ppb-Level SO2 Photoacoustic Sensor for SF6 Decomposition Analysis Utilizing a High-Power UV Laser with a Power Normalization Method
Abstract
1. Introduction
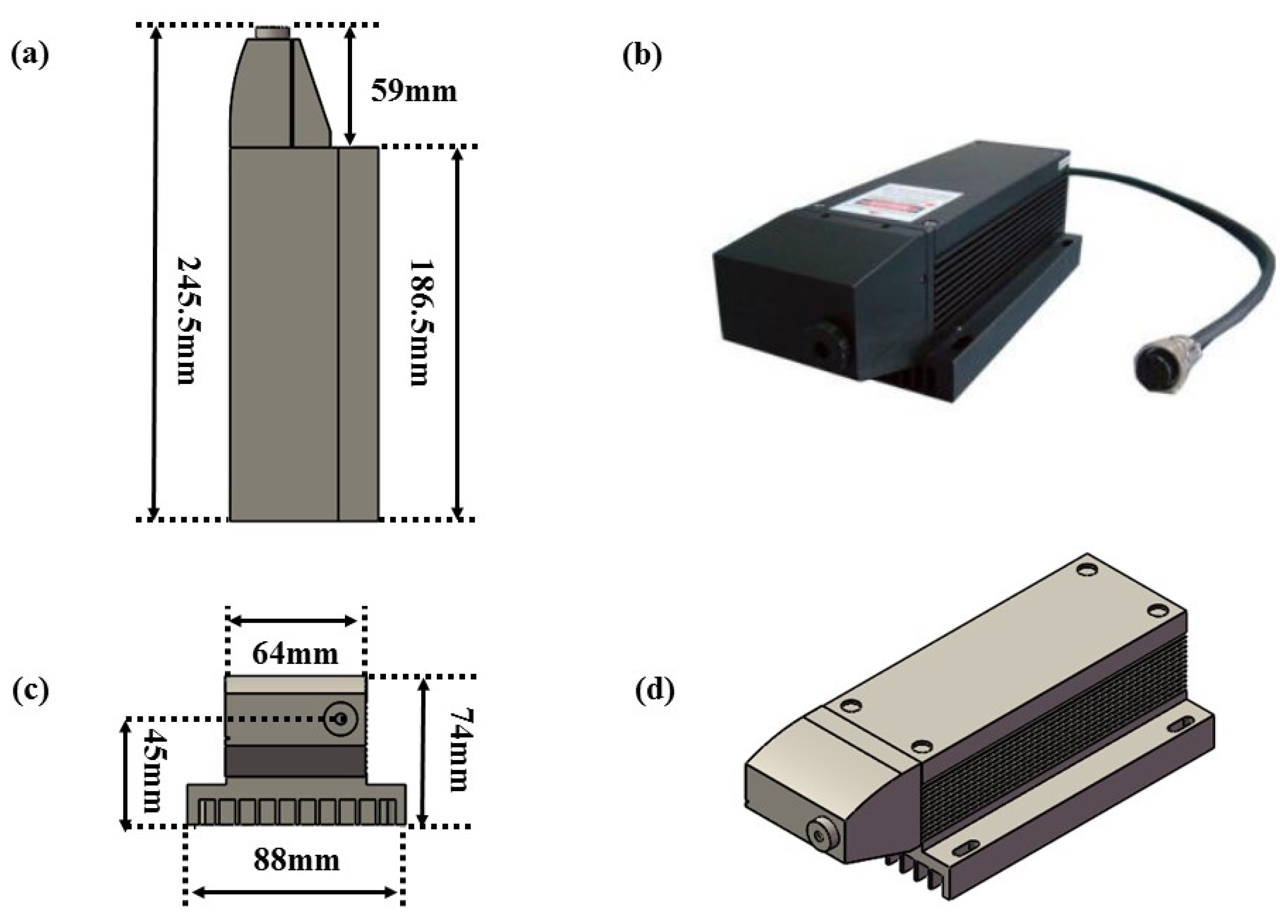
2. Selection of Optical Excitation Source for SO2 Detection
3. Photoacoustic Cell Design
4. The Establishment of the Sensing System
5. Evaluation of Sensor Performance
5.1. Photoacoustic Signal Optimization
5.2. Pressure Optimization
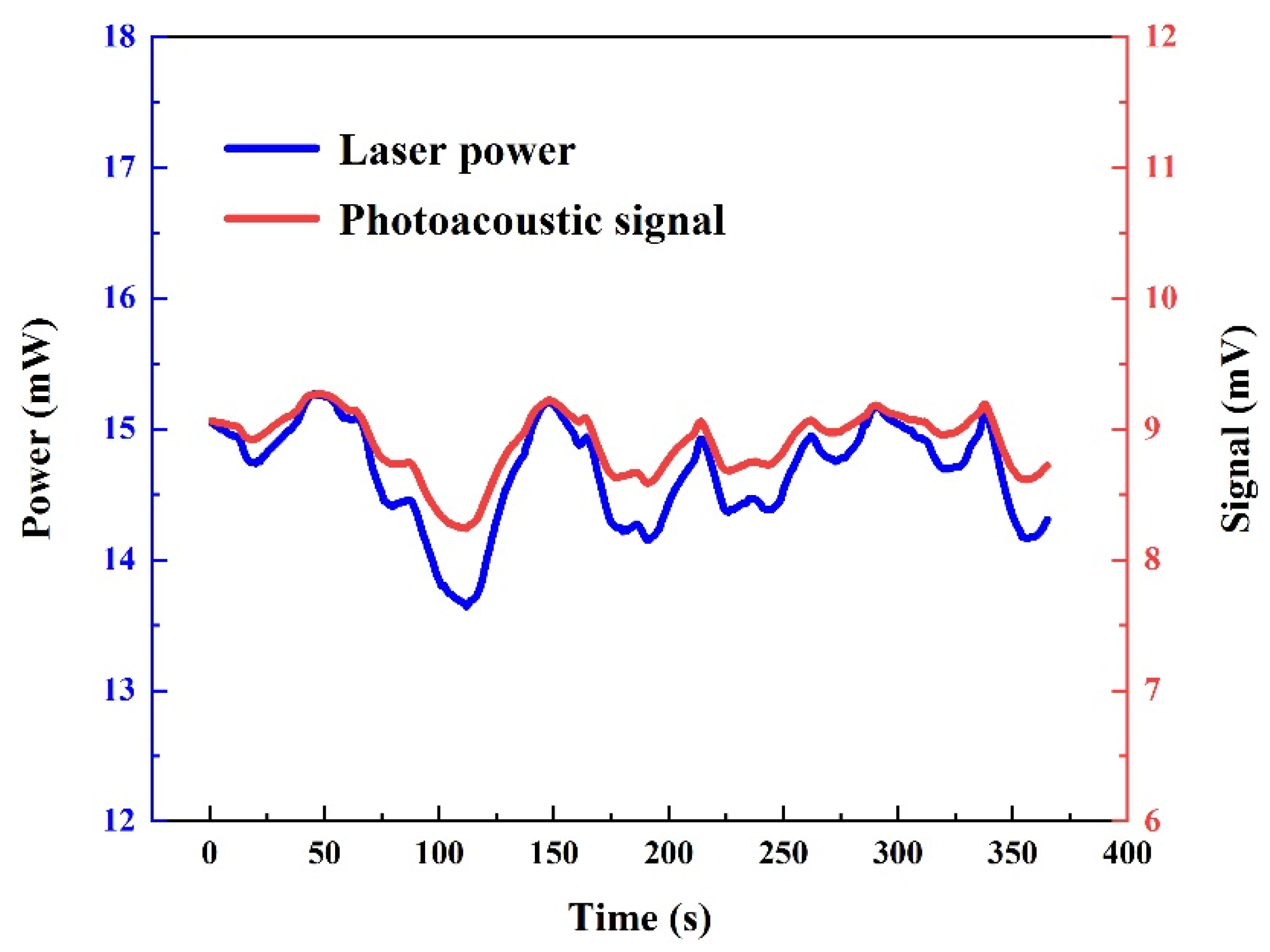
5.3. Performance Evaluation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Fang, Y.H.; Zhao, Y.D.; Wang, A.J.; Li, D.C.; Li, Y.Y.; Liu, Y.; Cui, F.X.; Wu, J.; Liu, J.X. Research on the detection of SF6 decomposition products based on non-resonant photoacoustic spectroscopy. Anal. Methods 2015, 7, 1200–1207. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.; Meng, Q.; Zhou, J. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Dai, H.F.; Xiao, P.; Lou, Q. Application of SnO2/MWCNTs nanocomposite for SF6 decomposition gas sensor. Phys. Status Solidi A Appl. Mater. Sci. 2011, 208, 1714–1717. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, B.; Wang, X.; Luo, C. Effect of plasma treatment on multi-walled carbon nanotubes for the detection of H2S and SO2. Sensors 2012, 12, 9375–9385. [Google Scholar] [CrossRef]
- Kurte, R.; Beyer, C.; Heise, H.M.; Klockow, D. Application of infrared spectroscopy to monitoring gas insulated high-voltage equipment: Electrode material-dependent SF6 decomposition. Anal. Bioanal. Chem. 2002, 373, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Duffour, E. Molecular dynamic simulations of the collision between copper ions, SF6 molecules and a polyethylene surface: A study of decomposition products and an evaluation of the self-diffusion coefficients. Macromol. Theory Simul. 2010, 19, 88–99. [Google Scholar] [CrossRef]
- Peng, S.; Wu, G.; Song, W.; Wang, Q. Application of flower-like ZnO nanorods gas sensor detecting SF6 decomposition products. J. Nanomater. 2013, 2013, 875. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Chen, C.; Zhang, Q.; Sultana, R.; Han, Y. Monitoring and Leak Diagnostics of Sulfur Hexafluoride and Decomposition Gases from Power Equipment for the Reliability and Safety of Power Grid Operation. Appl. Sci. 2024, 14, 3844. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G.; Lee, H.; Moon, D.; Lee, J.; Lim, J.S. Comparative study of various methods for trace SF6 measurement using GC-µECD: Demonstration of lab-pressure-based drift correction by preconcentrator. J. Atmos. Ocean. Technol. 2020, 37, 901–910. [Google Scholar] [CrossRef]
