1. Introduction
Video-based eye trackers determine gaze direction and ocular movements with high precision by measuring the relative position of the corneal reflection in relation to the pupil center [
1]. Available in both screen-based and head-mounted configurations, these eye trackers enable a wide range of experimental applications, such as in visual attention studies [
2], cognitive load assessment [
3], and applications in virtual and augmented reality [
4,
5]. The leading eye-tracking systems operate at high frequencies—up to 1000 Hz—making them the preferred choice for detailed eye movement analysis [
6]. With technological advancements, there is a growing interest in supplementing vergence measurements with simultaneous measurements of eye accommodation. Real-time data from these combined measurements can provide valuable insights into how visual processes adapt to different viewing conditions with various displays, as highlighted in studies investigating eye movements in headset-based digital environments [
4,
7,
8].
The video-based eccentric photorefractometry device PowerRef 3 (PlusOptix, Nuremberg, Germany), primarily designed to study dynamic eye accommodation, operates at a 50 Hz sampling rate and can be used for dynamic, simultaneous, non-invasive, and repeatable objective measurements of eye refraction, accommodation, gaze position, and pupil size, allowing the assessment of the near reflex triad. While a 50 Hz sampling rate does not sufficiently fulfill the required speed for detailed eye movement studies [
9], it remains the most promising commercially available device for researchers interested in real-time, simultaneous accommodation and vergence responses. Common vision research applications that employ PowerRef 3 include exploring infant visual system development [
10], investigating accommodation and binocular function disorders [
11,
12,
13], and assessing the effect of novel display technologies on the visual system [
14,
15].
The accuracy of the measured refraction and accommodation response in devices using eccentric photorefractometry depends on the calibration of the luminance slope formed across the pupil and the defocus calibration factor used to convert the luminance slope into diopters. The PowerRef 3 device uses a universal (population average) defocus calibration factor. Previous studies have highlighted that individual differences in pupil size, refractive error of the eye, and reflectance properties of the retina may, in certain cases, contribute to variations in the individual calibration factor, resulting in deviations from the population average [
16,
17]. Sravani et al. [
18] demonstrated that the accuracy of refraction measurements using photorefraction with a universal calibration factor varies significantly across people with different ethnic origins, suggesting that individual defocus calibration factors are crucial for accurate refraction estimation. The calibration of refraction and accommodation data to obtain an individual defocus calibration factor is performed using an infrared filter and trial lenses [
16,
17,
18]. Previous studies have highlighted that the range of lenses used for individual calibration significantly influences the variability of calibration estimates, where protocols incorporating both positive and negative trial lenses provide the best estimate of the calibration factor [
16]. Additionally, it has been suggested to scale the output data according to the magnitude of the induced magnification or minification when using positive and negative trial lenses during the calibration process or when performing any experimental design using ophthalmic lenses [
19]. While the accuracy of the measured refraction in video-based eccentric photorefractometry devices has been thoroughly described in the scientific literature [
16,
17,
18,
19], the accuracy of vergence measurements has received considerably less attention. To determine the gaze position in degrees, PowerRef 3 employs a calculation method that multiplies the decentration distance between the first Purkinje image and the center of the pupil by the Hirschberg ratio. This calculation utilizes a population-averaged Hirschberg ratio of 11.82°/mm [personal communication]. However, using the population-averaged Hirschberg ratio is susceptible to inaccuracies due to the inter-subject variability of the Hirschberg ratio, which can vary between 7 and 16°/mm [
20,
21]. Ntodie et al. [
21] suggested three different techniques for calculating the Hirschberg ratio: eccentric viewing, theoretical, and prism-based techniques. Their results showed that the eccentric viewing and the theoretical techniques have good repeatability, with respective values of ±0.4°/mm and ±0.3°/mm. However, even after applying the individual Hirschberg ratio, vergence data obtained from Purkinje image eye trackers show an offset between the obtained and expected physiological vergence angles. In humans, the pupillary and visual axes do not align, forming an angle known as the kappa angle, which plays an important role when interpreting the obtained vergence angle. Conventional eye trackers employing calibration procedures account for the kappa angle in gaze measurements [
22], whereas Purkinje image eye trackers, such as the PowerRef 3, do not incorporate individual calibration for gaze measurements.
