Strategy for Accurate Detection of Six Tropane Alkaloids in Honey Using Lateral Flow Immunosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of AuNPs
2.3. Preparation of AuNPs Immunoprobes
2.4. Preparation of Immunochromatographic Test Strips
2.5. Sample Preparation
2.6. Detection Procedure
2.7. Method Evaluation
3. Results and Discussion
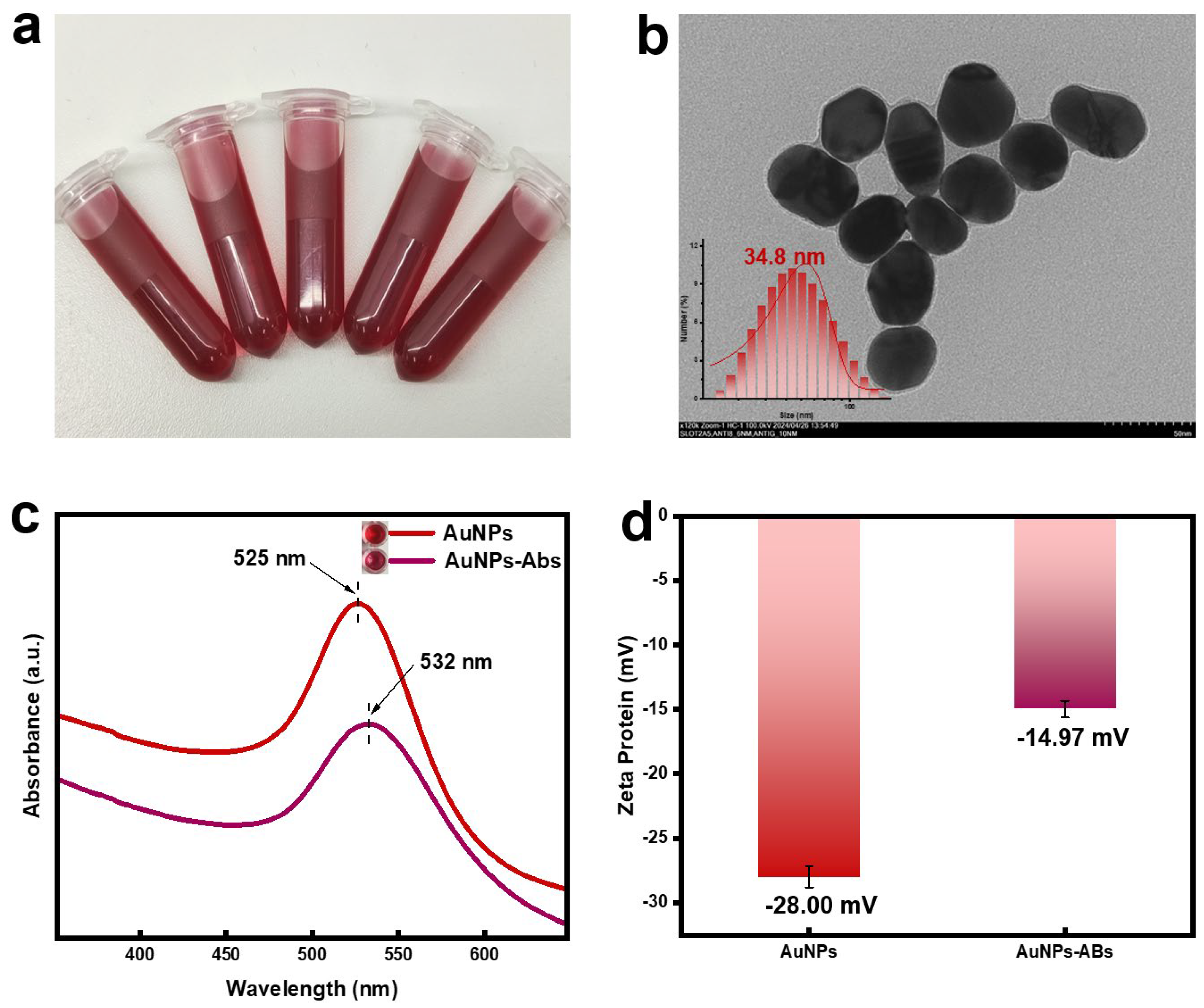
3.1. Characterization of AuNPs and AuNPs-Abs
3.2. Optimization of Conditions
3.2.1. pH Value
3.2.2. NC Membrane
3.2.3. Sample Pad
3.2.4. Optimization of the Input Amount of the TA Monoclonal Antibody
3.2.5. Amount of AuNPs-Abs
3.2.6. TRO-EDC-BSA Concentration
3.2.7. IgG Concentration
3.2.8. Sample Diluent
3.2.9. Incubation and Chromatography Time
3.3. Analytical Performance
3.3.1. Detection Sensitivity
3.3.2. Specificity
3.3.3. Accuracy and Reliability
3.3.4. Practicality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J.C. Tropane, and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Fodor, G.; Dharanipragada, R. Tropane Alkaloids. Nat. Prod. Rep. 1986, 3, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cai, Z.; Zou, Y.; Wu, D.; Wang, A.; Chang, J.; Wang, F.; Tian, Z.; Liu, G. Silver Nanoparticle-Based Surface-Enhanced Raman Spectroscopy for the Rapid and Selective Detection of Trace Tropane Alkaloids in Food. ACS Appl. Nano Mater. 2019, 2, 6592–6601. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Worldwide Occurrence and Investigations of Contamination of Herbal Medicines by Tropane Alkaloids. Toxins 2017, 9, 284. [Google Scholar] [CrossRef]
- Huang, J.-P.; Wang, Y.-J.; Tian, T.; Wang, L.; Yan, Y.; Huang, S.-X. Tropane Alkaloid Biosynthesis: A Centennial Review. Nat. Prod. Rep. 2021, 38, 1634–1658. [Google Scholar] [CrossRef]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Kwiatek, K. Simultaneous Determination of Pyrrolizidine and Tropane Alkaloids in Honey by Liquid Chromatography-Mass Spectrometry. J. Vet. Res. 2022, 66, 235–243. [Google Scholar] [CrossRef]
