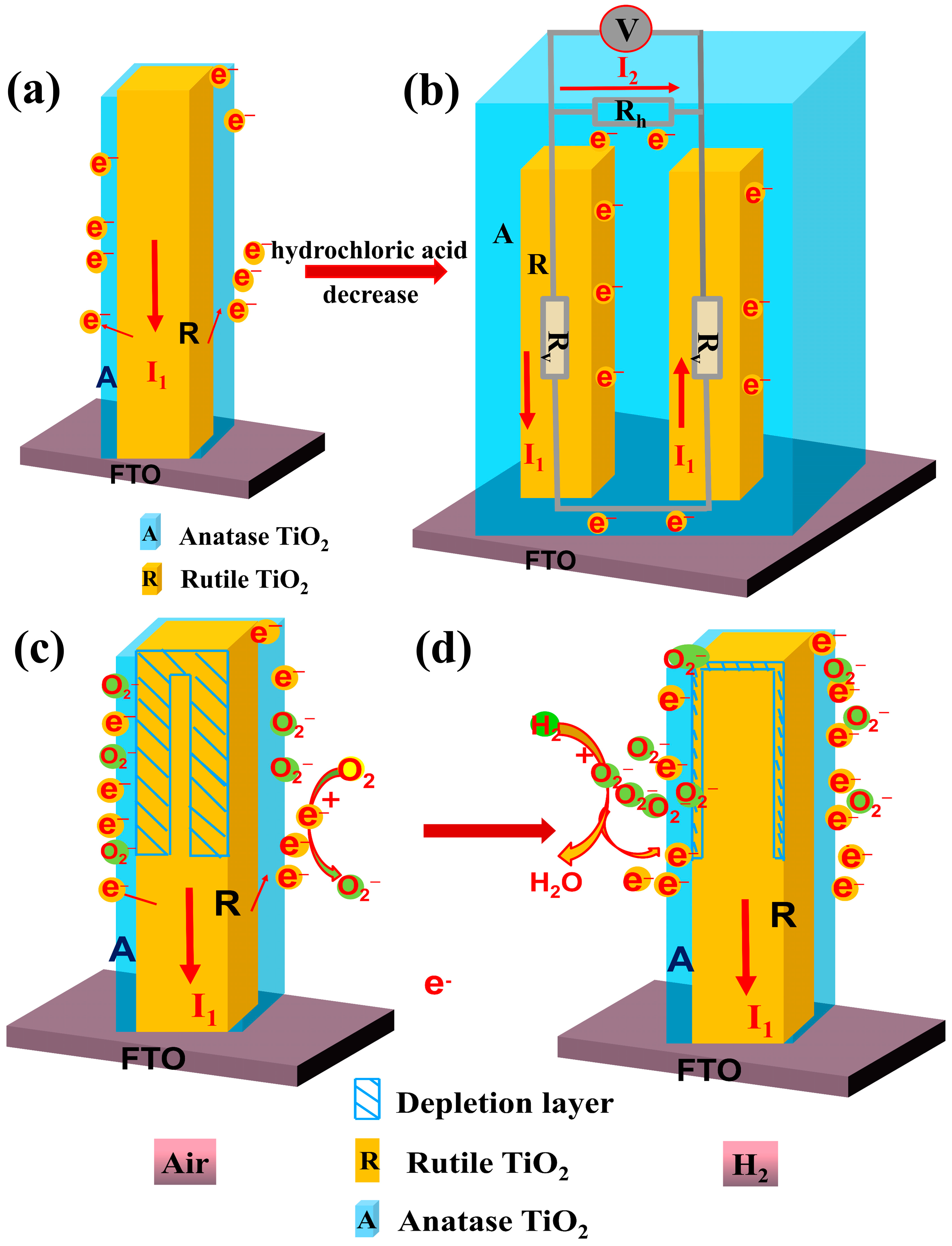
3.2. Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, P.; Duan, Q.; Peng, Q.; Jin, K.; Sun, J. Design of ultrasensitive gas sensor based on self-assembled Pd-SnO2/rGO porous ternary nanocomposites for ppb-level hydrogen. Sens. Actuators B Chem. 2022, 369, 132280. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultra-fast response and highly selectivity hydrogen gas sensor based on Pd/SnO2 nanoparticles. Int. J. Hydrogen Energy 2022, 47, 3157–3169. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Park, J.; Lee, K. MEMS hydrogen gas sensor for in-situ monitoring of hydrogen gas in transformer oil. Sens. Actuators B Chem. 2021, 326, 128989. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, M.S.; Jung, H.; Choe, Y.-S.; Kim, W.; Song, Y.G.; Kang, C.-Y.; Lee, H.-S.; Lee, W. Selective C2H2 detection with high sensitivity using SnO2 nanorod based gas sensors integrated with a gas chromatography. Sens. Actuators B Chem. 2020, 307, 127598. [Google Scholar] [CrossRef]
- Imonigie, J.A.; Walters, R.N.; Gribb, M.M. Rapid Isothermal Gas Chromatography-Mass Spectrometry Method for Validating a Small Ion Mobility Spectrometer Sensor. Instrum. Sci. Technol. 2006, 34, 677–695. [Google Scholar] [CrossRef]
- Wang, X.-d.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2015–2019). Anal. Chem. 2019, 92, 397–430. [Google Scholar] [CrossRef]
- Bakker, E. Electrochemical Sensors. Anal. Chem. 2004, 76, 3285–3298. [Google Scholar] [CrossRef]
- Tonezzer, M.; Dang, L.T.T.; Tran, H.Q.; Iannotta, S. Multiselective visual gas sensor using nickel oxide nanowires as chemiresistor. Sens. Actuators B Chem. 2018, 255, 2785–2793. [Google Scholar] [CrossRef]
- Bao, Y.; Wei, P.; Xia, X.; Huang, Z.; Homewood, K.; Gao, Y. Remarkably enhanced H2 response and detection range in Nb doped rutile/anatase heterophase junction TiO2 thin film hydrogen sensors. Sens. Actuators B Chem. 2019, 301, 127143. [Google Scholar] [CrossRef]
- Xia, X.; Wu, W.; Wang, Z.; Bao, Y.; Huang, Z.; Gao, Y. A hydrogen sensor based on orientation aligned TiO2 thin films with low concentration detecting limit and short response time. Sens. Actuators B Chem. 2016, 234, 192–200. [Google Scholar] [CrossRef]
- Li, Z.; Haidry, A.A.; Gao, B.; Wang, T.; Yao, Z. The effect of Co-doping on the humidity sensing properties of ordered mesoporous TiO2. Appl. Surf. Sci. 2017, 412, 638–647. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, Y.; Hwang, S.; Kim, J.K. Highly-sensitive H2 sensor operating at room temperature using Pt/TiO2 nanoscale Schottky contacts. Sens. Actuators B Chem. 2017, 241, 985–992. [Google Scholar] [CrossRef]
