MEMS and ECM Sensor Technologies for Cardiorespiratory Sound Monitoring—A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
3. Acoustic Properties of Heart and Lung
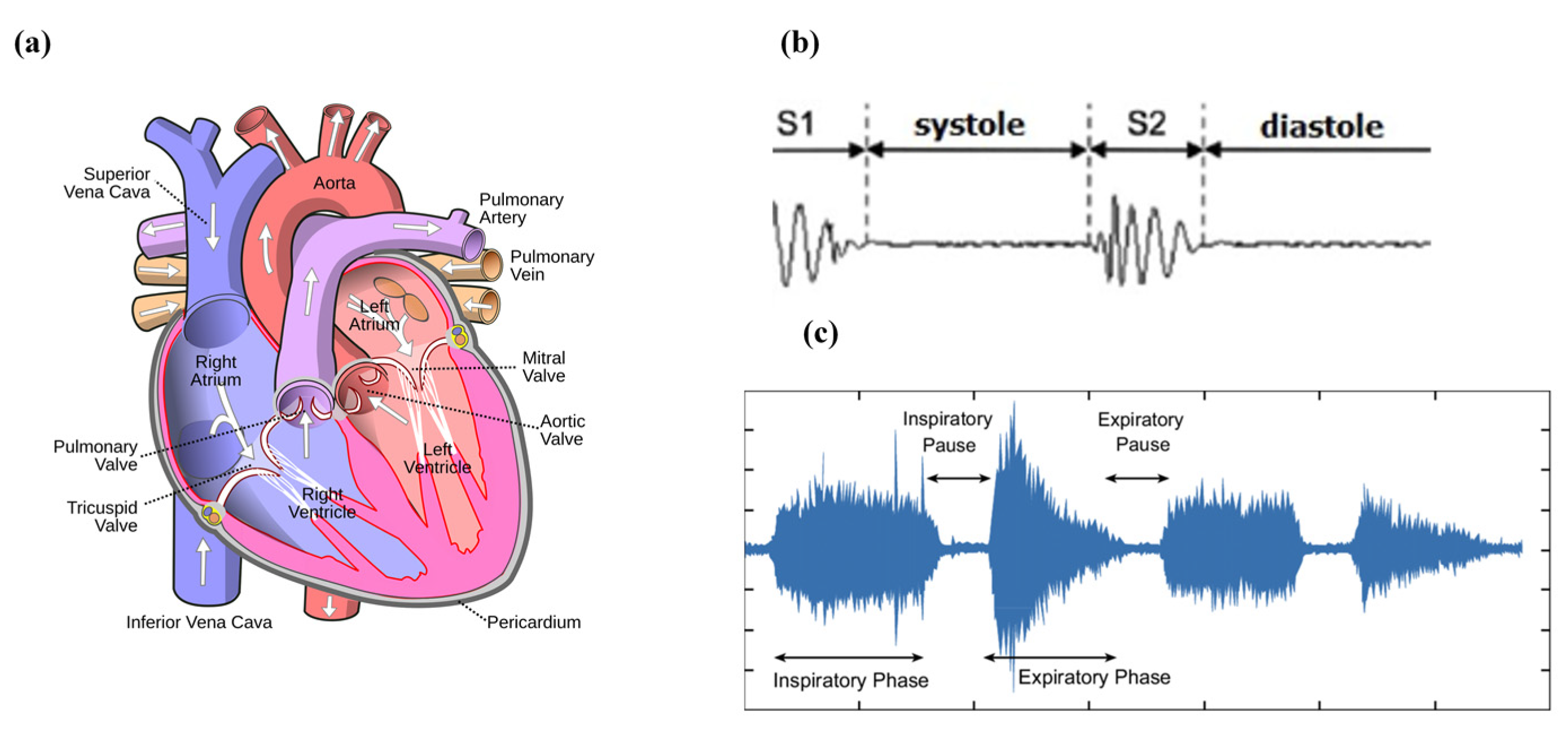
3.1. Cardiac Cycle
3.2. Respiratory Cycle
4. Evolution of the Stethoscope and Recent Advances
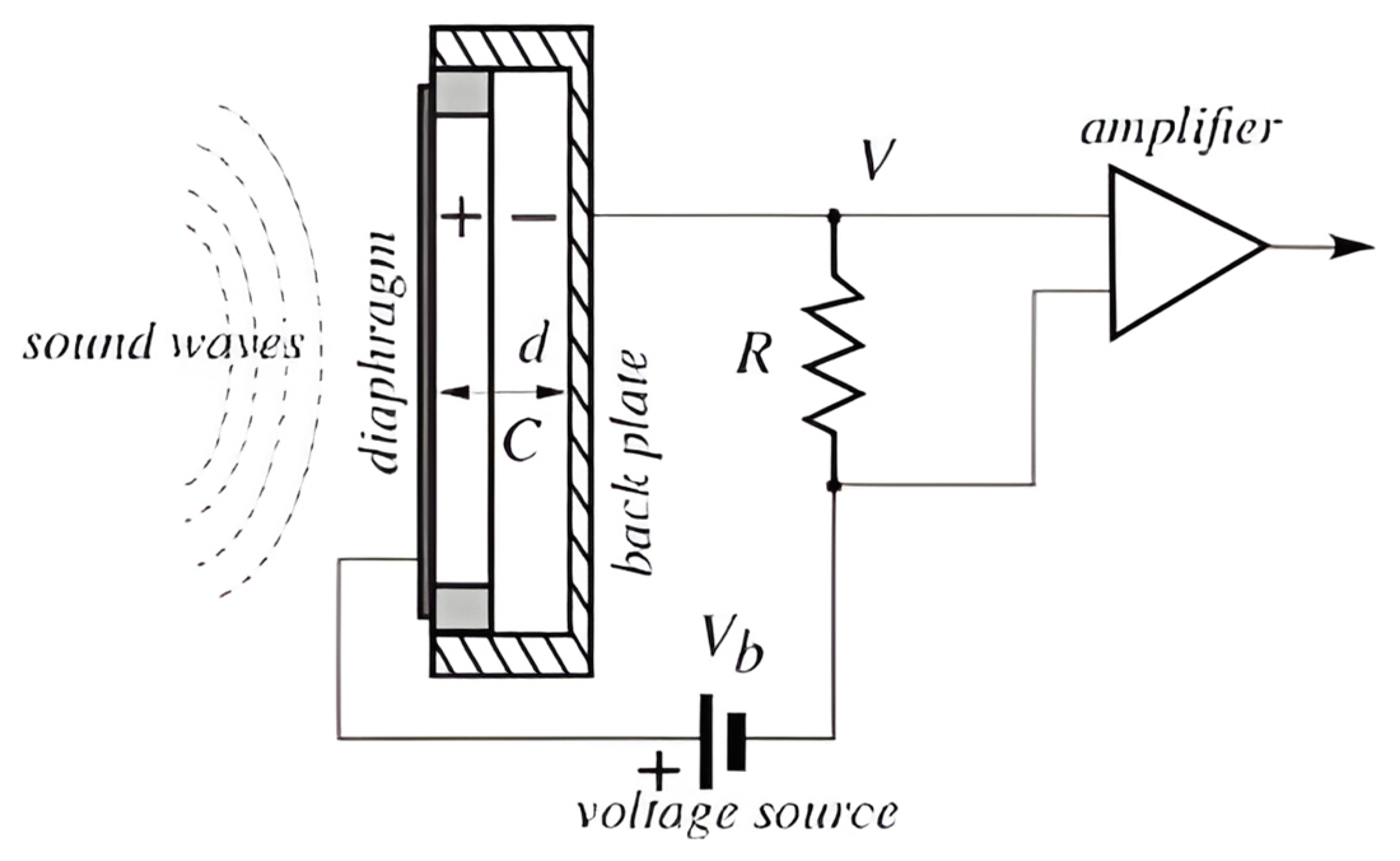
5. DC-Biased Condenser Microphone
