Non-Invasive Malaria Detection in Sub-Saharan Africa Using a DNA-Based Sensor System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. DNA Oligonucleotides
- 5′-Amine REEAD primer:5′-[AmC6] CCAACCAACCAACCAAGGAGCCAAACATGTGCATTGAGG-3′;
- pTOP1substrate:5′-TCTAGAAAGTATAGGAACTTCGAACGACTCAGAATGACTGTGAAGA TCGCTTATCCTCAATGCACATGTTTGGCTCCCATTCTGAGTCGTTCGAAGTTCCTATTCTTT-3′;
- hTOP1substrate:5′-AGAAAAATTTTTAAAAAAACTGTGAAGATCGCTTATTTTTTTAAAAAT TTTTCTAAGTCTTTTAGATCCCTCAATGCACATGTTTGGCTCCGATCTAAAAGACTTAGA-3′;
- Fluorescent probe:5′-[FAM] CCT CAA TGC ACA TGT TTG GCT CC-3′.
2.3. Saliva and Blood Samples from Malaria Patients and Uninfected Individuals
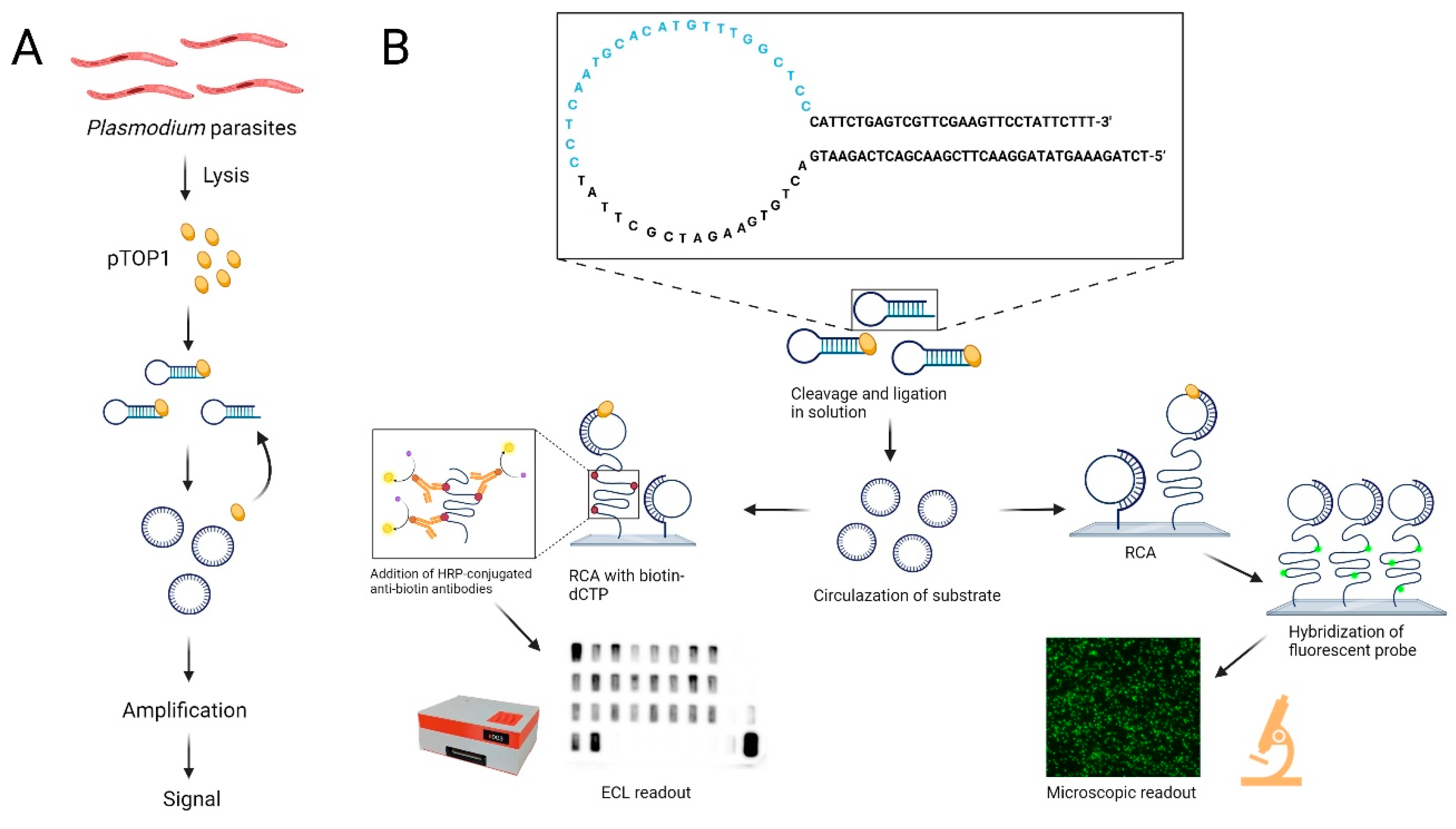
2.4. REEAD
2.4.1. Preparation of Slides
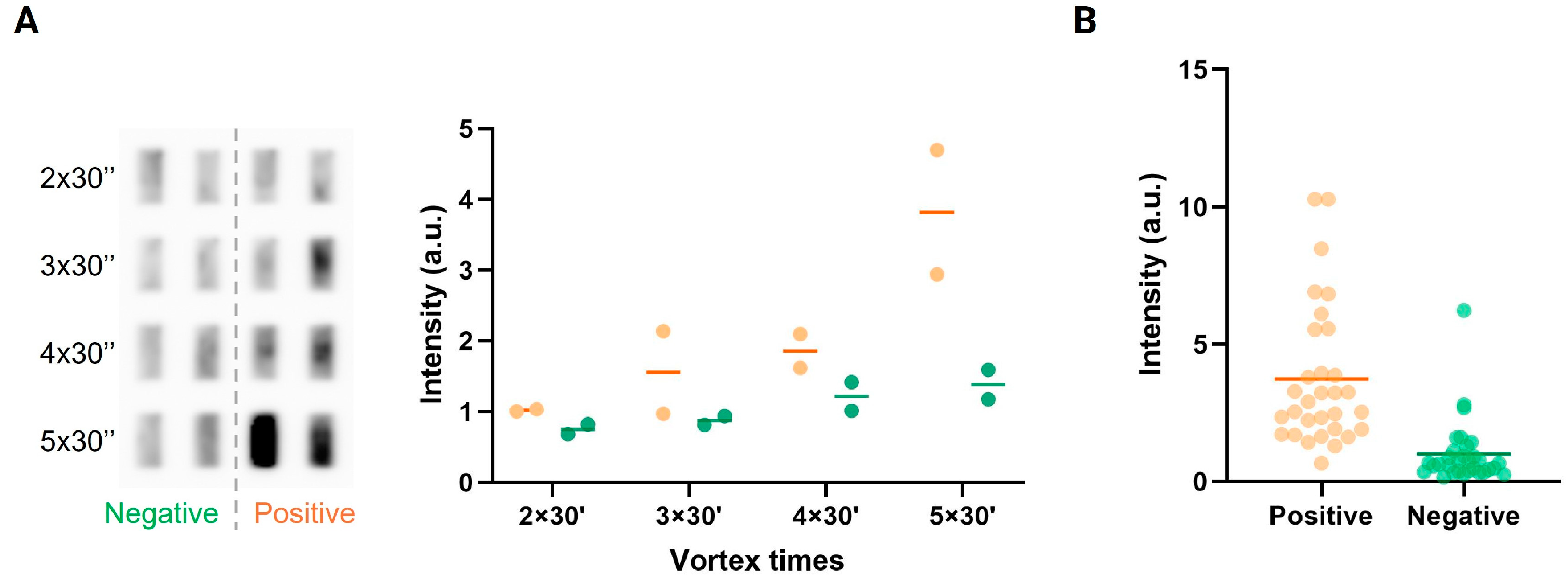
2.4.2. Extraction of Saliva Samples
2.4.3. Circularization with Saliva Samples
2.4.4. Circularization with P. falciparum TOP1 Spiked in Saliva
2.4.5. Circularization with Purified P. falciparum TOP1 and Human TOP1
2.4.6. Rolling Circle Amplification and Detection Using Fluorescence Microscope
2.4.7. Rolling Circle Amplification and Detection Using CCD Camera or VPCIReader
2.4.8. VPCIReader Usage