- Chu, J.; Yang, A.; Wang, Q.; Yang, X.; Wang, D.; Wang, X.; Yuan, H.; Rong, M. Multicomponent SF6 decomposition product sensing with a gas-sensing microchip. Microsyst. Nanoeng. 2021, 7, 18. [Google Scholar] [CrossRef]
- Sigrist, M.W. Trace gas monitoring by laser photoacoustic spectroscopy and related techniques (plenary). Rev. Sci. Instrum. 2003, 74, 486–490. [Google Scholar] [CrossRef]
- Wu, H.P.; Dong, L.; Zheng, H.D.; Yu, Y.J.; Ma, W.G.; Zhang, L.; Yin, W.B.; Xiao, L.T.; Jia, S.T.; Tittel, F.K. Calibration-free fast quartz-enhanced photoacoustic spectroscopy based on beat frequency effect for continuous trace gas monitoring. Nat. Commun. 2017, 8, 15331. [Google Scholar] [CrossRef]
- Yin, X.; Dong, L.; Zheng, H.; Liu, X.; Wu, H.; Yang, Y.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; et al. Impact of humidity on quartz-enhanced photoacoustic spectroscopy based CO detection using a near-IR telecommunication diode laser. Sensors 2016, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Berer, T.; Brandstetter, M.; Hochreiner, A.; Langer, G.; Märzinger, W.; Burgholzer, P.; Lendl, B. Remote mid-infrared photoacoustic spectroscopy with a quantum cascade laser. Opt. Lett. 2015, 40, 3476–3479. [Google Scholar] [CrossRef] [PubMed]
- Kottmann, J.; Rey, J.M.; Sigrist, M.W. Mid-Infrared photoacoustic detection of glucose in human skin: Towards non-invasive diagnostics. Sensors 2016, 16, 1663. [Google Scholar] [CrossRef]
- Ge, H.; Kong, W.; Wang, R.; Zhao, G.; Ma, W.; Chen, W.; Wan, F. Simple technique of coupling a diode laser into a linear power buildup cavity for Raman gas sensing. Opt. Lett. 2023, 48, 2186–2189. [Google Scholar] [CrossRef]
- Wang, C.; Lin, L.; Zeng, X.; Xie, J.; Luo, H.; Lin, H.; Wang, L.; Tang, J.; Cui, R.; Zhu, W.; et al. Ppb-level CO2 sensor based on a miniature multipass cell with eight-petaled spot pattern. Microw. Opt. Technol. Lett. 2024, 66, e34118. [Google Scholar] [CrossRef]