- Han, J.; Zhang, S.; Song, Q.; Yan, H.; Kang, J.; Guo, Y.; Liu, Z. The synergistic effect with S-vacancies and built-in electric field on a TiO2/MoS2 photoanode for enhanced photoelectrochemical performance. Sustain. Energy Fuels 2021, 5, 509–517. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Zhou, Y.; Shen, Q.; Ye, J.; Wu, C.; Zou, Z. Three-dimensional Bi2MoO6/TiO2 array heterojunction photoanode modified with cobalt phosphate cocatalyst for high-efficient photoelectrochemical water oxidation. Catal. Today 2019, 335, 262–268. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Yan, W.; Guo, Z.; Ruan, M. Decorating non-noble metal plasmonic Al on a TiO2/Cu2O photoanode to boost performance in photoelectrochemical water splitting. Chin. J. Catal. 2020, 41, 1884–1893. [Google Scholar] [CrossRef]
- He, Y.; Wang, P.; Zhu, J.; Yang, Y.; Liu, Y.; Chen, M.; Cao, D.; Yan, X. Synergistical Dual Strategies Based on in Situ-Converted Heterojunction and Reduction-Induced Surface Oxygen Vacancy for Enhanced Photoelectrochemical Performance of TiO2. ACS Appl. Mater. Interfaces 2019, 11, 37322–37329. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Gao, X.; Yu, B.; Gao, Y.; Han, Z.-G. TiO2-rutile/anatase homojunction with enhanced charge separation for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 26358–26366. [Google Scholar] [CrossRef]
- Tian, J.; Cao, G. Control of Nanostructures and Interfaces of Metal Oxide Semiconductors for Quantum-Dots-Sensitized Solar Cells. J. Phys. Chem. Lett. 2015, 6, 1859–1869. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Z. Synthesis and control strategies of nanomaterials for photoelectrochemical water splitting. Dalton Trans. 2021, 50, 1983–1989. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, J.; Ruan, M.; Guo, Z. An effective strategy of constructing a multi-junction structure by integrating a heterojunction and a homojunction to promote the charge separation and transfer efficiency of WO3. J. Mater. Chem. A 2020, 8, 6256–6267. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Dong, Z.; Meyuhas, O.; Chen, J.-K. Phosphorylation of ribosomal protein S6 mediates compensatory renal hypertrophy. Kidney Int. 2015, 87, 543–556. [Google Scholar] [CrossRef]
- Wang, F.; Pei, K.; Li, Y.; Li, H.; Zhai, T. 2D Homojunctions for Electronics and Optoelectronics. Adv. Mater. 2021, 33, 2005303. [Google Scholar] [CrossRef]
- Deák, P.; Aradi, B.; Frauenheim, T. Band Lineup and Charge Carrier Separation in Mixed Rutile-Anatase Systems. J. Phys. Chem. C 2011, 115, 3443–3446. [Google Scholar] [CrossRef]