2.5. Protein Purifications
2.5.1. P. falciparum TOP1
2.5.2. Human TOP1
2.5.3. Phi29 Polymerase
2.6. Statistical Analysis
3. Results and Discussion
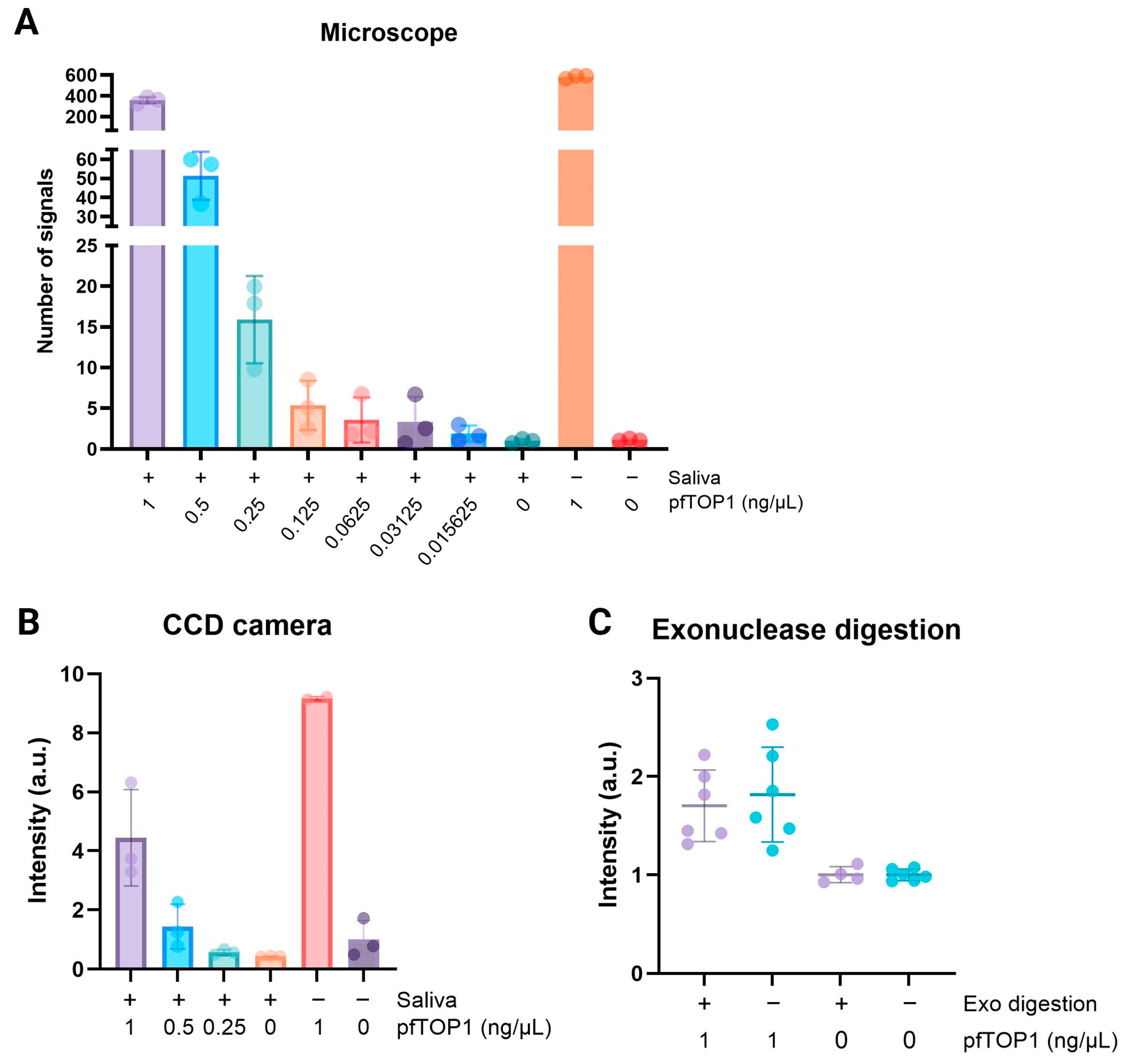
3.1. Semiquantitative Detection of the Plasmodium-SPECIFIC Biomarker pTOP1 by the Use of Chemiluminescence Readout
3.2. Detection of Plasmodium in Saliva from Malaria-Positive Individuals in High-Resource Laboratory Settings
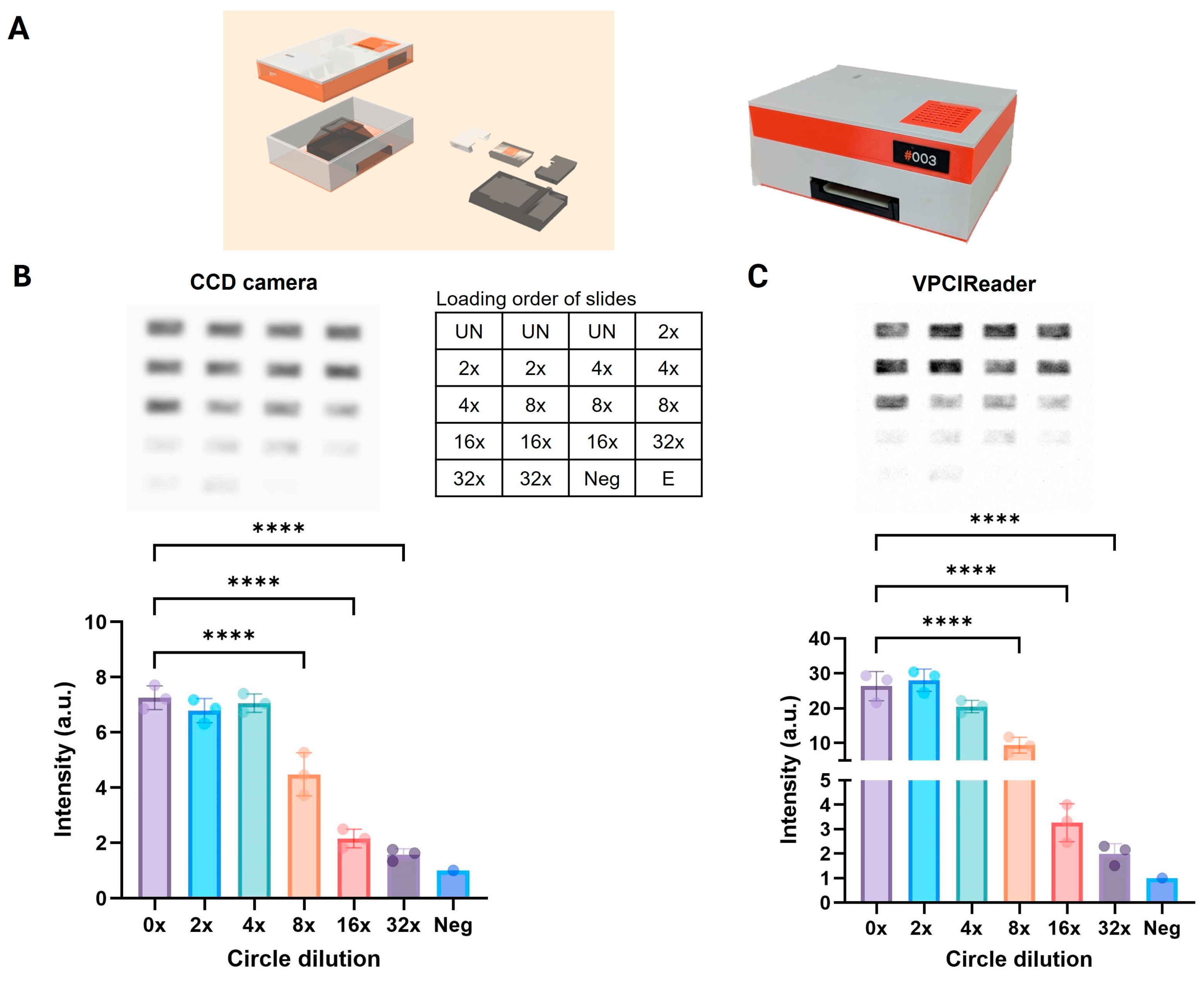
3.3. Development and Proof-of-Concept Testing of an Affordable Portable Reader for Measuring Chemiluminescence REEAD Signals