- Yin, X.; Dong, L.; Wu, H.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittle, F.K. Ppb-level H2S detection for SF6 decomposition based on a fiber-amplified telecommunication diode laser and a background-gas-induced high-Q photoacoustic cell. Appl. Phys. Lett. 2017, 111, 031109. [Google Scholar] [CrossRef]
- Yin, X.; Wu, H.; Dong, L.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittle, F.K. Ppb-level photoacoustic sensor system for saturation-free CO detection of SF6 decomposition by use of a 10 W fiber-amplified near-infrared diode laser. Sens. Actuators B Chem. 2019, 282, 567–573. [Google Scholar] [CrossRef]
- Wan, F.; Zhou, F.; Hou, J.; Wang, J.; Chen, W.; Zhu, C.; Liu, Y. Highly Sensitive and Precise Analysis of SF6 Decomposition Component CO by Multi-comb Optical-feedback Cavity Enhanced Absorption Spectroscopy with a 2.3 μm Diode Laser. Sci. Rep. 2019, 9, 9690. [Google Scholar] [CrossRef]
- Zifarelli, A.; Patimisco, P.; Russo, S.D.; Sampaolo, A.; Giglio, M.; Sun, B.; Wu, H.; Dong, L.; Spagnolo, V. Quartz-enhanced photoacoustic spectroscopy for CO detection in SF6 decomposition. In Photonic Instrumentation Engineering VIII; SPIE: Bellingham, WA, USA, 2021; Volume 11693. [Google Scholar]
- Yin, X.; Dong, L.; Wu, H.; Gao, M.; Zhang, L.; Zhang, X.; Liu, L.; Shao, X.; Tittel, F.K. Compact QEPAS humidity sensor in SF6 buffer gas for high-voltage gas power systems. Photoacoustics 2022, 25, 100319. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, B.; Zhao, B.; Yang, P. EC-QCL based photoacoustic spectroscopy for detection of SF6 decomposition components. Sens. Actuators B Chem. 2022, 369, 132351. [Google Scholar] [CrossRef]
- He, Q.; Deng, W.; Wang, W.; Hu, R.; Zhang, J.; Xia, D.; Li, Y. Mid-infrared photoacoustic spectrum analysis of SF6 gas-decomposition system. Electr. Eng. 2023, 105, 4311–4319. [Google Scholar] [CrossRef]
- Chen, K.; Wang, N.; Guo, M.; Zhao, X.; Qi, H.; Li, C.; Zhang, G.; Xu, L. Detection of SF6 gas decomposition component H2S based on fiber-optic photoacoustic sensing. Sens. Actuators B Chem. 2023, 378, 133174. [Google Scholar] [CrossRef]
- Sun, B.; Patimisco, P.; Sampaolo, A.; Zifarelli, A.; Spagnolo, V.; Wu, H.; Dong, L. Light-induced thermoelastic sensor for ppb-level H2S detection in a SF6 gas matrices exploiting a mini-multi-pass cell and quartz tuning fork photodetector. Photoacoustics 2023, 33, 100553. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, X.; Jiang, A.; Qian, W.; Zhang, C.; Zhang, X. Detection of SF6 Decomposition Components H2S and CO2 Based on WDM and CRDS. IEEE Trans. Dielectr. Electr. Insul. 2024. [Google Scholar] [CrossRef]