- Cirlini, M.; Demuth, T.M.; Biancardi, A.; Rychlik, M.; Dall’Asta, C.; Bruni, R. Are Tropane Alkaloids Present in Organic Foods? Detection of Scopolamine and Atropine in Organic Buckwheat (Fagopyron Esculentum L.) Products by UHPLC–MS/MS. Food Chem. 2018, 239, 141–147. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, P.; Wang, J.; He, S.; Ren, Z.; Zhang, Y.; Xiong, J.; Jiang, H. Indirect Competitive Enzyme-Linked Immunosorbent Assay Based on a Broad-Spectrum Monoclonal Antibody for Tropane Alkaloids Detection in Pig Urine, Pork and Cereal Flours. Food Chem. 2021, 337, 127617. [Google Scholar] [CrossRef]
- Eeva, M.; Salo, J.P.; Oksman-Caldentey, K.M. Determination of the Main Tropane Alkaloids from Transformed Hyoscyamus Muticus Plants by Capillary Zone Electrophoresis. J. Pharm. Biomed. Anal. 1998, 16, 717–722. [Google Scholar] [CrossRef]
- Garcia-Juan, A.; Leon, N.; Armenta, S.; Pardo, O. Development and Validation of an Analytical Method for the Simultaneous Determination of 12 Ergot, 2 Tropane, and 28 Pyrrolizidine Alkaloids in Cereal-Based Food by LC-MS/MS. Food Res. Int. 2023, 174, 113614. [Google Scholar] [CrossRef] [PubMed]
- Dräger, B. Analysis of Tropane and Related Alkaloids. J. Chromatogr. A 2002, 978, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Temerdashev, A.Z.; Kolychev, I.A.; Kiseleva, N.V. Chromatographic Determination of Some Tropane Alkaloids in Datura Metel. J. Anal. Chem. 2012, 67, 960–966. [Google Scholar] [CrossRef]
- Gadzikowska, M.; Jozwiak, G.W.; Waksmundzka-Hajnos, M. Effect of the Vapour Phase on the TLC Separation of Tropane Alkaloids. Acta Chromatogr. 2010, 22, 515–525. [Google Scholar] [CrossRef]
- Cirlini, M.; Cappucci, V.; Galaverna, G.; Dall’Asta, C.; Bruni, R. A Sensitive UHPLC-ESI-MS/MS Method for the Determination of Tropane Alkaloids in Herbal Teas and Extracts. Food Control 2019, 105, 285–291. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, F.; Li, F.; Jia, Y.; Wang, M.; Hua, X.; Wang, L. Development of Ic-ELISA and Colloidal Gold Lateral Flow Immunoassay for the Determination of Cypermethrin in Agricultural Samples. Biosensors 2022, 12, 1058. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Irie, M.; Yoshimatsu, K.; Ishimaru, K.; Shimomura, K.; Satake, M.; Sueyoshi, S.; Tanno, M.; Kamiya, S.; Sawada, J.; et al. A Monoclonal-Antibody to Scopolamine and Its Use for Competitive Enzyme-Linked-Immunosorbent-Assay. Phytochemistry 1991, 30, 3273–3276. [Google Scholar] [CrossRef]
- Hagemann, K.; Piek, K.; Stockigt, J.; Weiler, E. Monoclonal Antibody-Based Enzyme-Immunoassay for the Quantitative-Determination of the Tropane Alkaloid, Scopolamine. Planta Med. 1992, 58, 68–72. [Google Scholar] [CrossRef]
- Xu, X.; Ge, W.; Suryoprabowo, S.; Guo, X.; Zhu, J.; Liu, L.; Xu, C.; Kuang, H. Fluorescence-Based Immunochromatographic Test Strip for the Detection of Hyoscyamine. Analyst 2022, 147, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled Nucleation for Regulation of Particle-Size in Monodisperse Gold Suspensions. Nat. -Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Sun, X.L.; Zhao, X.L.; Tang, J.; Zhou, J.; Chu, F.S. Preparation of Gold-Labeled Antibody Probe and Its Use in Immunochromatography Assay for Detection of Aflatoxin B1. Int. J. Food Microbiol. 2005, 99, 185–194. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zheng, Z.; Liu, H.; Zhu, L.; Yang, M.; Chen, Y. Nanocellulose-Based Membranes for Highly Efficient Molecular Separation. Chem. Eng. J. 2023, 451, 138711. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Huang, T.-H.; Chen, C.-A.; Ho, N.Y.-J.; Chou, Y.-J.; Chen, C.-F. Development a Stacking Pad Design for Enhancing the Sensitivity of Lateral Flow Immunoassay. Sci. Rep. 2018, 8, 17319. [Google Scholar] [CrossRef] [PubMed]


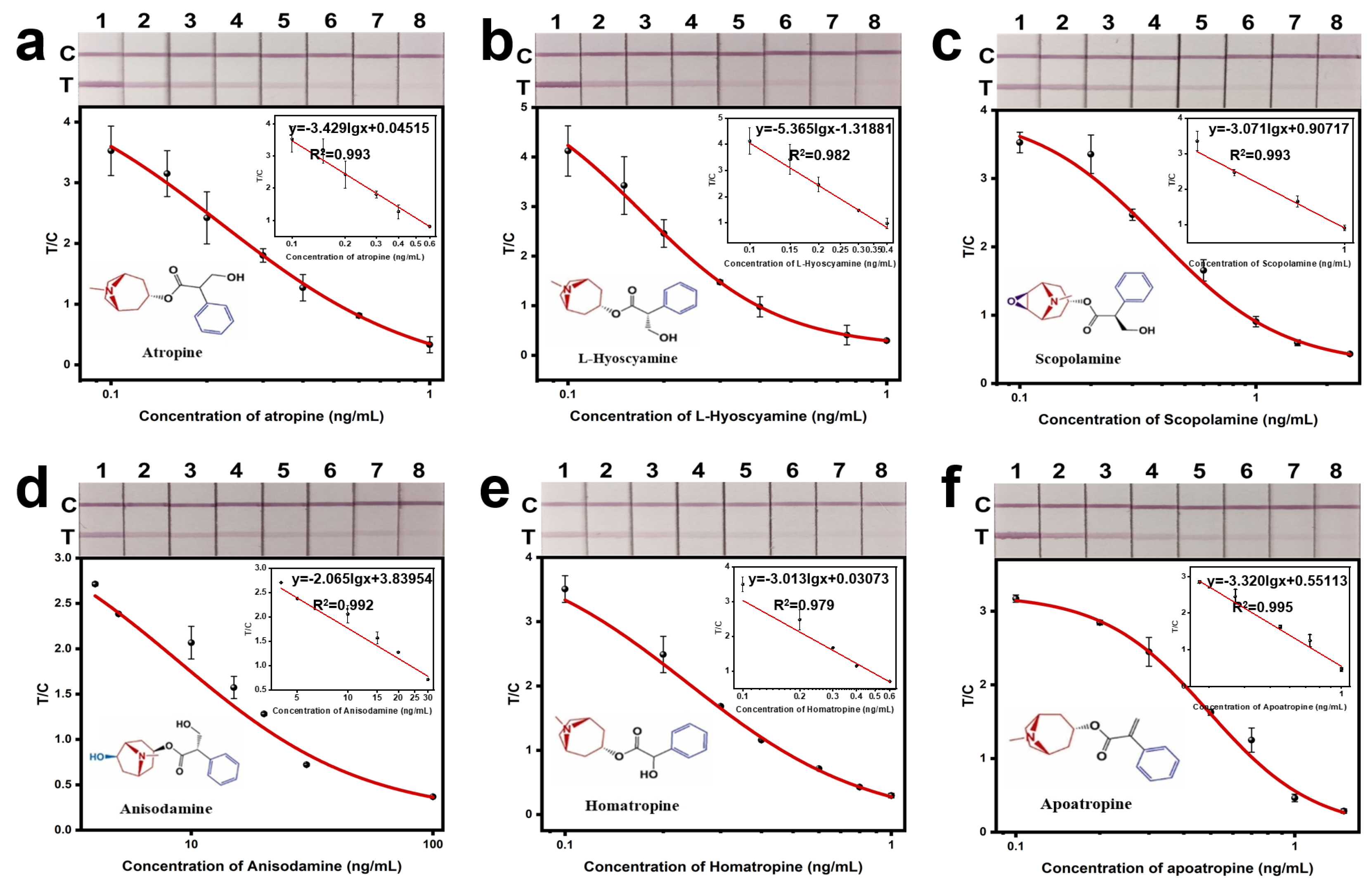

| Analytes | R2 | IC50 (ng/mL) | LOD (ng/mL) | Linear Range (ng/mL) | Cross-Reactivity |
|---|---|---|---|---|---|
| ATR | 0.998 | 1.11 | 0.22 | 0.40–3.03 | 100% |
| LHY | 0.999 | 0.86 | 0.29 | 0.43–1.70 | 129.1% |
| SCO | 0.998 | 1.78 | 0.51 | 0.83–4.48 | 62.2% |
| ANI | 0.996 | 44.14 | 6.34 | 12.98–150.14 | 2.5% |
| HOM | 0.997 | 1.19 | 0.30 | 0.49–2.87 | 93.1% |