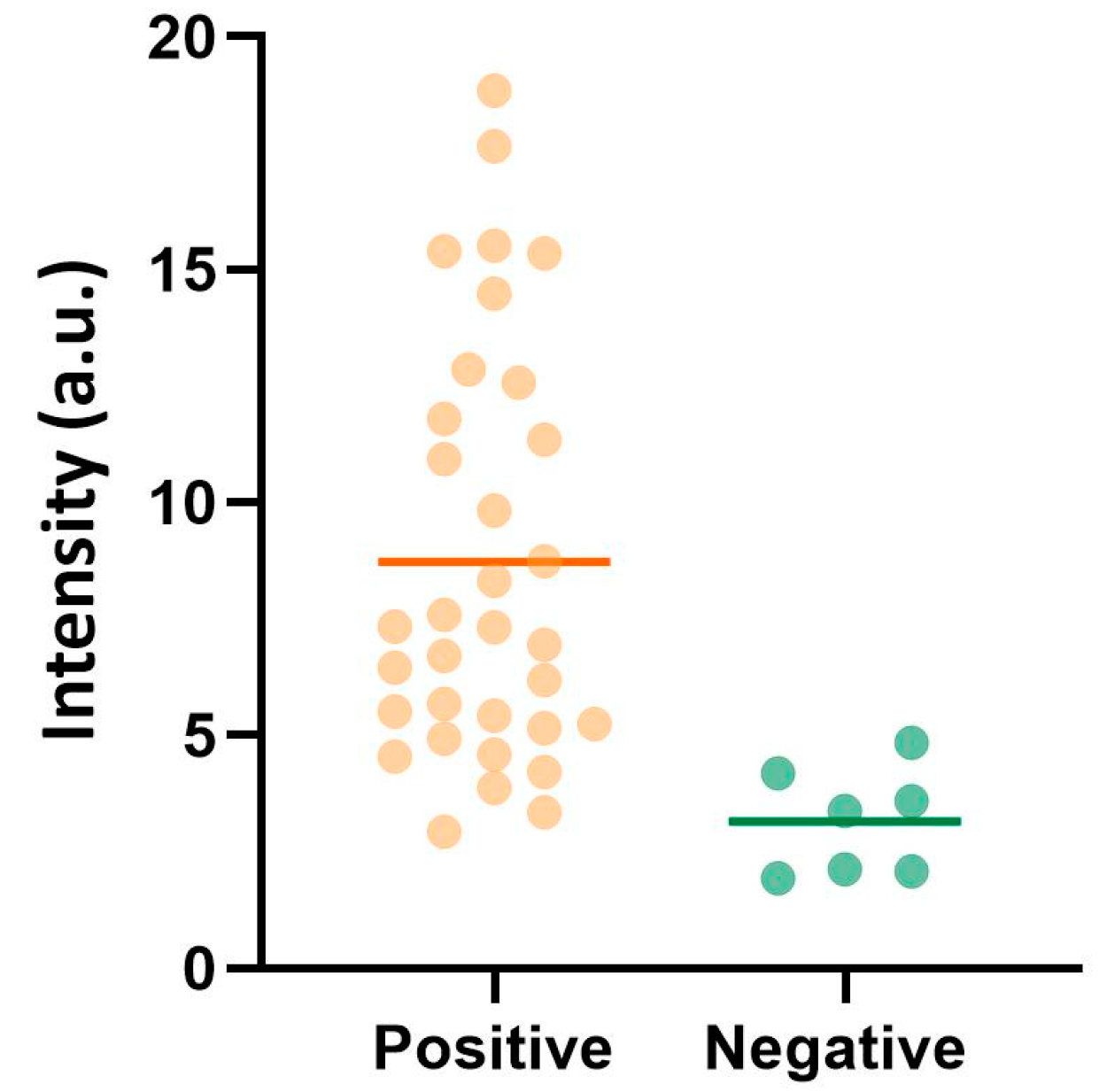
3.4. Detection of Malaria in Saliva from Infected Individuals in Sub-Saharan Africa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Wanjala, C.L.; Kweka, E.J. Impact of Highland Topography Changes on Exposure to Malaria Vectors and Immunity in Western Kenya. Front. Public Health 2016, 4, 227. [Google Scholar] [CrossRef]
- Rodó, X.; Martinez, P.P.; Siraj, A.; Pascua, M. Malaria trends in Ethiopian highlands track the 2000 ‘slowdown’ in global warming. Nat. Commun. 2021, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Bannon, E.; Mendenhall, E.; Newfield, T.; Bansal, S. Rapid range shifts in African Anopheles mosquitoes over the last century. Biol. Lett. 2023, 19, 20220365. [Google Scholar] [CrossRef] [PubMed]
- Gasimov, E. Update on Malaria Elimination, Including Zoonotic Malaria in Malaria Policy Advisory Group Meeting; World Health Organization: Yaoundé, Cameroon, 2024. [Google Scholar]
- WHO. Countries and Territories Certified Malaria-Free by WHO. 2024. Available online: https://www.who.int/teams/global-malaria-programme/elimination/countries-and-territories-certified-malaria-free-by-who (accessed on 10 July 2024).