Most individuals have a positive kappa angle, where the fovea is positioned temporally relative to the pupillary axis [
23]. When the pupillary axes are parallel (as expected during viewing at infinity), the visual axes converge by the magnitude of the positive kappa angle. As a result, for a positive kappa angle, the physiological vergence angle is typically larger than the visual angle observed and measured using first Purkinje image-based eye trackers. The kappa angle varies among individuals, with an average value of approximately 5° [
24]. The individual kappa angle is affected by various factors:
Type of refractive error: In hyperopic eyes, the kappa angle is larger compared to myopic eyes [
25].
Age: Kappa angle decreases by 0.015° per year [
26].
Measurement method: The principle of the measuring device significantly affects the measured kappa angle [
24,
25,
27].
Eye laterality: Previous studies have observed a larger kappa angle in the left eye [
25,
28].
Therefore, because devices such as PowerRef 3 lack individual calibration for gaze measurements, previous studies have been limited to assessing relative changes in vergence rather than the absolute vergence status [
12,
29]. This limitation causes the measured vergence angles to appear divergent from the target, even in binocularly aligned conditions at near distances, where convergence is expected, thereby hindering the evaluation of physiological vergence at the individual level. Moreover, the expected vergence angle, defined as ideal gaze alignment at the corresponding stimulus distance, is rarely achieved physiologically due to inherent visual system limitations, such as vergence error, requiring a more complex correction methodology than simple linear regression.
The absence of a methodology to address this issue has led researchers to focus on assessing relative changes or changes at the group level, hindering the ability to evaluate the physiological vergence angle at the individual level. To date, a universal method for determining physiological vergence angles from the visible vergence angle provided by the first Purkinje image eye trackers has not been described. Recently, Liu et al. [
22] suggested the use of an automated algorithm based on the initial assumption of a theoretical kappa angle of 5° with a promising but rather complex algorithm verification procedure that demonstrated a gaze accuracy of 1.30° in the horizontal plane and 1.34° in the vertical plane. Another approach was proposed by Kooijman et al. [
29], in which an 11.2° offset to the visual vergence angle at a distance of 1.8 m was used, assuming a population-averaged interpupillary distance of 63 mm; however, this offset value should be modified if a distance other than 1.8 m is used. Thus, the purpose of our study was to propose and compare new approaches for calculating the physiological vergence angle using data obtained with the first Purkinje image-based eye trackers.
3. Results
Our results demonstrate that the visual vergence data obtained with PowerRef 3 measured significantly more divergent gaze positions than the expected vergence angle at distances from 25 cm to 6 m (see
Table 1). The average difference between visual vergence data and the expected vergence angle at each distance was as follows: 9.64 ± 2.73° at 25 cm distance with a 95% confidence interval of 8.45° to 10.82° (t(22) = 16.91,
p < 0.01); 9.81 ± 2.63° at 30 cm distance with a 95% confidence interval of 8.68° to 10.95° (t(22) = 17.91
p < 0.01); 9.87 ± 2.60° at 50 cm distance with a 95% confidence interval of 8.74° to 10.99° (t(22) = 18.20,
p < 0.01); 9.49 ± 2.80° at 70 cm distance with a 95% confidence interval of 8.28° to 10.70° (t(22) = 16.24,
p < 0.01); and 9.25 ± 3.52° at 6 m distance with a 95% confidence interval of 7.72° to 10.77° (t(22) = 12.60,
p < 0.01). This discrepancy significantly differs from the expected physiological vergence angle and highlights the importance of performing additional calculations using visual data to accurately interpret the vergence results obtained with the first Purkinje image-based eye trackers.
