Comparison of EEG Signal Spectral Characteristics Obtained with Consumer- and Research-Grade Devices
Abstract
:1. Introduction
2. Methods
2.1. Data Acquisition and Experimental Procedure
2.2. EEG Data Analysis
2.3. Statistical Analysis
3. Results
3.1. Signal Quality Evaluation
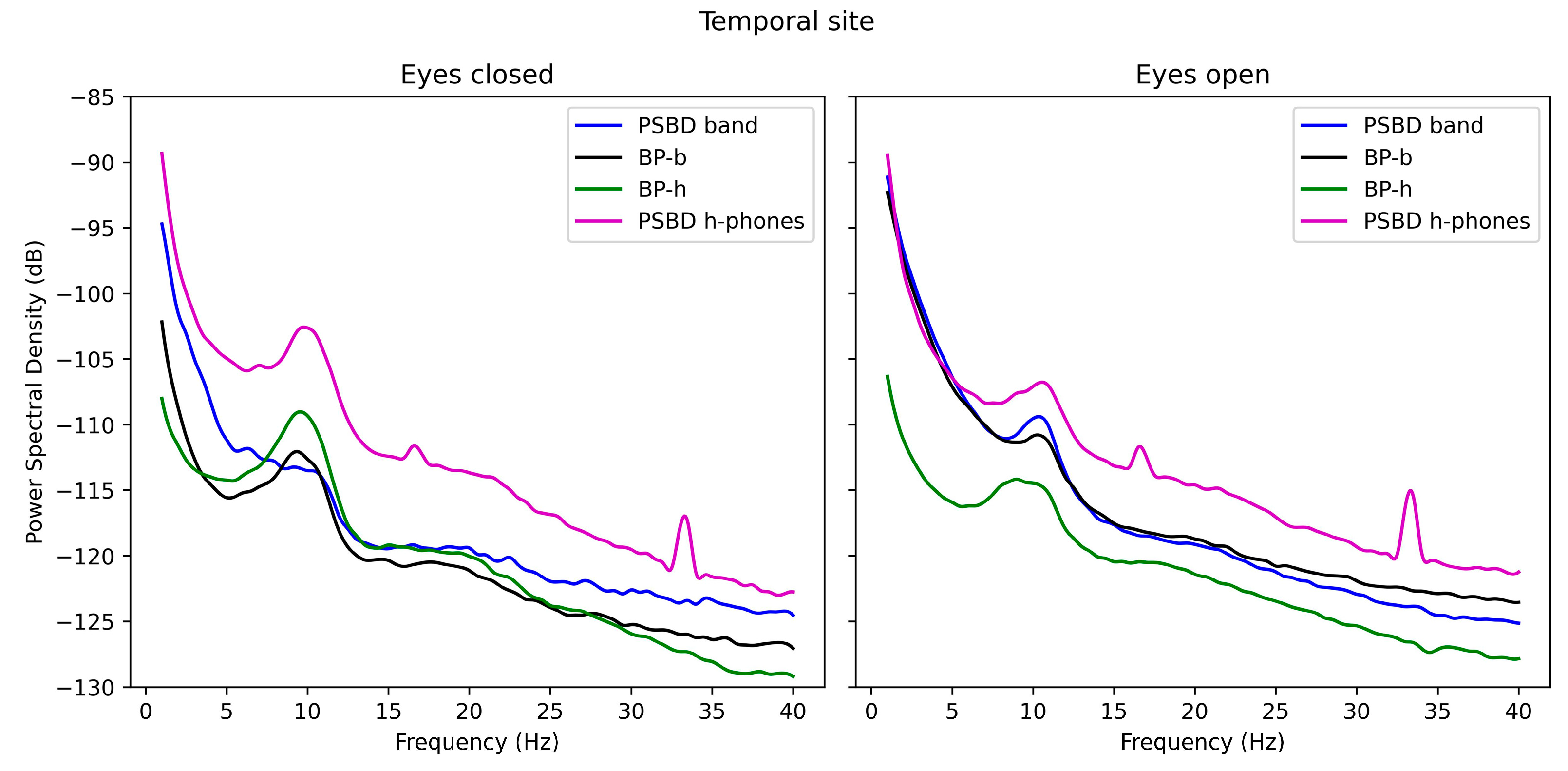
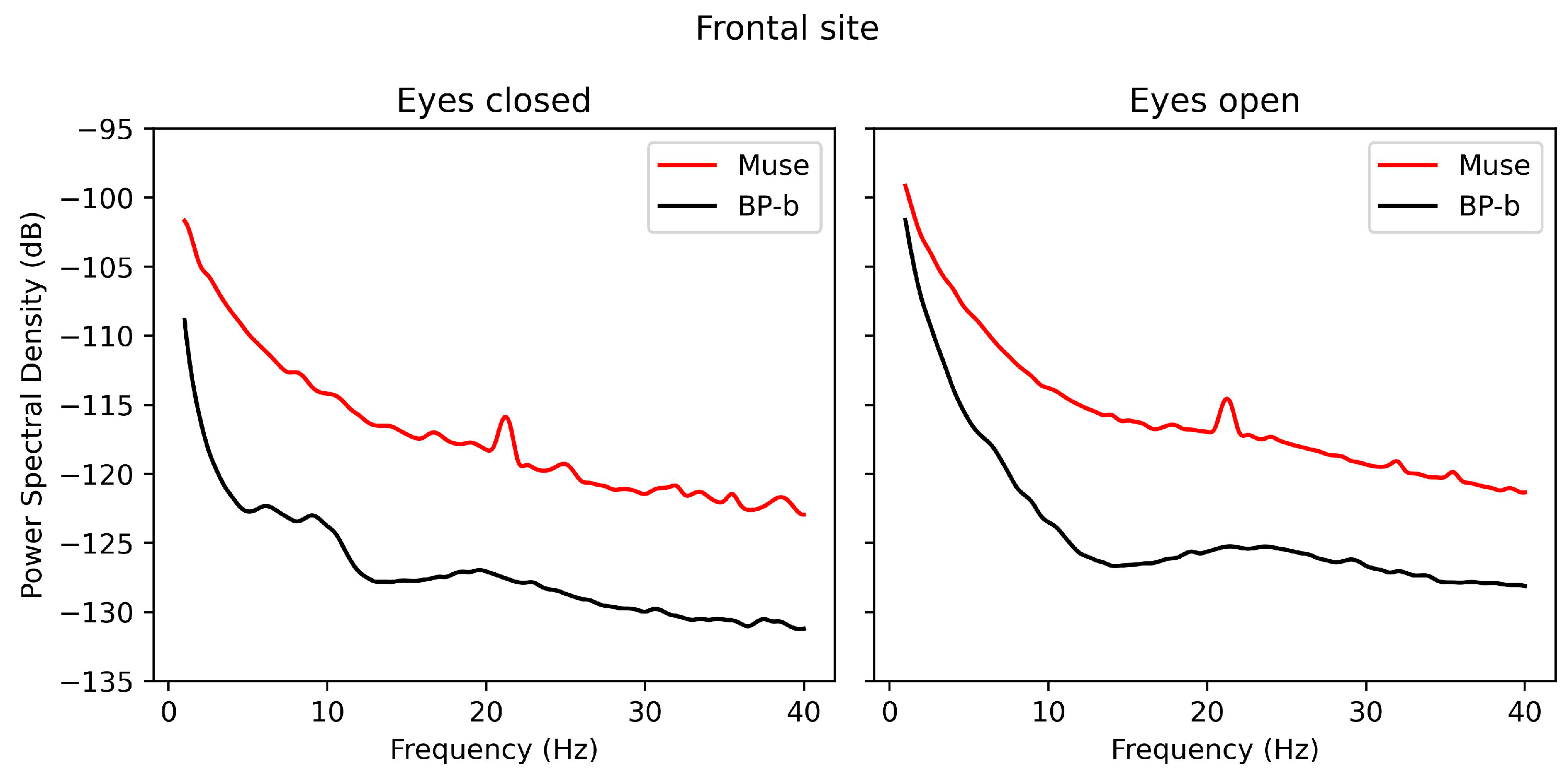
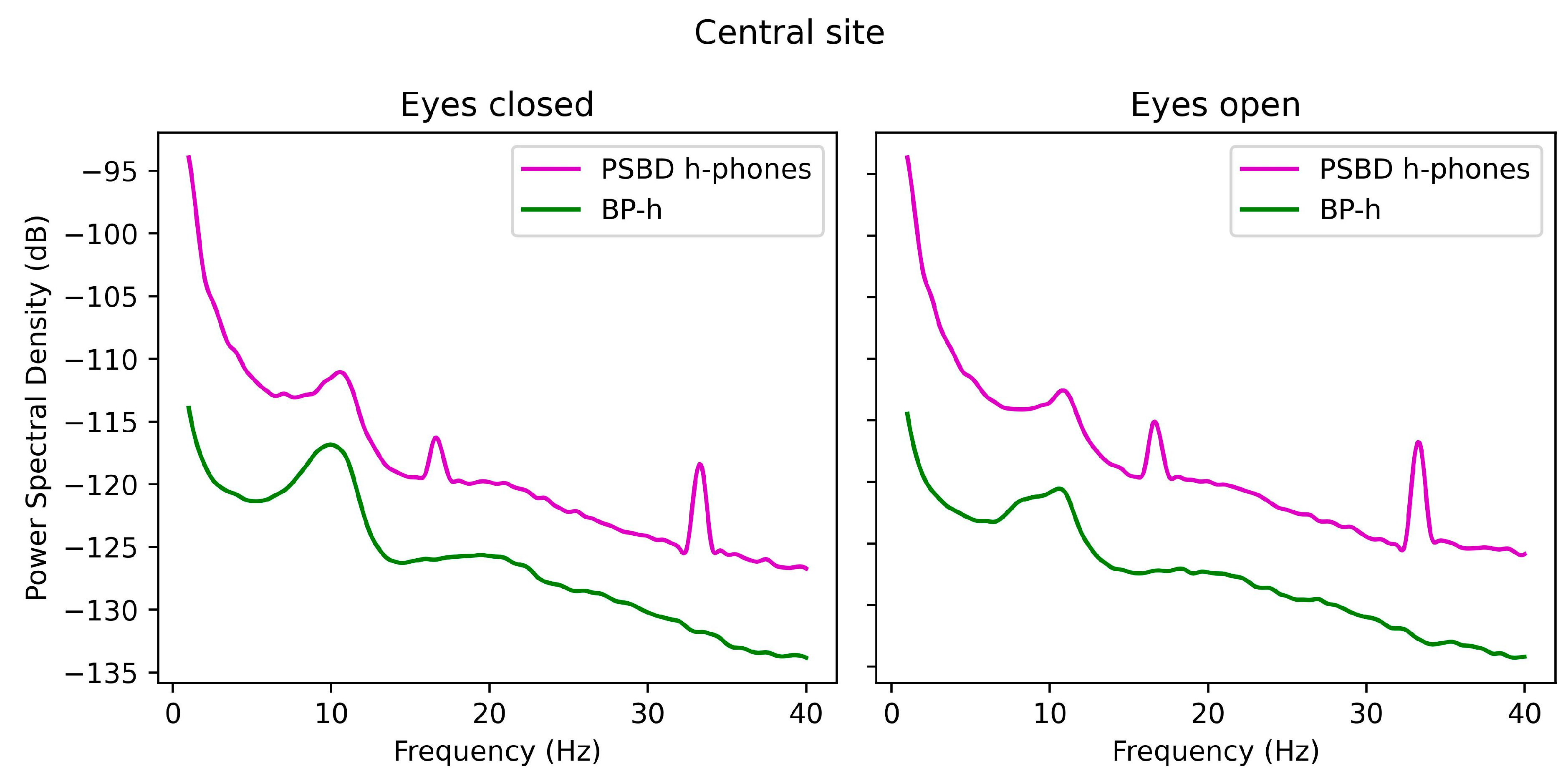
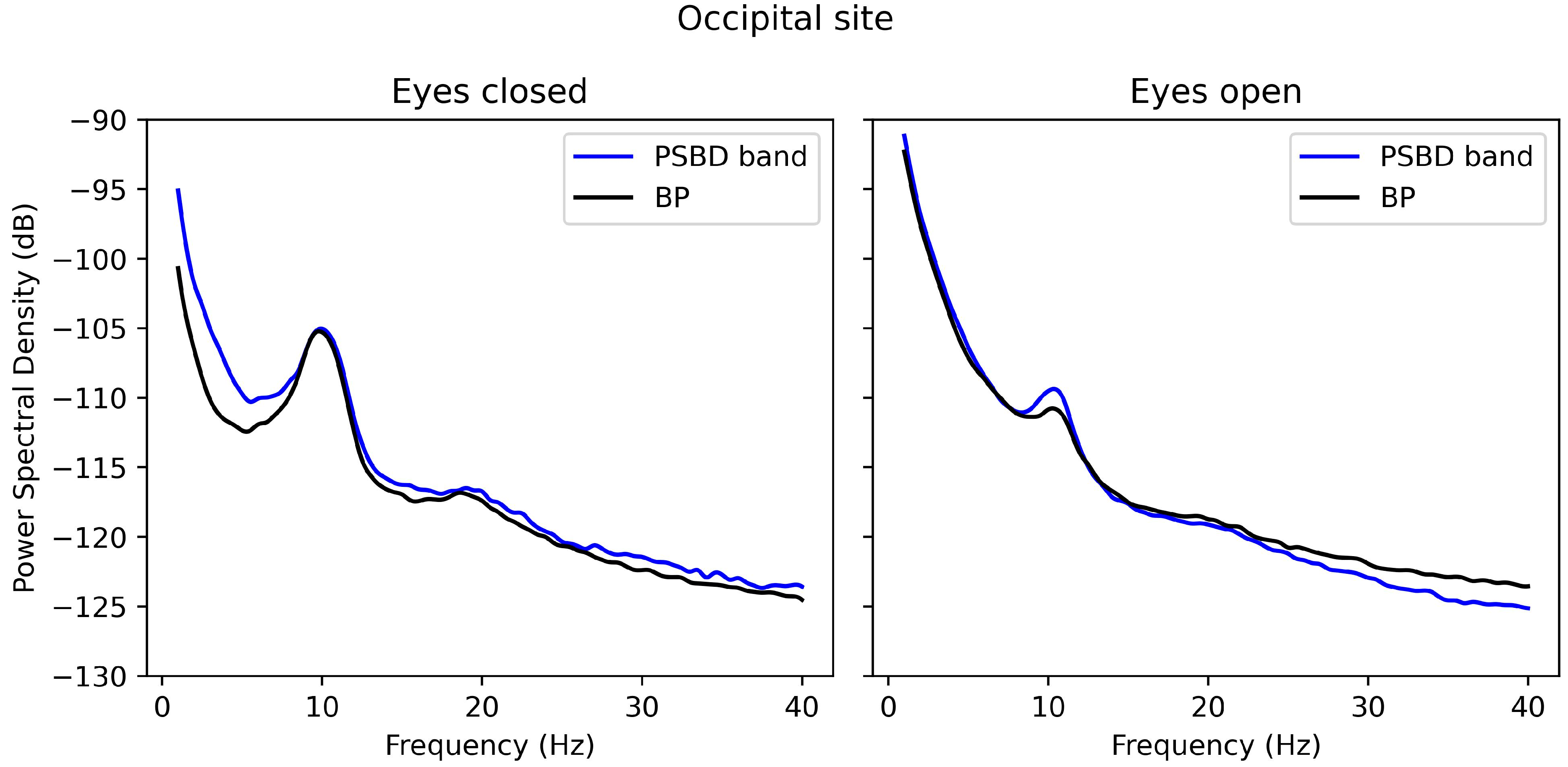
3.2. Power-Spectrum Plots
3.3. ANOVA of the Power in Specific Frequency Bands
3.3.1. Frontal Site: Muse and BP-Band