- Yin, X.; Dong, L.; Wu, H.; Zheng, H.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittle, F.K. Highly sensitive SO2 photoacoustic sensor for SF6 decomposition detection using a compact mW-level diode-pumped solid-state laser emitting at 303 nm. Opt. Express 2017, 25, 32581–32590. [Google Scholar] [CrossRef]
- Rothman, L.S.; Gordon, I.E.; Babikov, Y.; Barbe, A.; Benner, D.C.; Bernath, P.F.; Birk, M.; Bizzocchi, L.; Boudon, V.; Brown, L.R.; et al. The HITRAN 2012 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 4–50. [Google Scholar] [CrossRef]
- Pan, Y.; Dong, L.; Yin, X.; Wu, H. Compact and highly sensitive NO2 photoacoustic sensor for environmental monitoring. Molecules 2020, 25, 1201. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Huang, J.; Xu, Y.; Wang, Z.; Han, X.; Guo, M.; Chen, K. Integrated near-infrared fiber-optic photoacoustic sensing demodulator for ultra-high sensitivity gas detection. Photoacoustics 2023, 33, 100560. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Ma, F.; Zhao, X.; Wang, Z.; Qi, H.; Guo, M.; Chen, K. Multiplexed fiber-optic photoacoustic sensors for simultaneous detection of multi-point gases. Sens. Actuators B Chem. 2023, 399, 134801. [Google Scholar] [CrossRef]
- Chen, K.; Yu, Z.; Yu, Q.; Guo, M.; Zhao, Z.; Qu, C.; Gong, Z.; Yang, Y. Fast demodulated white-light interferometry-based fiber-optic Fabry-Perot cantilever microphone. Opt. Lett. 2018, 43, 3417–3420. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Luo, W.; Duan, L.; Zhao, S.; Jiang, S.; Bao, H.; Ho, H.; Zheng, C.; Zhang, Y.; Ye, W. High sensitivity and stability cavity-enhanced photoacoustic spectroscopy with dual-locking scheme. Sens. Actuators B Chem. 2024, 415, 135984. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Lv, H.; Xie, J.; Wang, C.; Lin, H.; Zhuang, R.; Zhu, W.; Zhong, Y.; Kan, R.; et al. Off-plane quartz-enhanced photoacoustic spectroscopy. Opt. Lett. 2024, 49, 3206–3209. [Google Scholar] [CrossRef]
