Hyperbranched Thermosensitive Polymer-AuNP Composite Probe for Temperature Colorimetric Detection
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
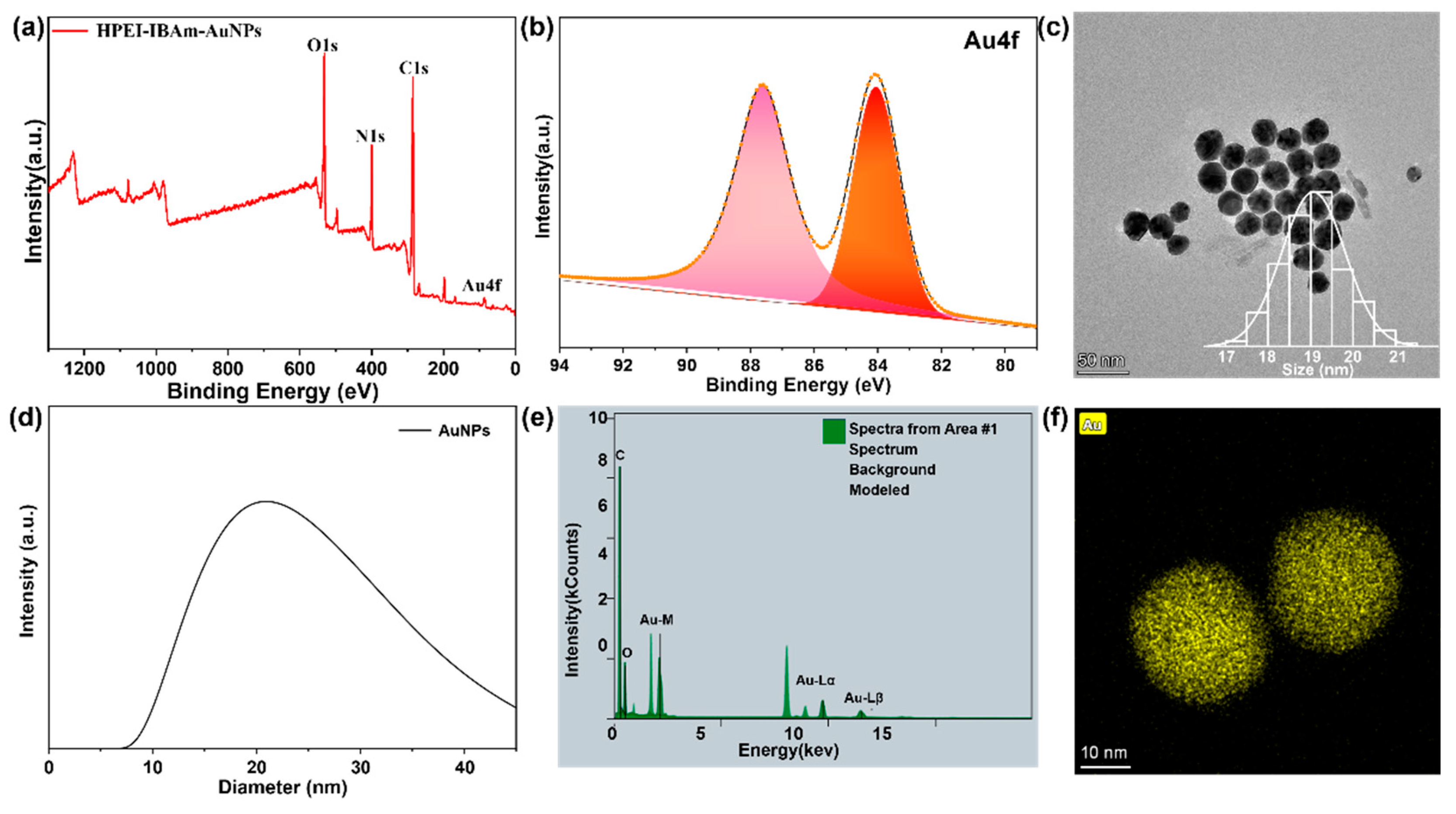
2.2. Preparation of AuNPs
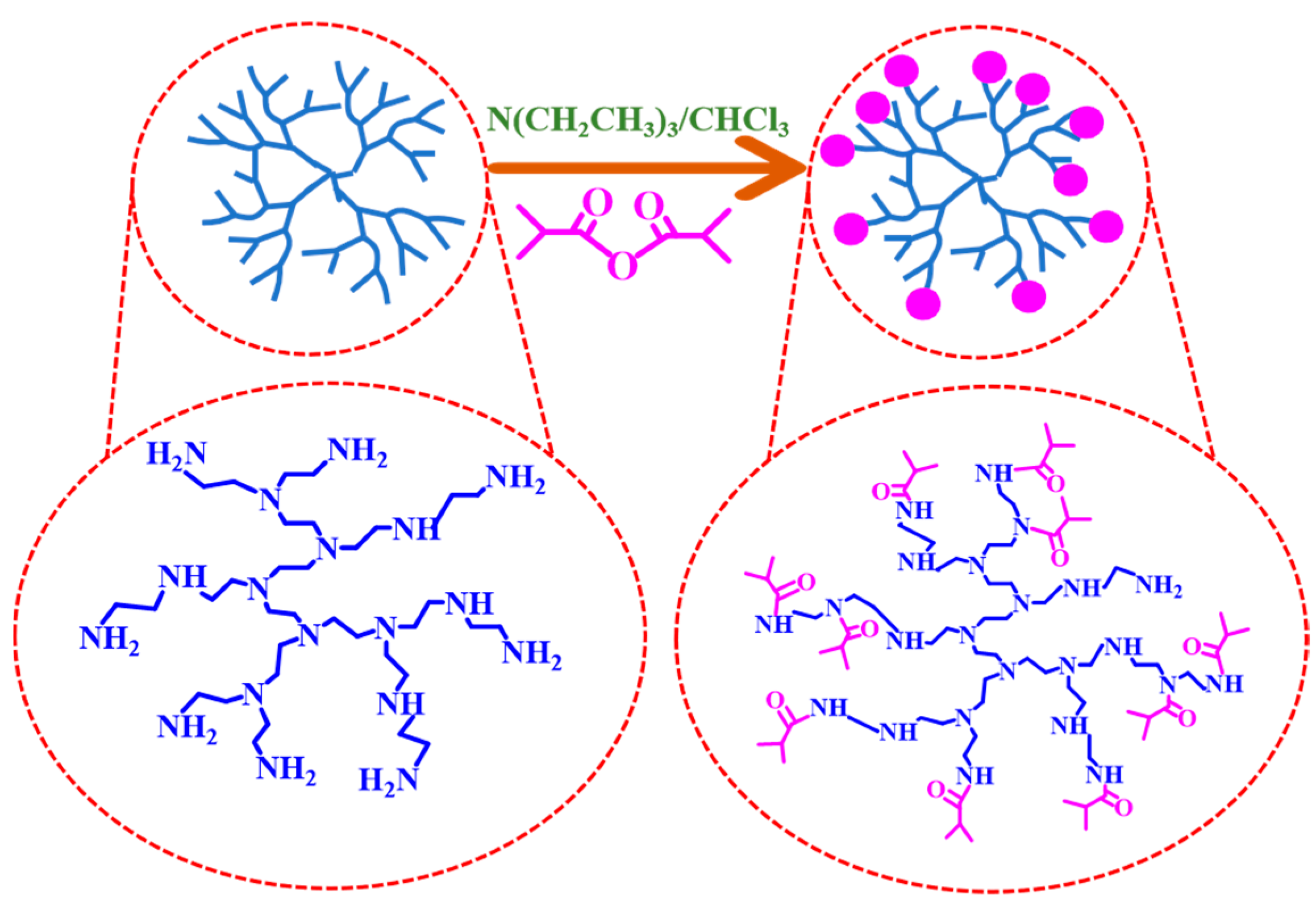
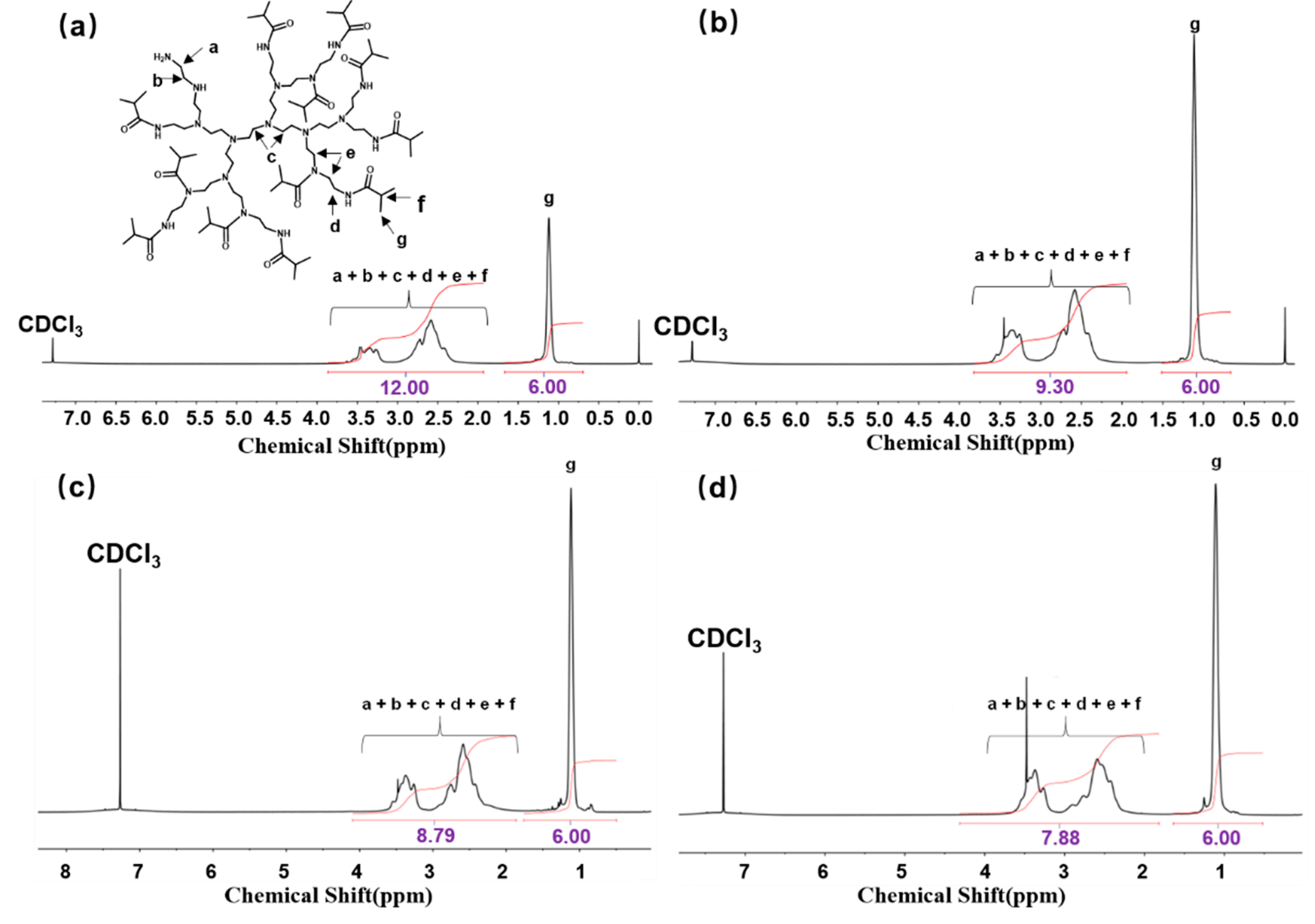
2.3. Preparation of HPEI-IBAm-AuNPs
2.4. Preparation of Polydimethylsiloxane Film-Based Flexible Probes
2.5. Optimization of Detection Conditions
2.6. Salt Stability of HPEI-IBAm-AuNP Probes
2.7. The Detection Sensitivity and Cycling Stability of the HPEI-IBAm-AuNP Probe
2.8. Practical Application of HPEI-IBAm-AuNP Probes for Temperature Detection
3. Results and Discussion
3.1. Characterization of HPEI-IBAm-AuNPs
3.2. Optimization of Experimental Conditions for Detection Temperature of HPEI-IBAm-AuNPs
3.3. The Salt Stability of the Probe
3.4. Sensitivity and Reversibility of Temperature Detection by Probes
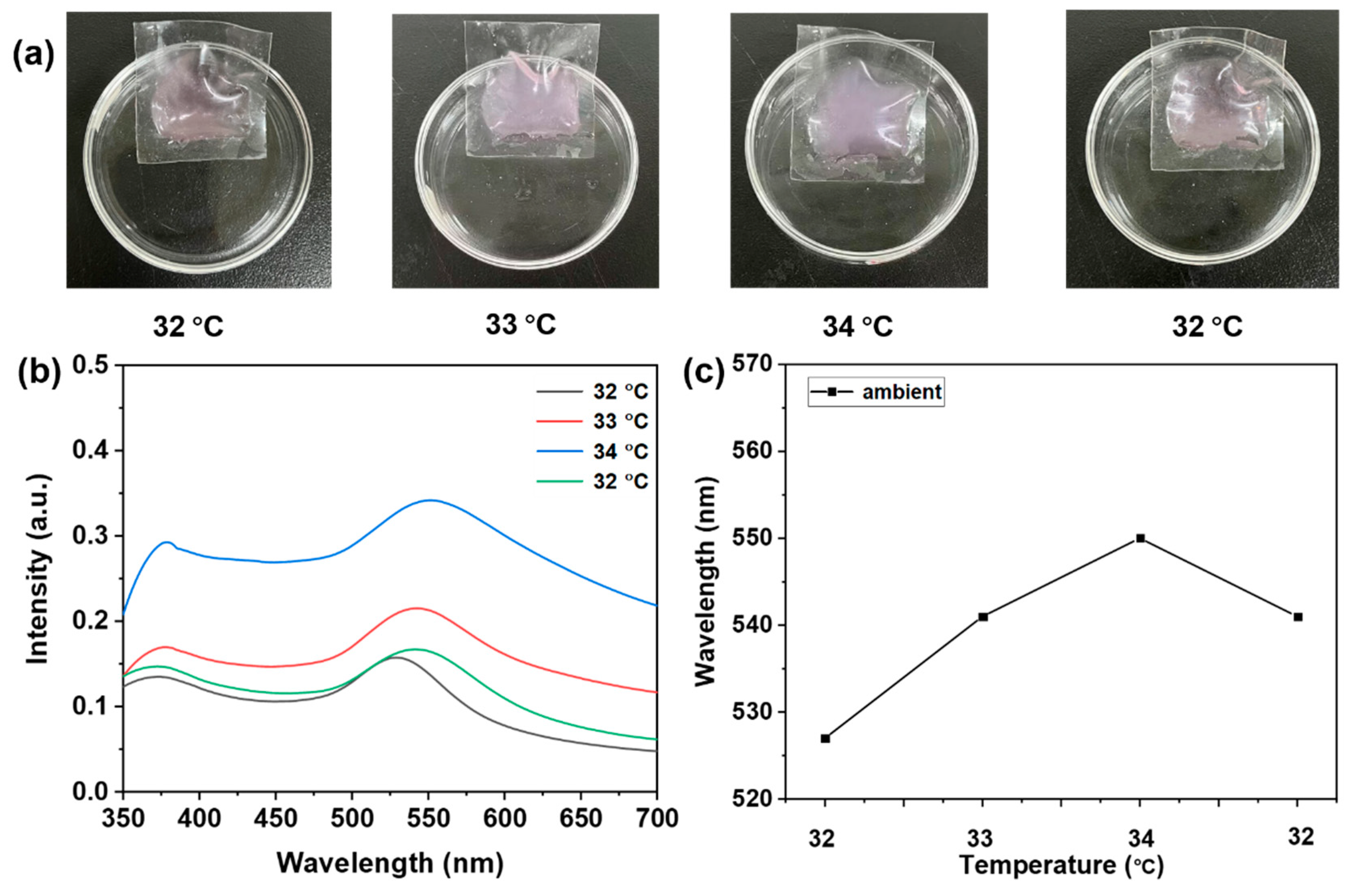
3.5. Probe Temperature Detection for Real-World Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and Challenges for Fluores-cence Nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent Probes and Sensors for Temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Vetrone, F. Luminescence Nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Konno, Y.; Nakanishi, T.; Kobayashi, A.; Kato, M.; Fushimi, K.; Hasegawa, Y. Chame-leon Luminophore for Sensing Temperatures: Control of Metal-to-Metal and Energy Back Transfer in Lanthanide Coordination Polymers. Angew. Chem. Int. Ed. Engl. 2013, 52, 6413–6416. [Google Scholar] [CrossRef] [PubMed]
- Saarimaa, R.; Wallin, P. Electronic Liquid-in-Glass Thermometer. Rev. Sci. Instrum. 1976, 47, 195–197. [Google Scholar] [CrossRef]
- Lojpur, V.; Nikolić, G.; Dramićanin, M.D. Luminescence Thermometry Below Room Temperature Via up-Conversion Emission of Y2o3:Yb3+, Er3+. Nanophosphors J. Appl. Phys. 2014, 115, 203106. [Google Scholar] [CrossRef]
- Dekoninck, S.; Blanpain, C. Stem Cell Dynamics, Migration and Plasticity During Wound Healing. Nat. Cell Biol. 2019, 21, 18–24. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Gan, D.; Xu, T.; Xing, W.; Ge, X.; Fang, L.; Wang, K.; Ren, F.; Lu, X. Mussel-Inspired Con-tact-Active Antibacterial Hydrogel with High Cell Affinity, Toughness, and Recoverability. Adv. Funct. Mater. 2019, 29, 1805964. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cuta-neous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Gong, M.; Wan, P.; Ma, D.; Zhong, M.; Liao, M.; Ye, J.; Shi, R.; Zhang, L. Flexible Breathable Na-nomesh Electronic Devices for on-Demand Therapy. Adv. Funct. Mater. 2019, 29, 1902127. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Zhang, T.; Zhang, W.; Chen, W.; Cao, Y.; Chen, M.; Zhou, X. Orange-Emissive Carbon Quantum Dots: Toward Application in Wound Ph Monitoring Based on Colorimetric and Fluorescent Changing. Small 2019, 15, 1902823. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Takahata, K. A Hydrogel-Based Passive Wireless Sensor Using a Flex-Circuit Inductive Transducer. Sens. Actuator A Phys. 2009, 155, 58–65. [Google Scholar] [CrossRef]
- Phair, J.; Benson, J.; McCormac, C.; Cundell, J.; Gracheva, S.; Wilkinson, D.; Forsythe, S.; Davis, J. Butyl Grafted Polyethylene Films Doped with Carbon Black: A Foundation for the Development of Smart Bandages. Sens. Actuator B Chem. 2014, 193, 764–769. [Google Scholar] [CrossRef]
- Salvo, P.; Dini, V.; Kirchhain, A.; Janowska, A.; Oranges, T.; Chiricozzi, A.; Lomonaco, T.; Di Francesco, F.; Romanelli, M. Sensors and Biosensors for C-Reactive Protein, Temperature and Ph, and Their Applications for Monitoring Wound Healing: A Review. Sensors 2017, 17, 2952. [Google Scholar] [CrossRef]