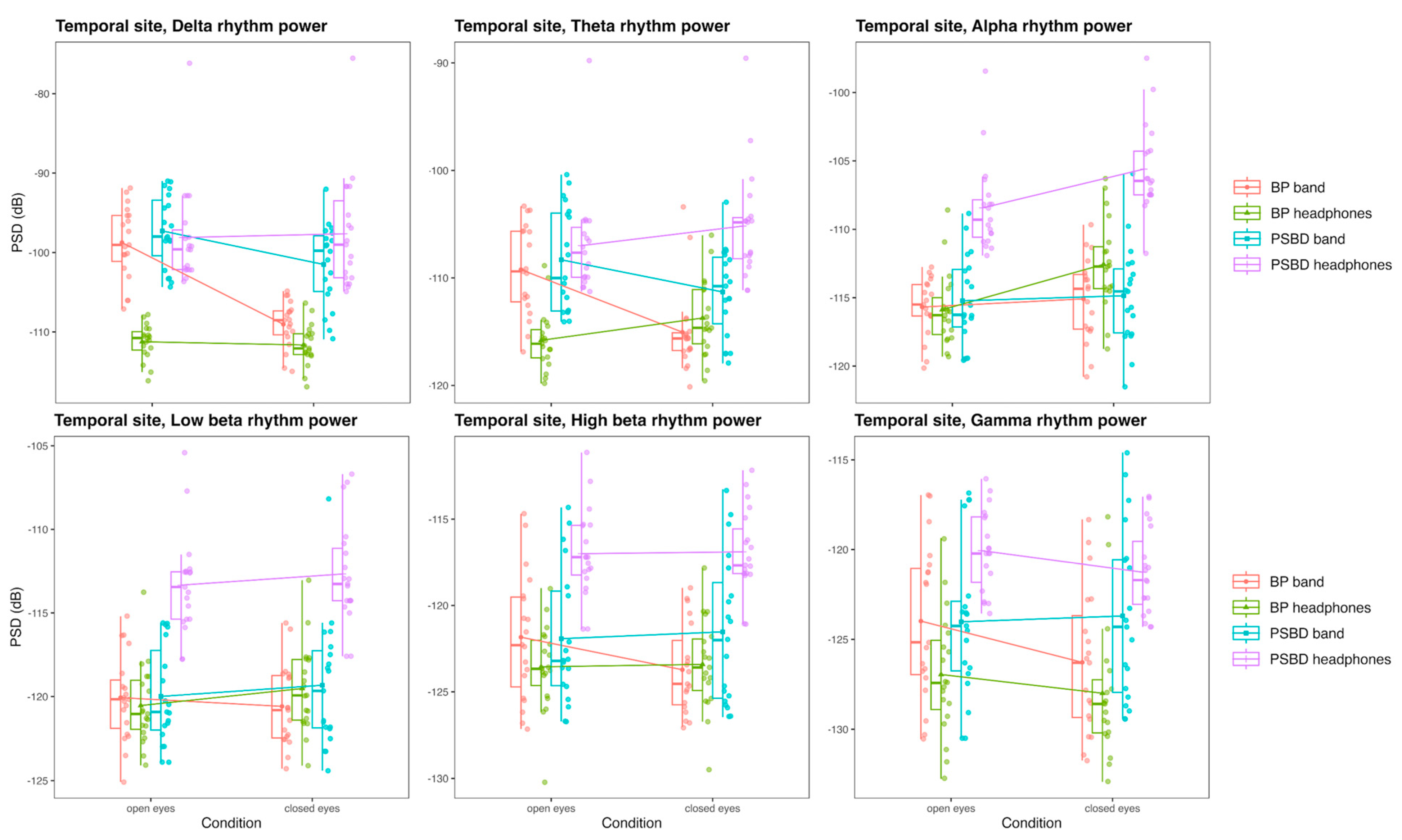
3.3.2. Temporal Site: PSBD-Band, BP-Band, PSBD-Headphones, and BP-Headphones
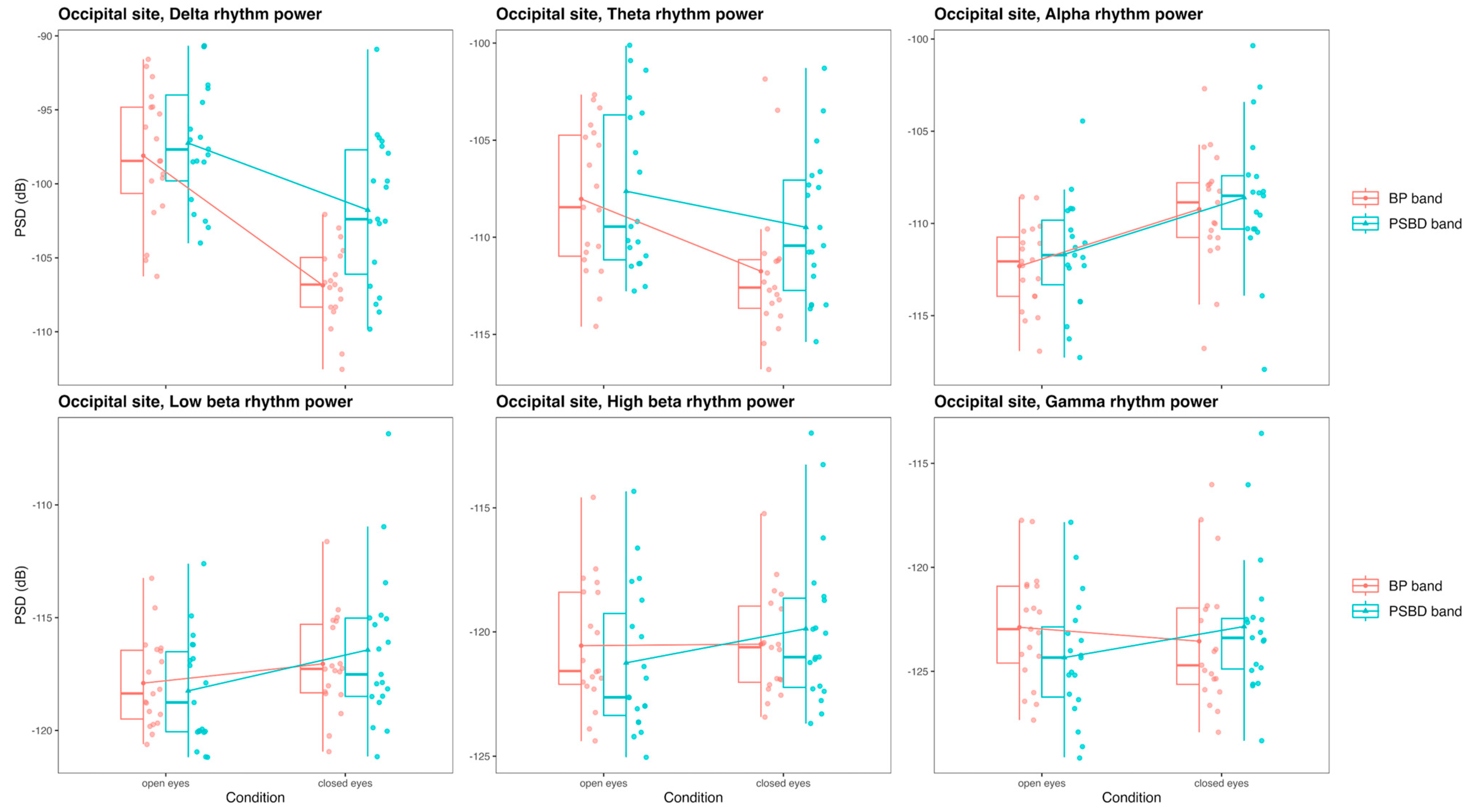
3.3.3. Occipital Site: PSBD Band and BP-Band
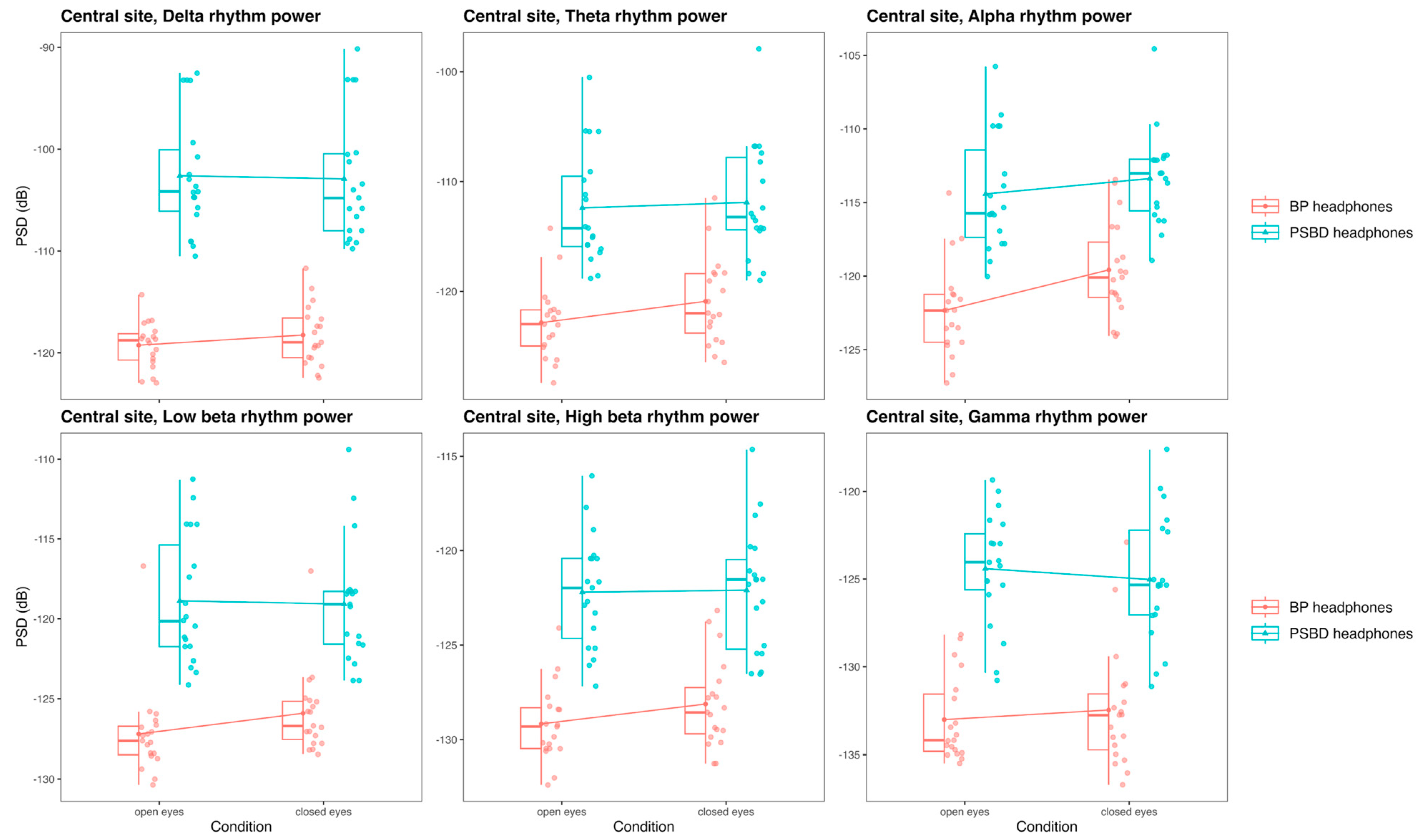