| APO | 0.998 | 2.46 | 0.94 | 1.35–4.51 | 45.0% |
| Analytes | Spiked Concentration (ng/mL) | Tested Concentration (ng/mL) | Mean Recovery (%) | Coefficient of Variation (%) |
|---|---|---|---|---|
| ATR | 0.5 | 0.55 ± 0.01 | 109.4 | 1.4 |
| 1.0 | 0.93 ± 0.08 | 93.4 | 8.5 | |
| 3.0 | 3.15 ± 0.03 | 105.3 | 1.0 | |
| LHY | 0.5 | 0.48 ± 0.02 | 95.6 | 3.8 |
| 1 | 1.01 ± 0.10 | 100.8 | 9.9 | |
| 1.5 | 1.54 ± 0.05 | 102.8 | 3.5 | |
| SCO | 1.0 | 1.03 ± 0.09 | 103.2 | 8.6 |
| 2.0 | 2.10 ± 0.19 | 104.8 | 9.1 | |
| 4.0 | 3.76 ± 0.15 | 93.9 | 3.9 | |
| ANI | 15 | 14.72 ± 1.66 | 98.1 | 11.2 |
| 45 | 45.74 ± 3.46 | 101.6 | 7.6 | |
| 150 | 140.26 ± 7.54 | 93.5 | 5.4 | |
| HOM | 0.5 | 0.53 ± 0.08 | 106.7 | 14.7 |
| 1 | 0.90 ± 0.03 | 90.1 | 2.9 | |
| 2.5 | 2.63 ± 0.25 | 105.1 | 9.3 | |
| APO | 1.5 | 1.56 ± 0.10 | 103.9 | 6.3 |
| 2.5 | 2.63 ± 0.17 | 105.2 | 6.4 | |
| 4.5 | 4.53 ± 0.14 | 100.7 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Wang, C.; Wang, Z.; Liang, J.; Han, K.; Zhang, S.; Yin, C.; Wang, X.; Liu, C.; Feng, Z.; et al. Strategy for Accurate Detection of Six Tropane Alkaloids in Honey Using Lateral Flow Immunosensors. Sensors 2024, 24, 7265. https://doi.org/10.3390/s24227265
Sun B, Wang C, Wang Z, Liang J, Han K, Zhang S, Yin C, Wang X, Liu C, Feng Z, et al. Strategy for Accurate Detection of Six Tropane Alkaloids in Honey Using Lateral Flow Immunosensors. Sensors. 2024; 24(22):7265. https://doi.org/10.3390/s24227265
Chicago/Turabian StyleSun, Boyan, Chuanlei Wang, Zile Wang, Jiayi Liang, Ke Han, Shuai Zhang, Chunchao Yin, Xiaomei Wang, Chujun Liu, Zhiyue Feng, and et al. 2024. "Strategy for Accurate Detection of Six Tropane Alkaloids in Honey Using Lateral Flow Immunosensors" Sensors 24, no. 22: 7265. https://doi.org/10.3390/s24227265
APA StyleSun, B., Wang, C., Wang, Z., Liang, J., Han, K., Zhang, S., Yin, C., Wang, X., Liu, C., Feng, Z., Wang, S., & Jiang, H. (2024). Strategy for Accurate Detection of Six Tropane Alkaloids in Honey Using Lateral Flow Immunosensors. Sensors, 24(22), 7265. https://doi.org/10.3390/s24227265