- Sun, T.; He, J.; Qian, S.; Zheng, Y.; Zhang, K.; Luo, J.; Tian, F. Collaborative Detection for Wound Infections Using Electronic Nose and Faims Technology Based on a Rat Wound Model. Sens. Actuator B Chem. 2020, 320, 128595. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef]
- Yang, F.Q.; Ge, L. Colorimetric Sensors: Methods and Applications. Sensors 2023, 23, 9887. [Google Scholar] [CrossRef]
- Kojima, C.; Xia, H.; Yamamoto, Y.; Shiigi, H. Front Cover: A Naked-Eye Colorimetric Ph and Temperature Sensor Based on Gold Nanoparticle-Loaded Stimuli-Sensitive Dendrimers (Chem-nanomat 3/2022). Chem. Nano Mat. 2022, 8, e202200040. [Google Scholar] [CrossRef]
- Zheng, X.C.; Wu, C.L.; Xiong, J.; Lei, H. Uv Photoinitiated Temperature-Sensitive Modification of Polypropylene Grafted with Poly(N-Isopropylacrylamide). Polym. Sci. Serious B 2022, 64, 644–650. [Google Scholar] [CrossRef]
- Jones, S.T.; Walsh-Korb, Z.; Barrow, S.J.; Henderson, S.L.; del Barrio, J.; Scherman, O.A. The Importance of Excess Poly(N-Isopropylacrylamide) for the Aggregation of Poly(N-Isopropylacrylamide)-Coated Gold Nanoparticles. ACS Nano 2016, 10, 3158–3165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Q.; Wang, L.Q.; Exarhos, G.J.; Li, A.D.Q. Thermosensitive Gold Nanoparticles. J. Am. Chem. Soc. 2004, 126, 2656–2657. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, X.; Zha, L. Synthesis, Properties and Applications of Gold or Silver Nanoparticles Loaded Intelligent Hybrid Microgels. Prog. Chem. 2013, 25, 2038–2052. [Google Scholar]
- Chen, Y.; Wang, Z.; Harn, Y.W.; Pan, S.; Li, Z.; Lin, S.; Peng, J.; Zhang, G.; Lin, Z. Resolving Op-tical and Catalytic Activities in Thermoresponsive Nanoparticles by Permanent Ligation with Temperature-Sensitive Polymers. Angew. Chem. Int. Ed. 2019, 58, 11910–11917. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ding, H.; Tao, K.; Wei, Y.; Gui, X.; Shi, W.; Xie, X.; Wu, J. Ultrasensitive, Stretchable, and Fast-Response Temperature Sensors Based on Hydrogel Films for Wearable Applications. ACS Appl. Mater. Inter. 2021, 13, 21854–21864. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, C.; Xu, L.; Dai, Y.; Liu, Y.; Liu, Y. Green and Facile Synthesis of Highly Stable Gold Nanoparticles Via Hyperbranched Polymer in-Situ Reduction and Their Application in Ag+ Detec-tion and Separation. Polymers 2018, 10, 42. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Zhang, J.; Huo, K.; Gu, J.; Zhou, Y.; Liu, Y.; Liu, Y.; Liu, X. Bimetallic-Based Colorimetric Sensor for Highly Selective, Stable and Sensitive Detection of Iodide Ions. Microchem. J. 2024, 199, 110098. [Google Scholar] [CrossRef]
- Bian, J.; Li, Y.; Zhu, C.; Liu, X.; Liu, Y. Graphene Oxide-Hyperbranched Polyethyleneimine Fabri-cated and Stabilized Aunps Nanocomposites for Colorimetric Detection of Silver Ions Based on a Non-Aggregation Mechanism. Chem. Nano Mat. 2021, 7, 85–94. [Google Scholar]
- Liu, Y.; Dai, J.; Xu, L.; Liu, X.; Liu, J.; Li, G. Red to Brown to Green Colorimetric Detection of Ag+ Based on the Formation of Au-Ag Core-Shell Nps Stabilized by a Multi-Sulfhydryl Functionalized Hyperbranched Polymer. Sens. Actuator B Chem. 2016, 237, 216–223. [Google Scholar] [CrossRef]
- Christau, S.; Moeller, T.; Genzer, J.; Koehler, R.; von Klitzing, R. Salt-Induced Aggregation of Negatively Charged Gold Nanoparticles Confined in a Polymer Brush Matrix. Macromolecules 2017, 50, 7333–7343. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, Z.; Liu, J.; Xu, L.; Liu, X. An Unusual Red-to-Brown Colorimetric Sensing Method for Ultrasensitive Silver(I) Ion Detection Based on a Non-Aggregation of Hyperbranched Polyethylenimine Derivative Stabilized Gold Nanoparticles. Analyst 2015, 140, 5335–5343. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Li, P.J.; Wang, M.; Liang, Y.D.; Yang, X.D.; Pang, S.J. Carbonized Polymer Dots-Silver Nanoclusters Nanocomposite with Dual-Emission for Property Ratiometric Fluorescence and Visual Detection of Temperature. Diam. Relat. Mater. 2024, 141, 110559. [Google Scholar] [CrossRef]
- Lan, J.; Zou, H.; Liu, Z.; Gao, M.; Chen, B.; Li, Y.; Huang, C. A Visual Physiological Temperature Sensor Developed with Gelatin-Stabilized Luminescent Silver Nanoclusters. Talanta 2015, 143, 469–473. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Xiang, H.; Zhai, G.; Zhu, M. Fabrication of Visual Textile Temperature Indicators Based on Reversible Thermochromic Fibers. Dyes Pigm 2019, 162, 705–711. [Google Scholar] [CrossRef]





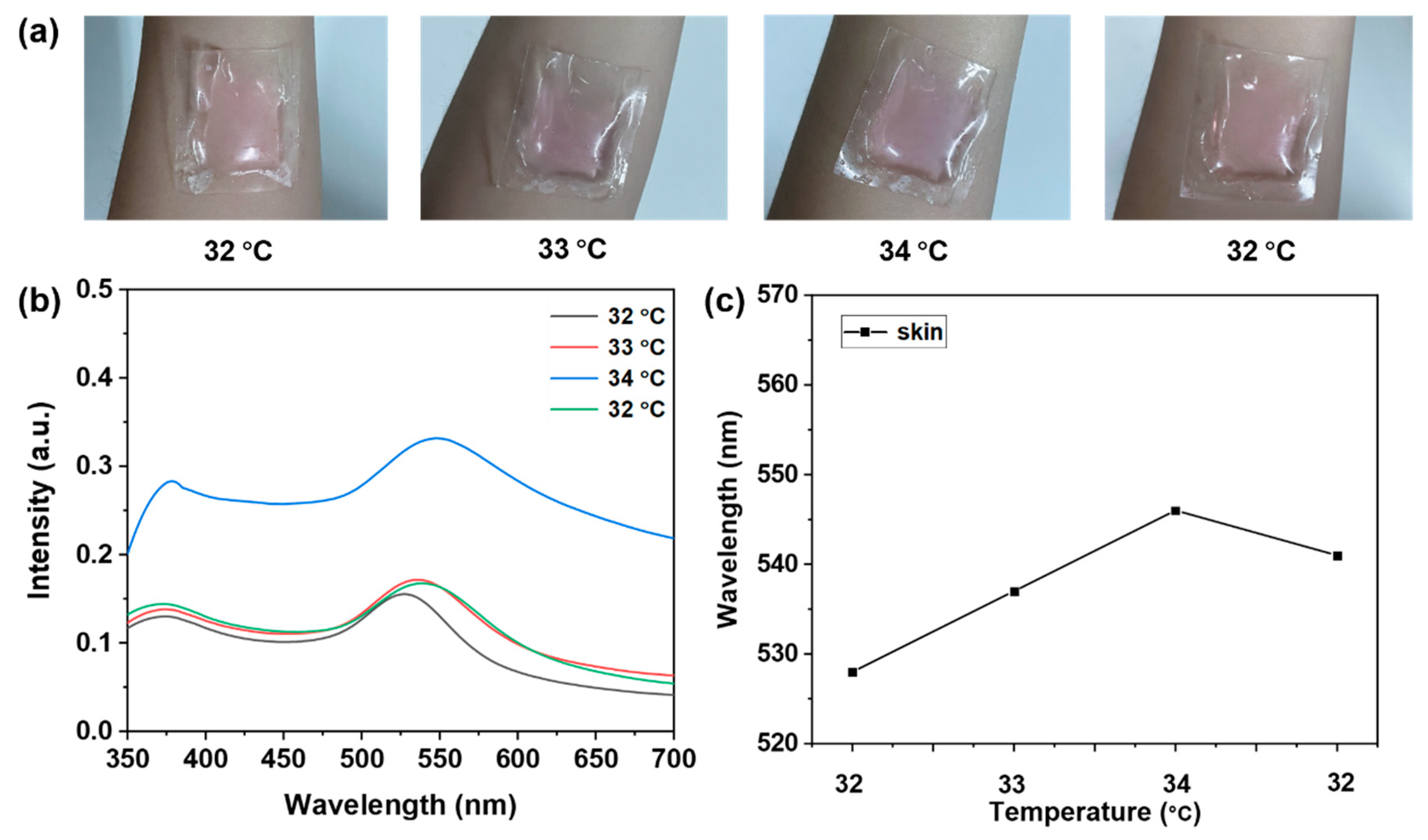
| Sample | Entry | Probe Detection Temperature (UV–Vis) (°C) | Thermometer Detects Temperature (°C) | Recovery (%) | RSD% (n = 3) |
|---|---|---|---|---|---|
| skin | 1 | 32 | 32.3 | 99.07 | 0.8105 |
| 2 | 33 | 32.8 | 100.61 | 0.7389 | |
| 3 | 34 | 34.2 | 99.41 | 0.5114 | |