3.3.4. Central Site: PSBD-Headphones and BP-Headphones
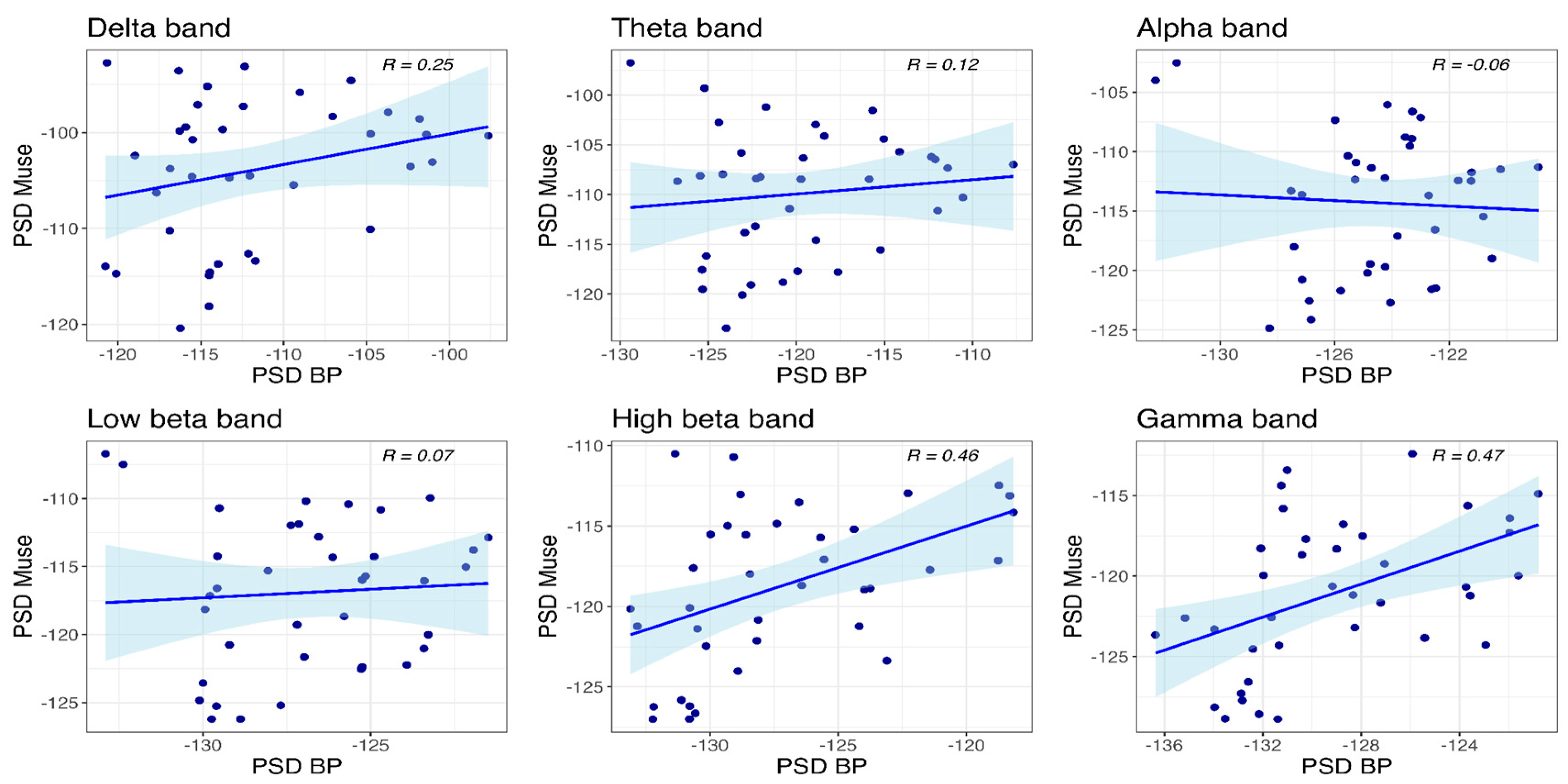
3.4. Correlational Analysis
3.4.1. Muse and BP-Band
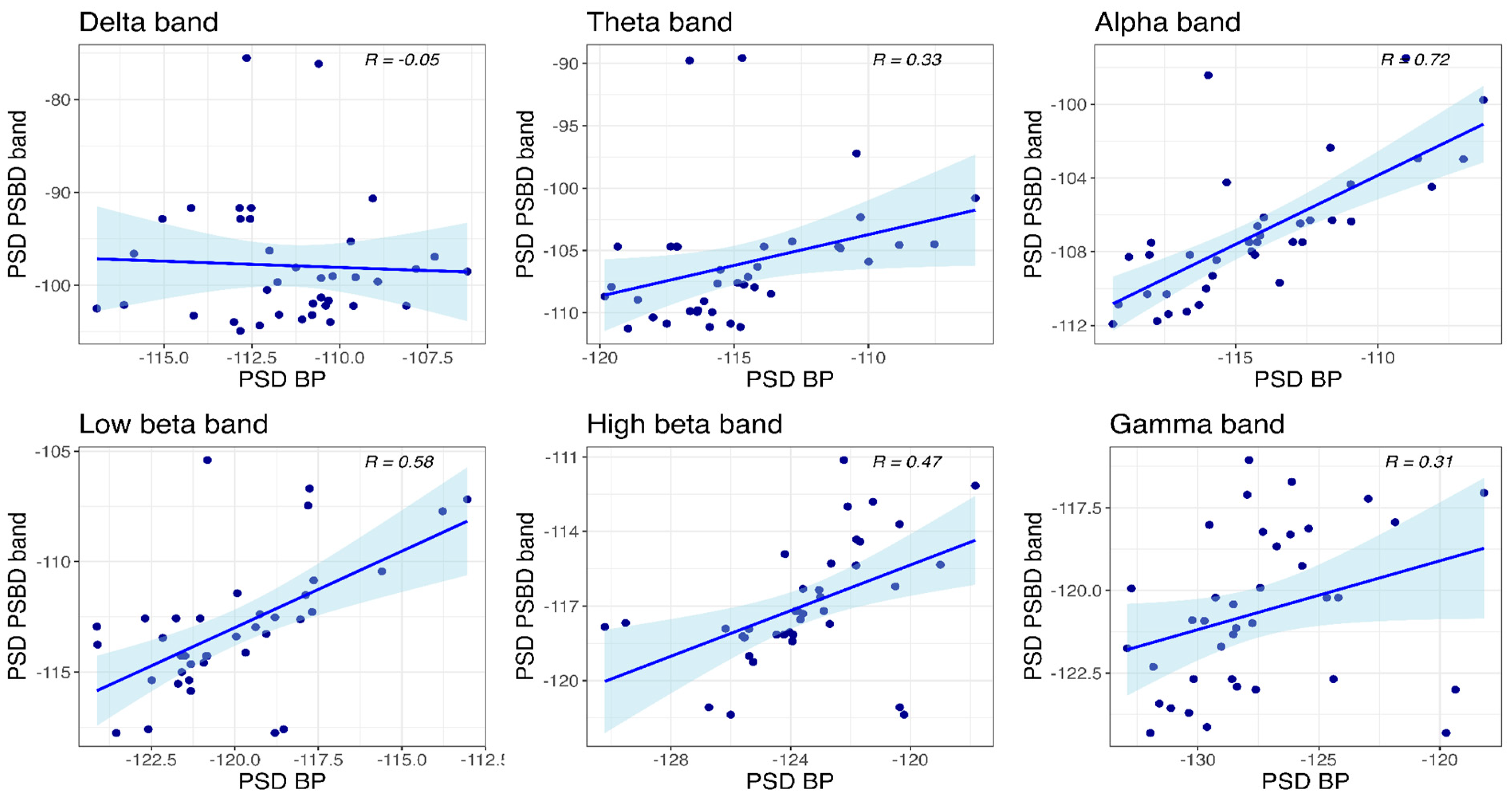
3.4.2. PSBD Band and BP-Band
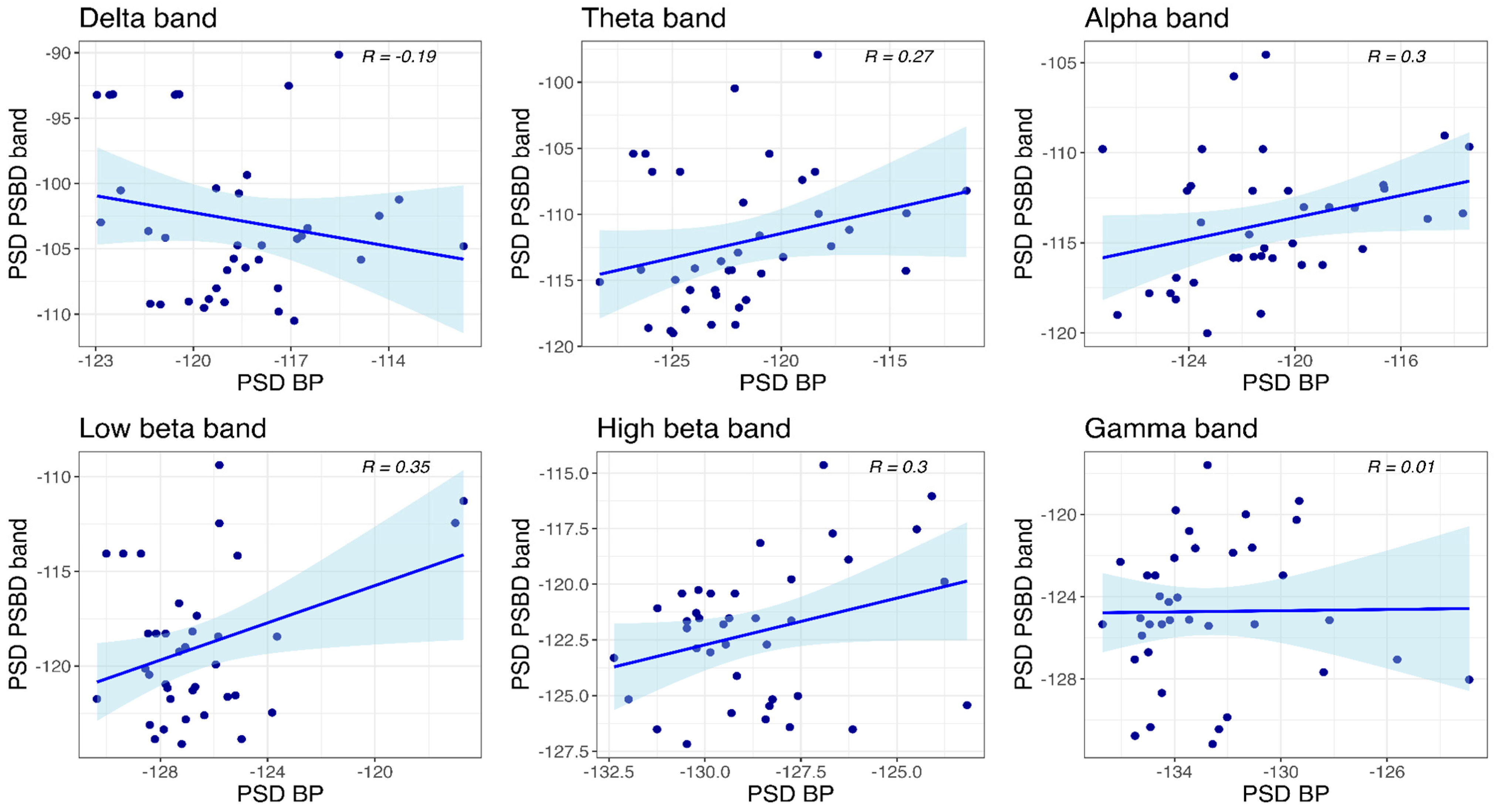
3.4.3. PSBD-Headphones and BP-Headphones
4. Discussion
4.1. Device-Specific Differences and Contributing Factors
4.1.1. Electrode Design
- Muse uses flat electrodes coated with conductive silver ink, which are prone to signal degradation and may result in poor contact, particularly at temporal sites.
- PSBD devices use multi-pin dry electrodes, which improve contact stability and reduce impedance to some extent. However, the higher impedance compared to gel electrodes still leads to increased noise, especially in lower-frequency bands such as delta and theta.