- Cooke, B.M.; Mohandas, N.; Coppel, R.L. The malaria-infected red blood cell: Structural and functional changes. Adv. Parasitol. 2001, 50, 1–86. [Google Scholar] [PubMed]
- Gilson, P.R.; Crabb, B.S. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int. J. Parasitol. 2009, 39, 91–96. [Google Scholar] [CrossRef]
- Warhurst, D.C.; Williams, J.E. ACP Broadsheet no 148. July 1996. Laboratory diagnosis of malaria. J. Clin. Pathol. 1996, 49, 533–538. [Google Scholar] [CrossRef]
- Tangpukdee, N.; Duangdee, C.; Wilairatana, P.; Krudsood, S. Malaria diagnosis: A brief review. Korean J. Parasitol. 2009, 47, 93–102. [Google Scholar] [CrossRef]
- Murray, C.K.; Gasser, R.A., Jr.; Magill, A.J.; Miller, R.S. Update on rapid diagnostic testing for malaria. Clin. Microbiol. Rev. 2008, 21, 97–110. [Google Scholar] [CrossRef]
- Gitta, B.; Kilian, N. Diagnosis of Malaria Parasites Plasmodium spp. in Endemic Areas: Current Strategies for an Ancient Disease. Bioessays 2020, 42, e1900138. [Google Scholar] [CrossRef]
- Baker, B.R.; Lai, R.Y.; Wood, M.S.; Doctor, E.H.; Heeger, A.J.; Plaxco, K.W. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J. Am. Chem. Soc. 2006, 128, 3138–3139. [Google Scholar] [CrossRef]
- Halder, S.; Krishnan, Y. Design of ultrasensitive DNA-based fluorescent pH sensitive nanodevices. Nanoscale 2015, 7, 10008–10012. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Jiang, Y.; Hu, Y.; Li, J.; Yang, S.; Li, Y.; Yang, R.; Tan, W.; Huang, C.Z. Graphene signal amplification for sensitive and real-time fluorescence anisotropy detection of small molecules. Anal. Chem. 2013, 85, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Zayats, M.; Huang, Y.; Gill, R.; Ma, C.A.; Willner, I. Label-free and reagentless aptamer-based sensors for small molecules. J. Am. Chem. Soc. 2006, 128, 13666–13667. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Li, L.; Zhang, S. Triggered polycatenated DNA scaffolds for DNA sensors and aptasensors by a combination of rolling circle amplification and DNAzyme amplification. Anal. Chem. 2010, 82, 9447–9454. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, L.; Tesauro, C.; Kurkina, T.; Fiorani, P.; Yu, H.K.; Knudsen, B.R.; Kern, K.; Desideri, A.; Balasubramanian, K. Real-Time Label-Free Direct Electronic Monitoring of Topoisomerase Enzyme Binding Kinetics on Graphene. ACS Nano 2015, 9, 11166–11176. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.F.; Stougaard, M.; Jørgensen, H.L.; Bendsen, S.; Juul, S.; Hald, K.; Andersen, A.H.; Koch, J.; Knudsen, B.R. Multiplexed detection of site specific recombinase and DNA topoisomerase activities at the single molecule level. ACS Nano 2009, 3, 4043–4054. [Google Scholar] [CrossRef]
- Hardin, A.H.; Sarkar, S.K.; Seol, Y.; Liou, G.F.; Osheroff, N.; Neuman, K.C. Direct measurement of DNA bending by type IIA topoisomerases: Implications for non-equilibrium topology simplification. Nucleic AcidsRes. 2011, 39, 5729–5743. [Google Scholar] [CrossRef]
- Jakobsen, A.-K.; Stougaard, M. Combining a Nanosensor and ELISA for Measurement of Tyrosyl-DNA Phosphodiesterase 1 (TDP1) Activity and Protein Amount in Cell and Tissue Extract. Nano Life 2015, 5, 1541001. [Google Scholar] [CrossRef]
- Jensen, P.W.; Falconi, M.; Kristoffersen, E.L.; Simonsen, A.T.; Cifuentes, J.B.; Marcussen, L.B.; Frøhlich, R.; Vagner, J.; Harmsen, C.; Juul, S.; et al. Real-time detection of TDP1 activity using a fluorophore-quencher coupled DNA-biosensor. Biosens. Bioelectron. 2013, 48, 230–237. [Google Scholar] [CrossRef]
- Jepsen, M.L.; Harmsen, C.; Godbole, A.A.; Nagaraja, V.; Knudsen, B.R.; Ho, Y.P. Specific detection of the cleavage activity of mycobacterial enzymes using a quantum dot based DNA nanosensor. Nanoscale 2016, 8, 358–364. [Google Scholar] [CrossRef]