The average kappa angle of the right eye (0.32 ± 0.16 mm or 2.51 ± 1.29°) was significantly smaller than the average kappa angle of the left eye (0.42 ± 0.16 mm or 3.31 ± 1.31°) (t(22) = −3.48, p < 0.01). The average difference in kappa angle between the right and left eye was −0.80 ± 1.10°, with a 95% confidence interval of −1.28° to −0.32°.
At all distances, both methods yielded results that were significantly different from the expected vergence angle: at 25 cm (F(94.75, 2) = 103.65, p < 0.01), at 30 cm (F(94.69, 2) = 89.94, p < 0.01), at 50 cm (F(140.31, 2) = 90.12, p < 0.01), at 70 cm (F(105.35, 2) = 76.17, p < 0.01), and at 600 cm (F(100.13, 2) = 97.88, p < 0.01). The amount by which the kappa angle results deviate from the EVA remains relatively consistent across various distances, whereas, for the linear regression method, the difference is distance-dependent. For the kappa calibration method, the mean difference between the EVA was 3.96 ± 0.31° at 25 cm distance, 3.84 ± 0.29° at 30 cm, 3.96 ± 0.21° at 50 cm, 3.62 ± 0.24° at 70 cm, and 3.70 ± 0.36° at 600 cm. For the linear regression method, the mean difference between the EVA progressively decreases with increasing distance: 3.30 ± 0.25° at 25 cm distance, 2.75 ± 0.23° at 30 cm, 2.00 ± 0.20° at 50 cm, 1.46 ± 0.19° at 70 cm, and 0.26 ± 0.01° at 600 cm.
To compare the results of the total physiological vergence angle obtained with the kappa and linear regression methods at all distances, repeated measures ANOVA was conducted. Mauchly’s test of sphericity indicated that the assumption of sphericity was violated for the interaction effect of distance χ2(9) = 128.16, p < 0.01. Therefore, the degrees of freedom were corrected using the Greenhouse–Geisser estimate of sphericity (ε = 0.46). The main effect of distance was significant, F(52.99, 1.86) = 28.54, p < 0.01, indicating that the physiological vergence angles varied between distances, regardless of the method, as expected. At the closest distance (25 cm), both methods provided similar results (F(3.43, 1) = 5.07, p = 0.07), while for farther distances, there was a statistically significant difference between the two methods, where the total physiological angle with both methods was smaller (more divergent) than the EVA, and the linear regression method gave a slightly larger (more convergent) angle compared to the kappa angle method; therefore, the angle was closer to the expected vergence angle. At a 30 cm distance, the difference between both methods was −1.09 ± 1.09° (F(10.35, 1) = 13.55, p < 0.01), 50 cm −1.96 ± 1.01° (F(48.10, 1) = 44.30, p < 0.01), 70 cm −2.16 ± 1.01° (F(50.35, 1) = 53.51, p < 0.01), and 600 cm −3.43 ± 1.72° (F(92.54, 1) = 135.66, p < 0.01).
To determine whether the difference between the expected vergence angle and the total physiological vergence angle could be attributed to independent variables, we tested the subjective fixation disparity (measured with a Saladin card at a 40 cm distance (Bernell Corporation, Mishawaka, Indiana, USA)) by plotting individual differences at measured distances (25 cm, 30 cm, 50 cm, and 70 cm) and extrapolating the difference at 40 cm using a regression slope, as this distance was not directly measured, while also considering the potential influence of the kappa angle. For the linear regression calibration method, the ANOVA test showed no significant effect of the independent variables on the difference from the EVA (F(2, 13) = 1.04, p = 0.38), with neither the fixation disparity (t = 1.32, p = 0.21) nor the kappa angle (t = −0.58, p = 0.57) being significant contributors. For the kappa calibration method, the fixation disparity was used as a variable and showed no significant effect (F(1, 14) = 3.38, p = 0.09).