- Sun, H.; Qiao, S.; He, Y.; Liu, Y.; Ma, Y. Highly sensitive CH4, C2H2 and CO simultaneous measurement LITES sensor based on multi-pass cell with overlapped spots pattern and QTFs with low resonant frequency. Opt. Express 2024, 32, 28183–28194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Han, X.; Qi, H.; Ma, F.; Chen, K. Pressure-Compensated Fiber-Optic Photoacoustic Sensors for Trace SO2 Analysis in Gas Insulation Equipment. Anal. Chem. 2024, 96, 10995–11001. [Google Scholar] [CrossRef]
- Gondal, M.A.; Mastromarino, J. Pulsed laser photoacoustic detection of SO2 near 225.7 nm. Appl. Opt. 2001, 40, 2010–2016. [Google Scholar] [CrossRef]
- Somesfalean, G.; Zhang, Z.G.; Sjöholm, M.; Svanberg, S. All-diode-laser ultraviolet absorption spectroscopy for sulfur dioxide detection. Appl. Phys. B 2005, 80, 1021–1025. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Zhao, X.; Gao, M.; Cheng, K.; Shao, X.; Wu, H.; Dong, L.; Yin, X. Trace photoacoustic SO2 gas sensor in SF6 utilizing a 266 nm UV laser and an acousto-optic power stabilizer. Opt. Express 2023, 31, 6974–6981. [Google Scholar] [CrossRef]
- Keller-Rudek, H.; Moortgat, G.K.; Sander, R.; Sörensen, R. The MPI-Mainz UV/VIS spectral atlas of gaseous molecules of atmospheric interest. Earth Syst. Sci. Data 2013, 5, 365–373. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chen, B.; He, Y.; Zhu, C.; Zhou, X.; Liang, Y.; Li, B.; Yin, X. ppb-Level SO2 Photoacoustic Sensor for SF6 Decomposition Analysis Utilizing a High-Power UV Laser with a Power Normalization Method. Sensors 2024, 24, 7911. https://doi.org/10.3390/s24247911
Yang X, Chen B, He Y, Zhu C, Zhou X, Liang Y, Li B, Yin X. ppb-Level SO2 Photoacoustic Sensor for SF6 Decomposition Analysis Utilizing a High-Power UV Laser with a Power Normalization Method. Sensors. 2024; 24(24):7911. https://doi.org/10.3390/s24247911
Chicago/Turabian StyleYang, Xiu, Baisong Chen, Yuyang He, Chenchen Zhu, Xing Zhou, Yize Liang, Biao Li, and Xukun Yin. 2024. "ppb-Level SO2 Photoacoustic Sensor for SF6 Decomposition Analysis Utilizing a High-Power UV Laser with a Power Normalization Method" Sensors 24, no. 24: 7911. https://doi.org/10.3390/s24247911
APA StyleYang, X., Chen, B., He, Y., Zhu, C., Zhou, X., Liang, Y., Li, B., & Yin, X. (2024). ppb-Level SO2 Photoacoustic Sensor for SF6 Decomposition Analysis Utilizing a High-Power UV Laser with a Power Normalization Method. Sensors, 24(24), 7911. https://doi.org/10.3390/s24247911