- Kristoffersen, E.L.; Jørgensen, L.A.; Franch, O.; Etzerodt, M.; Frøhlich, R.; Bjergbæk, L.; Stougaard, M.; Ho, Y.P.; Knudsen, B.R. Real-time investigation of human topoisomerase I reaction kinetics using an optical sensor: A fast method for drug screening and determination of active enzyme concentrations. Nanoscale 2015, 7, 9825–9834. [Google Scholar] [CrossRef] [PubMed]
- Stougaard, M.; Juul, S.; Andersen, F.F.; Knudsen, B.R. Strategies for highly sensitive biomarker detection by Rolling Circle Amplification of signals from nucleic acid composed sensors. Integr. Biol. 2011, 3, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, C.; Juul, S.; Arnò, B.; Nielsen, C.J.; Fiorani, P.; Frøhlich, R.F.; Andersen, F.F.; Desideri, A.; Stougaard, M.; Petersen, E.; et al. Specific detection of topoisomerase I from the malaria causing P. falciparum parasite using isothermal rolling circle amplification. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2416–2419. [Google Scholar]
- Jäger, S.; Rasched, G.; Kornreich-Leshem, H.; Engeser, M.; Thum, O.; Famulok, M. A versatile toolbox for variable DNA functionalization at high density. J. Am. Chem. Soc. 2005, 127, 15071–15082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tuleouva, N.; Ramanculov, E.; Revzin, A. Aptamer-based electrochemical biosensor for interferon gamma detection. Anal. Chem. 2010, 82, 8131–8136. [Google Scholar] [CrossRef]
- Blanco, L.; Bernad, A.; Lázaro, J.M.; Martín, G.; Garmendia, C.; Salas, M. Highly efficient DNA synthesis by the phage phi 29 DNA polymerase. Symmetrical mode of DNA replication. J. Biol. Chem. 1989, 264, 8935–8940. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Kataoka, Y.; Tobita, S.; Kuwahara, M.; Sugimoto, N. Novel One-Tube-One-Step Real-Time Methodology for Rapid Transcriptomic Biomarker Detection: Signal Amplification by Ternary Initiation Complexes. Anal. Chem. 2016, 88, 7137–7144. [Google Scholar] [CrossRef]
- Wei, H.; Tang, S.; Hu, T.; Zhao, G.; Guan, Y. Production of dumbbell probe through hairpin cleavage-ligation and increasing RCA sensitivity and specificity by circle to circle amplification. Sci. Rep. 2016, 6, 29229. [Google Scholar] [CrossRef]
- Franch, O.; Han, X.; Marcussen, L.B.; Givskov, A.; Andersen, M.B.; Godbole, A.A.; Harmsen, C.; Nørskov-Lauritsen, N.; Thomsen, J.; Pedersen, F.S.; et al. A new DNA sensor system for specific and quantitative detection of mycobacteria. Nanoscale 2019, 11, 587–597. [Google Scholar] [CrossRef]
- Hede, M.S.; Fjelstrup, S.; Lötsch, F.; Zoleko, R.M.; Klicpera, A.; Groger, M.; Mischlinger, J.; Endame, L.; Veletzky, L.; Neher, R.; et al. Detection of the Malaria causing Plasmodium Parasite in Saliva from Infected Patients using Topoisomerase I Activity as a Biomarker. Sci. Rep. 2018, 8, 4122. [Google Scholar] [CrossRef]
- Keller, J.G.; Petersen, K.V.; Mizielinski, K.; Thiesen, C.; Bjergbæk, L.; Reguera, R.M.; Pérez-Pertejo, Y.; Balaña-Fouce, R.; Trejo, A.; Masdeu, C.; et al. Gel-Free Tools for Quick and Simple Screening of Anti-Topoisomerase 1 Compounds. Pharmaceuticals 2023, 16, 657. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Thomsen, J.; Selnihhin, D.; Hede, M.S.; Kirsebom, F.C.; Franch, O.; Fjelstrup, S.; Stougaard, M.; Ho, Y.P.; et al. Novel DNA sensor system for highly sensitive and quantitative retrovirus detection using virus encoded integrase as a biomarker. Nanoscale 2017, 9, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rani, K.; Gotmare, A.; Bhattacharyya, S. A tale of topoisomerases and the knotty genetic material in the backdrop of Plasmodium biology. Biosci. Rep. 2022, 42, BSR20212847. [Google Scholar] [CrossRef] [PubMed]