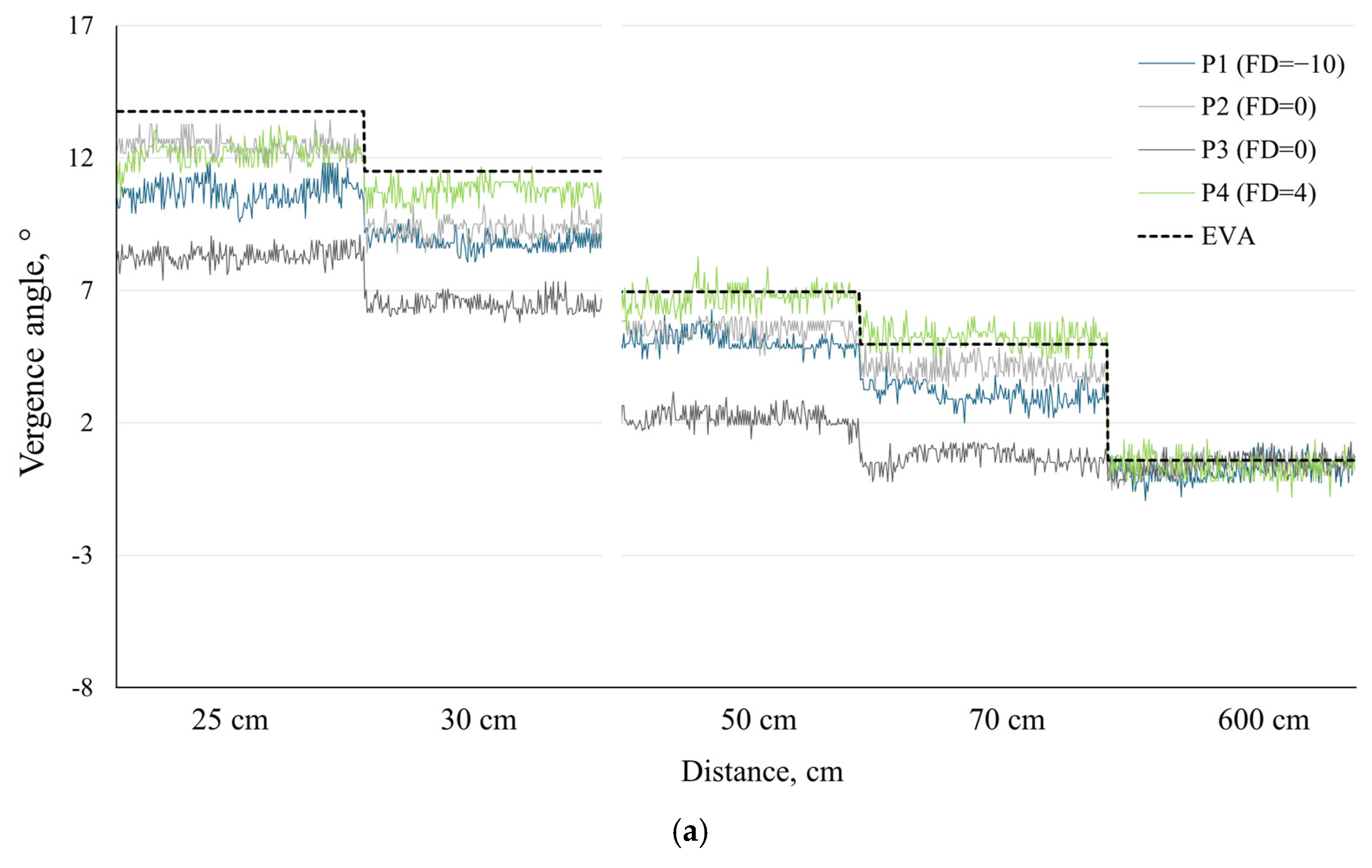
To illustrate and demonstrate the potential applications of our proposed methods, we chose to present individual data for four participants from our sample: P1 and P4, who had the highest exo and eso fixation disparities, respectively, and two participants both with zero fixation disparity—one exhibiting the largest and the other the smallest deviations from the expected vergence angle (
EVA) among our participants (see
Figure 2).