| 4 | 32 | 31.9 | 100.31 | 1.0337 | |
| environment | 5 | 32 | 32.2 | 99.37 | 1.3351 |
| 6 | 33 | 33.3 | 99.10 | 0.5881 | |
| 7 | 34 | 33.8 | 100.59 | 0.6632 | |
| 8 | 32 | 32.1 | 99.69 | 0.9483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhou, Y.; Gu, J.; Zhong, W.; Li, X.; Liu, X.; Qiao, Z.; Liu, Y. Hyperbranched Thermosensitive Polymer-AuNP Composite Probe for Temperature Colorimetric Detection. Sensors 2024, 24, 7124. https://doi.org/10.3390/s24227124
Li H, Zhou Y, Gu J, Zhong W, Li X, Liu X, Qiao Z, Liu Y. Hyperbranched Thermosensitive Polymer-AuNP Composite Probe for Temperature Colorimetric Detection. Sensors. 2024; 24(22):7124. https://doi.org/10.3390/s24227124
Chicago/Turabian StyleLi, Huidong, Yao Zhou, Junqi Gu, Wenjie Zhong, Xinlong Li, Xunyong Liu, Zhuhui Qiao, and Yi Liu. 2024. "Hyperbranched Thermosensitive Polymer-AuNP Composite Probe for Temperature Colorimetric Detection" Sensors 24, no. 22: 7124. https://doi.org/10.3390/s24227124
APA StyleLi, H., Zhou, Y., Gu, J., Zhong, W., Li, X., Liu, X., Qiao, Z., & Liu, Y. (2024). Hyperbranched Thermosensitive Polymer-AuNP Composite Probe for Temperature Colorimetric Detection. Sensors, 24(22), 7124. https://doi.org/10.3390/s24227124