- Mondal, B.; Basumatari, B.; Das, J.; Roychaudhury, C.; Saha, H.; Mukherjee, N. ZnO–SnO2 based composite type gas sensor for selective hydrogen sensing. Sens. Actuators B Chem. 2014, 194, 389–396. [Google Scholar] [CrossRef]
- Yin, X.-T.; Li, J.; Dastan, D.; Zhou, W.-D.; Garmestani, H.; Alamgir, F.M. Ultra-high selectivity of H2 over CO with a p-n nanojunction based gas sensors and its mechanism. Sens. Actuators B Chem. 2020, 319, 128330. [Google Scholar] [CrossRef]
- Wu, C.-H.; Zhu, Z.; Chang, H.-M.; Jiang, Z.-X.; Hsieh, C.-Y.; Wu, R.-J. Pt@NiO core–shell nanostructure for a hydrogen gas sensor. J. Alloys Compd. 2020, 814, 151815. [Google Scholar] [CrossRef]
- Yin, X.-T.; Zhou, W.-D.; Li, J.; Wang, Q.; Wu, F.-Y.; Dastan, D.; Wang, D.; Garmestani, H.; Wang, X.-M.; Ţălu, Ş. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J. Alloys Compd. 2019, 805, 229–236. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sens. Actuators B Chem. 2022, 359, 131612. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, S.; Wu, G.; Jiao, F.; Li, W.; Wang, X.; An, Y.; Hu, Y.; Sun, J.; Dong, X.; et al. Highly sensitive H2 sensor based on PdO-decorated WO3 nanospindle p-n heterostructure. Int. J. Hydrogen Energy 2020, 45, 31327–31340. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.; Sheng, X.; Chen, L.; Xu, T.; Zhu, K.; Feng, X. 100-Fold Enhancement of Charge Transport in Uniaxially Oriented Mesoporous Anatase TiO2 Films. Chem.—A Eur. J. 2017, 24, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Z.; Xia, X.; Shao, G.; Homewood, K.; Gao, Y. Synergistic Cooperation of Rutile TiO2 {002}, {101}, and {110} Facets for Hydrogen Sensing. ACS Appl. Mater. Interfaces 2018, 10, 28199–28209. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Aydil, E. Growth of Oriented Single-Crystalline Rutile TiO2 Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Zhang, M.; Wang, X. Hydrothermal synthesis and characterization of TiO2 nanorod arrays on glass substrates. Mater. Res. Bull. 2009, 44, 1232–1237. [Google Scholar] [CrossRef]
- Sacco, A.; Mandrile, L.; Tay, L.-L.; Itoh, N.; Raj, A.; Moure, A.; Del Campo, A.; Fernandez, J.F.; Paton, K.R.; Wood, S.; et al. Quantification of titanium dioxide (TiO2) anatase and rutile polymorphs in binary mixtures by Raman spectroscopy: An interlaboratory comparison. Metrologia 2023, 60, 055011. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Li, X.; He, H.; Tan, T.; Zou, Z.; Tian, Z.; Zhou, W.; Bao, Y.; Xia, X.; Gao, Y. Annealing effect on the methane sensing performance of Pt–SnO2/ZnO double layer sensor. Appl. Surf. Sci. 2023, 640, 158428. [Google Scholar] [CrossRef]