Figure 2a,b present the
TPVA results for each participant using the linear regression method and kappa angle calibration method, respectively, at five different distances.
4. Discussion
The simultaneous evaluation of dynamic accommodation and vergence responses, permitted by eccentric photorefractometry, is of high interest in vision research, offering valuable insights into real-time ocular behavior under diverse experimental conditions and designs. Our study has demonstrated that calibration calculations are essential for determining the physiological vergence angle from PowerRef 3 measurements, as the recorded visual vergence data output by the first Purkinje image-based eye-tracking principle provides a visual vergence angle that is substantially more divergent than the expected values at corresponding distances.
Both proposed methods are influenced by different parameters. For the kappa angle calibration method, the primary influencing factors are the decentration value obtained with the PowerRef 3 and the assumed location of the nodal point. Given the substantial variability in the results produced by different devices measuring the kappa angle [
25,
27], one of the limitations of the kappa angle calibration method is that it is directly influenced by the specific technique used for kappa angle measurement. For example, Domínguez-Vicent et al. [
27] evaluated the difference in kappa angle results between Orbscan II and Galilei G4 devices and found that the kappa angle difference was 0.16 ± 0.08 mm. A change of ±0.16 mm in horizontal decentration measured with PowerRef3 would lead to a corresponding 1.27 ± 0.003° change in the kappa angle of each eye, which would result in a total change of 2.54 ± 0.01° in the total physiological vergence angle (
TPVA). Since significant differences (up to 3.6°) [
27] in the kappa angle can be caused by the differences between measuring devices, and to our knowledge there is no previous research comparing the kappa angle results obtained from PowerRef 3 and other devices, this may explain the discrepancy between the kappa angle calibration method and the expected vergence angle (
EVA), as the obtained result differences ranged from 3.62 to 3.96°. For kappa angle measurements derived from the distance between the first Purkinje image and the pupil center, high repeatability is expected (SD = 0.04 mm over 10 trials) [
34]. However, when using devices with different measurement principles, it is advisable to take multiple kappa angle measurements and use their average to improve accuracy [
24,
26]. The calculations using the kappa angle calibration method are influenced by how the stimulus is centered relative to the participant, which was ensured by using a sliding rail aligned with the midline of the participant’s head. Additionally, the kappa angle value calculated at a far distance was applied to the total physiological vergence angle (
TPVA) calculation at near distances, as recent studies have shown no significant variation in kappa angle across varying distances [
27]. Consequently, this could clarify why the differences between the
TPVA and
EVA results remained constant across different distances. Stability in the stimulus setup, including a forehead rest, is critical, especially at closer distances where small shifts (e.g., 1 cm at 25 cm) can alter the vergence angle by ~0.5°.
In the linear regression method, the results are affected by the individual
ACD, whereby an increase in the
ACD results in a reduction in the expected vergence angle, with a decrease of approximately 0.03° for every 0.5 mm increase in the
ACD at a 25 cm distance. The
ACD affected the results less at distances beyond 25 cm, but the
TPVA calculated with the linear regression method increases with a larger
ACD. Therefore, if the
ACD of the participant is larger than the assumed value of 3 mm, the resulting vergence angle is expected to be closer to the
EVA. Conversely, if the
ACD is less than 3 mm, the vergence angle will deviate further from the
EVA. Given that
ACD values vary individually, typically ranging between 2.80 and 3.75 mm [
35], when evaluating the difference in
TPVA at the typical minimum and maximum
ACD values, the result would be 0.9° closer to the
EVA when the
ACD = 3.75 mm compared to the
ACD = 2.8 mm. It should be noted that the far
IPD value used for the expected interpupillary distance calculations in the linear regression method is obtained at a far distance using PowerRef 3. Based on this far-distance
IPD, the expected
IPD at closer distances in the pupil plane is theoretically calculated. Although
IPD is a variable in the linear regression method, the calculated
IPD and the
IPD obtained from the PowerRef 3 did not differ significantly (F(0.52, 1) = 12.74,
p = 0.47). Therefore, although
IPD may not be a primary contributing factor to the differences between the methods, slight variations still exist. Specifically, if the far-distance
IPD is smaller, the expected
IPD value difference across various distances changes slightly less, and participants with smaller far
IPD values tend to have a lower intercept value than those with larger far
IPD values.