4.1.2. Reference Electrode Placement
- In Muse, the frontal placement of reference electrodes increases susceptibility to eye-movement and blink artifacts. This likely contributed to the poor signal quality observed at the temporal electrodes.
- In the PSBD Headphones, the central reference placement (Cz) is less prone to frontal artifacts but still shows some susceptibility to muscle-related noise at the central site.
- The PSBD Headband demonstrated better alignment with the BP system, particularly at the occipital site, where alpha suppression (Berger’s effect) was clearly observed. This highlights the importance of electrode and reference placement in mitigating artefacts and improving reliability.
4.1.3. Signal Processing Algorithms
- Muse relies on proprietary algorithms for signal quality metrics, which may not always accurately reflect impedance or noise levels, as observed in our study.
- Research-grade BP systems employ robust preprocessing, filtering, and impedance monitoring techniques that significantly enhance signal fidelity.
- While the PSBD devices performed better than Muse, slight deviations in spectral power, particularly in the low-frequency bands, suggest opportunities for improving their signal processing algorithms to account for dry-electrode noise and artifacts.
4.1.4. Artifact Susceptibility
- Temporal and central recordings with PSBD Headphones showed higher noise levels and artifact spikes at 16 Hz and 34 Hz, which are likely linked to external interference or device-specific design constraints.
- Signal quality in Muse’s temporal site was completely compromised, suggesting a need for better artifact handling algorithms or improved electrode placement.
5. Future Directions
6. Conclusions
- This study demonstrates that consumer-grade EEG devices vary in their ability to capture high-quality brain activity signals. Among the tested devices, the PSBD Headband exhibited the strongest alignment with research-grade equipment, while the PSBD Headphones demonstrated moderate performance and the Muse device showed the poorest performance. These results emphasize the importance of understanding the specific design limitations of consumer-grade EEG devices for researchers and consumers. The accuracy and sensitivity of these devices in detecting brain signals are limited compared to research-grade systems, particularly regarding noise levels and signal artifacts.
- This study underscores the need for ongoing research and development to improve the reliability of consumer EEG devices and enhance their signal processing algorithms to reduce artifacts. This is especially pertinent for devices like Muse, which exhibit significant differences in signal quality. While PSBD devices hold promise for applications such as neurofeedback training or cognitive state monitoring, users and developers should consider limitations in lower-frequency bands, especially during tasks that may involve eye movement or muscle tension.
- This study recommends that users should utilize such devices in controlled environments, particularly during calm periods with minimal eye or body movements, to reduce potential errors in signal interpretation, particularly in low-frequency bands like delta and theta rhythms.
- The PSBD Headband Pro demonstrated strong alignment with the research-grade system, particularly in the occipital region, making it reliable for tasks such as alpha suppression during calm recording conditions. However, its susceptibility to low-frequency artifacts requires careful use. The PSBD Headphones Lite provided moderate signal quality, particularly for temporal EEG, but noise artifacts at specific frequencies limit its use in central EEG analysis. The Muse S Gen 2, despite its affordability and accessibility, displayed the poorest performance due to significant artifacts and poor temporal signal quality. As such, the Muse device is not recommended for high-fidelity EEG studies but may be used for basic frontal-region neurofeedback tasks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, E.K.S.; Frey, L.C.; Britton, J.W.; Hopp, J.L.; Korb, P.; Koubeissi, M.Z.; Lievens, W.E.; Pestana-Knight, E.M.; Foundation, C.C.C. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; American Epilepsy Society: William Davis Gaillard, MD, USA, 2016; Available online: http://europepmc.org/books/NBK390354 (accessed on 14 March 2024).
- Zanetti, R.; Arza, A.; Aminifar, A.; Atienza, D. Real-Time EEG-Based Cognitive Workload Monitoring on Wearable Devices. IEEE Trans. Biomed. Eng. 2022, 69, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain–computer interface paradigms. J. Neural. Eng. 2019, 16, 011001. [Google Scholar] [CrossRef] [PubMed]
- Kamp, A.; Pfurtscheller, G.; Edlinger, G.; da Silva, F.H.L. Technological Basis of EEG Recording, Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; 2005; Volume 1; pp. 127–138. Available online: https://books.google.com/books/about/Electroencephalography.html?id=tndqYGPHQdEC (accessed on 7 October 2024).
- Taheri, B.A.; Knight, R.T.; Smith, R.L. A dry electrode for EEG recording. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kam, J.W.; Griffin, S.; Shen, A.; Patel, S.; Hinrichs, H.; Heinze, H.-J.; Deouell, L.Y.; Knight, R.T. Systematic comparison between a wireless EEG system with dry electrodes and a wired EEG system with wet electrodes. Neuroimage 2019, 184, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Giannadou, A.; Jones, M.; Freeth, M.; Samson, A.C.; Milne, E. Investigating neural dynamics in autism spectrum conditions outside of the laboratory using mobile electroencephalography. Psychophysiology 2022, 59, e13995. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.U.; Lin, C.; Lee, S.-H.; Huang, H.-W.; Wu, S.-C.; Madathil, D.; Huang, C.-M. Review of EEG-based neurofeedback as a therapeutic intervention to treat depression. Psychiatry Res. Neuroimaging 2023, 329, 111591. [Google Scholar] [CrossRef] [PubMed]
- Värbu, K.; Muhammad, N.; Muhammad, Y. Past, Present, and Future of EEG-Based BCI Applications. Sensors 2022, 22, 3331. [Google Scholar] [CrossRef] [PubMed]
- Acabchuk, R.L.; Simon, M.A.; Low, S.; Brisson, J.M.; Johnson, B.T. Measuring Meditation Progress with a Consumer-Grade EEG Device: Caution from a Randomized Controlled Trial. Mindfulness 2021, 12, 68–81. [Google Scholar] [CrossRef]
- Wexler, A.; Thibault, R. Mind-Reading or Misleading? Assessing Direct-to-Consumer Electroencephalography (EEG) Devices Marketed for Wellness and Their Ethical and Regulatory Implications. J. Cogn. Enhanc. 2019, 3, 131–137. [Google Scholar] [CrossRef]
- Mathewson, K.E.; Harrison, T.J.; Kizuk, S.A.J.P. High and dry? Comparing active dry EEG electrodes to active and passive wet electrodes. In Psychophysiology; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2017; pp. 74–82. [Google Scholar] [CrossRef]
- Kleeva, D.; Ninenko, I.; Lebedev, M.A. Resting-state EEG recorded with gel-based vs. consumer dry electrodes: Spectral characteristics and across-device correlations. Front. Neurosci. 2024, 18, 1326139. [Google Scholar] [CrossRef]
- Niso, G.; Romero, E.; Moreau, J.T.; Araujo, A.; Krol, L.R. Wireless EEG: A survey of systems and studies. NeuroImage 2023, 269, 119774. [Google Scholar] [CrossRef] [PubMed]
- Anwar, D.; Garg, P.; Naik, V.; Gupta, A.; Kumar, A. Use of Portable EEG Sensors to Detect Meditation. In Proceedings of the 2018 10th International Conference on Communication Systems & Networks (COMSNETS), Bengaluru, India, 3–7 January 2018; pp. 705–710. Available online: https://www.researchgate.net/publication/ (accessed on 14 March 2024).