- Tosh, K.; Cheesman, S.; Horrocks, P.; Kilbey, B. Plasmodium falciparum: Stage-related expression of topoisomerase I. Exp. Parasitol. 1999, 91, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.G.; Mizielinski, K.; Petersen, K.V.; Stougaard, M.; Knudsen, B.R.; Tesauro, C. Simple and Fast Rolling Circle Amplification-Based Detection of Topoisomerase 1 Activity in Crude Biological Samples. J. Vis. Exp. 2022, e64484. [Google Scholar] [CrossRef]
- Juul, S.; Nielsen, C.J.; Labouriau, R.; Roy, A.; Tesauro, C.; Jensen, P.W.; Harmsen, C.; Kristoffersen, E.L.; Chiu, Y.L.; Frøhlich, R.; et al. Droplet microfluidics platform for highly sensitive and quantitative detection of malaria-causing Plasmodium parasites based on enzyme activity measurement. ACS Nano 2012, 6, 10676–10683. [Google Scholar] [CrossRef]
- Ramharter, M.; Agnandji, S.T.; Adegnika, A.A.; Lell, B.; Mombo-Ngoma, G.; Grobusch, M.P.; McCall, M.; Muranaka, R.; Kreidenweiss, A.; Velavan, T.P.; et al. Development of sustainable research excellence with a global perspective on infectious diseases: Centre de Recherches Médicales de Lambaréné (CERMEL), Gabon. Wien. Klin. Wochenschr. 2021, 133, 500–508. [Google Scholar] [CrossRef]
- Planche, T.; Krishna, S.; Kombila, M.; Engel, K.; Faucher, J.F.; Ngou-Milama, E.; Kremsner, P.G. Comparison of methods for the rapid laboratory assessment of children with malaria. Am. J. Trop. Med. Hyg. 2001, 65, 599–602. [Google Scholar] [CrossRef]
- Juul-Kristensen, T.; Keller, J.G.; Borg, K.N.; Hansen, N.Y.; Foldager, A.; Ladegaard, R.; Ho, Y.P.; Loeschcke, V.; Knudsen, B.R. Topoisomerase 1 Activity Is Reduced in Response to Thermal Stress in Fruit Flies and in Human HeLa Cells. Biosensors 2023, 13, 950. [Google Scholar] [CrossRef]
- Josephine Geertsen, K.; Vandsø, P.K.; Birgitta, R.K.; Cinzia, T. Simple and Fast DNA-Based Tool to Investigate Topoisomerase 1 Activity, a Biomarker for Drug Susceptibility in Colorectal Cancer. In Recent Understanding of Colorectal Cancer Treatment; Keun-Yeong, J., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 3. [Google Scholar]
- Putaporntip, C.; Buppan, P.; Jongwutiwes, S. Improved performance with saliva and urine as alternative DNA sources for malaria diagnosis by mitochondrial DNA-based PCR assays. Clin. Microbiol. Infect. 2011, 17, 1484–1491. [Google Scholar] [CrossRef]
- McMorrow, M.L.; Aidoo, M.; Kachur, S.P. Malaria rapid diagnostic tests in elimination settings—Can they find the last parasite? Clin. Microbiol. Infect. 2011, 17, 1624–1631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juul-Kristensen, T.; Thiesen, C.; Wulff Haurum, L.; Keller, J.G.; Lendamba, R.W.; Zoleko Manego, R.; Betouke Ongwe, M.E.; Knudsen, B.R.; Pareja, E.; Pareja-Tobes, E.; et al. Non-Invasive Malaria Detection in Sub-Saharan Africa Using a DNA-Based Sensor System. Sensors 2024, 24, 7947. https://doi.org/10.3390/s24247947
Juul-Kristensen T, Thiesen C, Wulff Haurum L, Keller JG, Lendamba RW, Zoleko Manego R, Betouke Ongwe ME, Knudsen BR, Pareja E, Pareja-Tobes E, et al. Non-Invasive Malaria Detection in Sub-Saharan Africa Using a DNA-Based Sensor System. Sensors. 2024; 24(24):7947. https://doi.org/10.3390/s24247947
Chicago/Turabian StyleJuul-Kristensen, Trine, Celine Thiesen, Line Wulff Haurum, Josephine Geertsen Keller, Romeo Wenceslas Lendamba, Rella Zoleko Manego, Madeleine Eunice Betouke Ongwe, Birgitta Ruth Knudsen, Eduardo Pareja, Eduardo Pareja-Tobes, and et al. 2024. "Non-Invasive Malaria Detection in Sub-Saharan Africa Using a DNA-Based Sensor System" Sensors 24, no. 24: 7947. https://doi.org/10.3390/s24247947
APA StyleJuul-Kristensen, T., Thiesen, C., Wulff Haurum, L., Keller, J. G., Lendamba, R. W., Zoleko Manego, R., Betouke Ongwe, M. E., Knudsen, B. R., Pareja, E., Pareja-Tobes, E., Labouriau, R., Mombo-Ngoma, G., & Tesauro, C. (2024). Non-Invasive Malaria Detection in Sub-Saharan Africa Using a DNA-Based Sensor System. Sensors, 24(24), 7947. https://doi.org/10.3390/s24247947