Both methods show a slight underestimation of the vergence angle, which reflects under-convergence, as the
TPVA is smaller than the
EVA. A binocular calibration will not detect this physiological condition, as it assumes the absence of a fixation disparity—where the two lines of sight cross precisely on the fixation target [
36]. The average objective fixation disparity has been estimated to rarely exceed 30 arcmin, when measured subjectively and converted to degrees that do not exceed 0.5°, and up to 60 arcmin (1°) or more when measured objectively [
36,
37]. Under high vergence demands or visual strain, vergence errors can reach 2° [
38]. However, in this study, the difference between the
TPVA and expected vergence angles was 3.30 ± 0.86° at 25 cm, 2.75 ± 0.77° at 30 cm, 2.00 ± 0.83° at 50 cm, and 1.46 ± 0.85° at 70 cm using the linear regression method and 3.93 ± 1.19° at 25 cm, 3.84 ± 1.12° at 30 cm, 3.96 ± 0.89° at 50 cm, and 3.62 ± 1.08° at 70 cm using the kappa angle calibration method. While it was anticipated that the obtained total physiological vergence angle (
TPVA) would not perfectly align with the expected angle due to various physiological factors, it is important to note that the observed difference exceeds what could be attributed to the fixation disparity alone. Examining the results, the kappa angle calibration method induces a divergent eye position (−3.10 ± 1.71°) at a 6 m distance, and the difference between the kappa angle calibration method and the expected vergence angle (
EVA) remains relatively consistent at closer distances, while also demonstrating greater variability in the
TPVA data (see
Figure 2b) compared to the
TPVA obtained using the linear regression calibration method (see
Figure 2a). This may indicate a consistent underestimation of the kappa angle, which, in this case, was based on the measurements provided by the PowerRef 3. In contrast, with the linear regression method, the difference between the
TPVA and
EVA was not constant across all distances—it decreased for farther stimuli. The difference between both methods at the closest distance was small (−0.65 ± 1.18° at 25 cm), while at a distance of 6 m, the difference increased to −3.43 ± 1.72°, making the
TPVA obtained with the linear regression method closer to the
EVA and therefore more precise at farther distances.
This study has certain limitations that should be acknowledged. Firstly, although the experimental setup was well centered, it did not fully control minor head movements, as the head of the participant was on a chin and head rest rather than in a fully fixed position. Secondly, the kappa angle was obtained from PowerRef 3 data, and to the best of our knowledge, no study has compared these results with those from more commonly used measurement techniques, thus leaving open the possibility that the kappa angle may have been underestimated. Furthermore, applying a single kappa angle value obtained at a far distance to near-distance calculations may introduce inaccuracies if the kappa angle varies across distances. Thirdly, the individual variability in parameters such as the anterior chamber depth, nodal point, and center of eye rotation were assumed to correspond to the average population values, potentially overlooking minor differences that could affect the TPVA calculation. Finally, binocular IPD values were utilized, as PowerRef 3 does not provide monocular IPD values; consequently, in cases with an asymmetric monocular IPD, the influence of asymmetric fixation disparity might be overlooked.