- Nasralla, N.H.S.; Yeganeh, M.; Astuti, Y.; Piticharoenphun, S.; Šiller, L. Systematic study of electronic properties of Fe-doped TiO2 nanoparticles by X-ray photoemission spectroscopy. J. Mater. Sci. Mater. Electron. 2018, 29, 17956–17966. [Google Scholar] [CrossRef]
- Liu, J.; He, L.; Dong, F.; Hudson-Edwards, K.A. The role of nano-sized manganese coatings on bone char in removing arsenic(V) from solution: Implications for permeable reactive barrier technologies. Chemosphere 2016, 153, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Schierbaum, K.; Kirner, U.; Kirner, J.; Gdrel, W. Schottky-barrier and conductivity gas sensors based upon Pd/SnO2 and Pt/TiO2. Sens. Actuators B Chem. 1991, 4, 87–94. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Bao, Y.; Xia, X.; Tao, T.; Homewood, K.P.; Li, R.; Gao, Y. Comprehensively improved hydrogen sensing performance via constructing the facets homojunction in rutile TiO2 hierarchical structure. Sens. Actuators B Chem. 2022, 350, 130869. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, L.; Zhang, Y.; Wu, X.; Zhang, M.; Liang, J.; Bao, Y.; Xia, X.; Gu, H.; Homewood, K.; et al. Homojunction TiO2 thin film-based room-temperature working H2 sensors with non-noble metal electrodes. Sens. Actuators B Chem. 2024, 398, 134675. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, H.W.; Kim, S.S. Grain-Size-Tuned Highly H2-Selective Chemiresistive Sensors Based on ZnO–SnO2 Composite Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 2486–2494. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhang, Y.; Zhang, M.; Liang, J.; Bao, Y.; Xia, X.; Homewood, K.; Lourenco, M.; Gao, Y. An Ultrasensitive Room-Temperature H2 Sensor Based on a TiO2 Rutile–Anatase Homojunction. Sensors 2024, 24, 978. https://doi.org/10.3390/s24030978
Wu X, Zhang Y, Zhang M, Liang J, Bao Y, Xia X, Homewood K, Lourenco M, Gao Y. An Ultrasensitive Room-Temperature H2 Sensor Based on a TiO2 Rutile–Anatase Homojunction. Sensors. 2024; 24(3):978. https://doi.org/10.3390/s24030978
Chicago/Turabian StyleWu, Xuefeng, Ya Zhang, Menghan Zhang, Jianhu Liang, Yuwen Bao, Xiaohong Xia, Kevin Homewood, Manon Lourenco, and Yun Gao. 2024. "An Ultrasensitive Room-Temperature H2 Sensor Based on a TiO2 Rutile–Anatase Homojunction" Sensors 24, no. 3: 978. https://doi.org/10.3390/s24030978
APA StyleWu, X., Zhang, Y., Zhang, M., Liang, J., Bao, Y., Xia, X., Homewood, K., Lourenco, M., & Gao, Y. (2024). An Ultrasensitive Room-Temperature H2 Sensor Based on a TiO2 Rutile–Anatase Homojunction. Sensors, 24(3), 978. https://doi.org/10.3390/s24030978