- Krigolson, O.E.; Williams, C.C.; Norton, A.; Hassall, C.D.; Colino, F.L. Choosing MUSE: Validation of a low-cost, portable EEG system for ERP research. Front. Neurosci. 2017, 11, 109. [Google Scholar] [CrossRef]
- Lin, C.-T.; Chang, C.-J.; Lin, B.-S.; Hung, S.-H.; Chao, C.-F.; Wang, I.-J. A real-time wireless brain-computer interface system for drowsiness detection. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Marini, F.; Lee, C.; Wagner, J.; Makeig, S.; Gola, M. A comparative evaluation of signal quality between a research-grade and a wireless dry-electrode mobile EEG system. J. Neural Eng. 2019, 16, 054001. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef]
- Przegalinska, A.; Ciechanowski, L.; Magnuski, M.; Gloor, P. Muse Headband: Measuring Tool or a Collaborative Gadget? In Studies on Entrepreneurship, Structural Change and Industrial Dynamics; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 93–101. [Google Scholar] [CrossRef]
- Ratti, E.; Waninger, S.; Berka, C.; Ruffini, G.; Verma, A. Comparison of medical and consumer wireless EEG systems for use in clinical trials. Front. Hum. Neurosci. 2017, 11, 398. [Google Scholar] [CrossRef]
- Di Flumeri, G.; Aricò, P.; Borghini, G.; Sciaraffa, N.; Di Florio, A.; Babiloni, F. The dry revolution: Evaluation of three different eeg dry electrode types in terms of signal spectral features, mental states classification and usability. Sensors 2019, 19, 1365. [Google Scholar] [CrossRef]
- Rieiro, H.; Diaz-Piedra, C.; Morales, J.M.; Catena, A.; Romero, S.; Roca-Gonzalez, J.; Fuentes, L.J.; Di Stasi, L.L. Validation of electroencephalographic recordings obtained with a consumer-grade, single dry electrode, low-cost device: A comparative study. Sensors 2019, 19, 2808. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Cannard, C.; Wahbeh, H.; Delorme, A. Validating the wearable MUSE headset for EEG spectral analysis and Frontal Alpha Asymmetry. In Proceedings of the 2021 IEEE International Conference on Bioinformatics and Biomedicine, BIBM 2021, Houston, TX, USA, 9–12 December 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021; pp. 3603–3610. [Google Scholar]
- Arvaneh, M.; Guan, C.; Ang, K.K.; Quek, C. Optimizing the channel selection and classification accuracy in EEG-based BCI. IEEE Trans. Biomed. Eng. 2011, 58, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Mitiureva, D.; Bobrov, P.; Rebreikina, A.; Sysoeva, O. An inclusive paradigm to study mu-rhythm properties. Int. J. Psychophysiol. 2023, 190, 42–55. [Google Scholar] [CrossRef]
- Hinrichs, H.; Scholz, M.; Baum, A.K.; Kam, J.W.Y.; Knight, R.T.; Heinze, H.-J. Comparison between a wireless dry electrode EEG system with a conventional wired wet electrode EEG system for clinical applications. Sci. Rep. 2020, 10, 5218. [Google Scholar] [CrossRef]
- Minguillon, J.; Lopez-Gordo, M.A.; Pelayo, F. Trends in EEG-BCI for daily-life: Requirements for artifact removal. Biomed. Signal Process. Control. 2017, 31, 407–418. [Google Scholar] [CrossRef]
- Doan, Q.M.; Dinh, T.H.; Singh, A.K.; Lin, C.-T.; Trung, N.L. Cascaded Thinning in Upscale and Downscale Representation for EEG Signal Processing. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 3677–3688. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhang, L.; Yang, X.; Zhang, W.-A. EEG-Based Driver Fatigue Detection Using Spatio-Temporal Fusion Network With Brain Region Partitioning Strategy. IEEE Trans. Intell. Transp. Syst. 2024, 25, 9618–9630. [Google Scholar] [CrossRef]



| Device | Number of Electrodes | Ref. | GND | BP Mirror-Montage Specification |
|---|---|---|---|---|
| PSBD Headband Pro | 4: T3, T4, O1, O2 | FPz | FP1, FP2 | BP-band Electrodes: O1, O2, T7 (~T3), T8 (~T4) Ref.: FPz GND: FP1 |
| Muse S Gen 2 | 4: AF7, AF8, TP9, TP10 | FPz | FP1, FP2 | BP-band Electrodes: F7 (~AF7), F8 (~AF8), T7 (~TP9), T8 (~TP10) Ref.: FPz GND: FP1 |
| PSBD Headphones Lite | 4: C3, C4, A1, A2 | Cz | FT9 | BP-headphones Electrodes: C3, C4, TP9 (~A1), TP10 (~A2) Ref.: Cz GND: FT9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhaylov, D.; Saeed, M.; Husain Alhosani, M.; F. Al Wahedi, Y. Comparison of EEG Signal Spectral Characteristics Obtained with Consumer- and Research-Grade Devices. Sensors 2024, 24, 8108. https://doi.org/10.3390/s24248108
Mikhaylov D, Saeed M, Husain Alhosani M, F. Al Wahedi Y. Comparison of EEG Signal Spectral Characteristics Obtained with Consumer- and Research-Grade Devices. Sensors. 2024; 24(24):8108. https://doi.org/10.3390/s24248108
Chicago/Turabian StyleMikhaylov, Dmitry, Muhammad Saeed, Mohamed Husain Alhosani, and Yasser F. Al Wahedi. 2024. "Comparison of EEG Signal Spectral Characteristics Obtained with Consumer- and Research-Grade Devices" Sensors 24, no. 24: 8108. https://doi.org/10.3390/s24248108
APA StyleMikhaylov, D., Saeed, M., Husain Alhosani, M., & F. Al Wahedi, Y. (2024). Comparison of EEG Signal Spectral Characteristics Obtained with Consumer- and Research-Grade Devices. Sensors, 24(24), 8108. https://doi.org/10.3390/s24248108