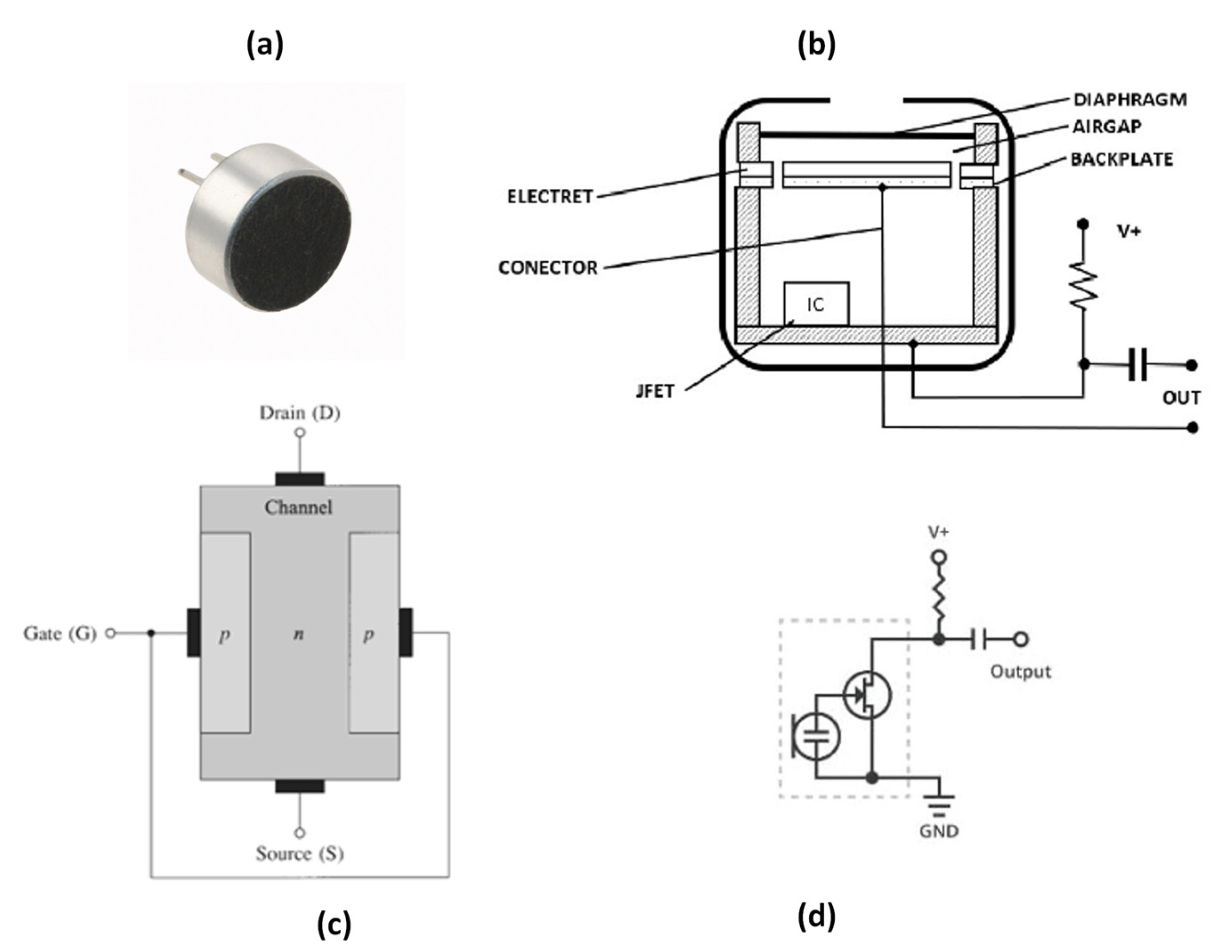
6. Electret Condenser Microphones (ECM)
7. ECM-Based Sensors for Cardiorespiratory Sound Acquisition

8. Microelectromechanical Systems (MEMSs)
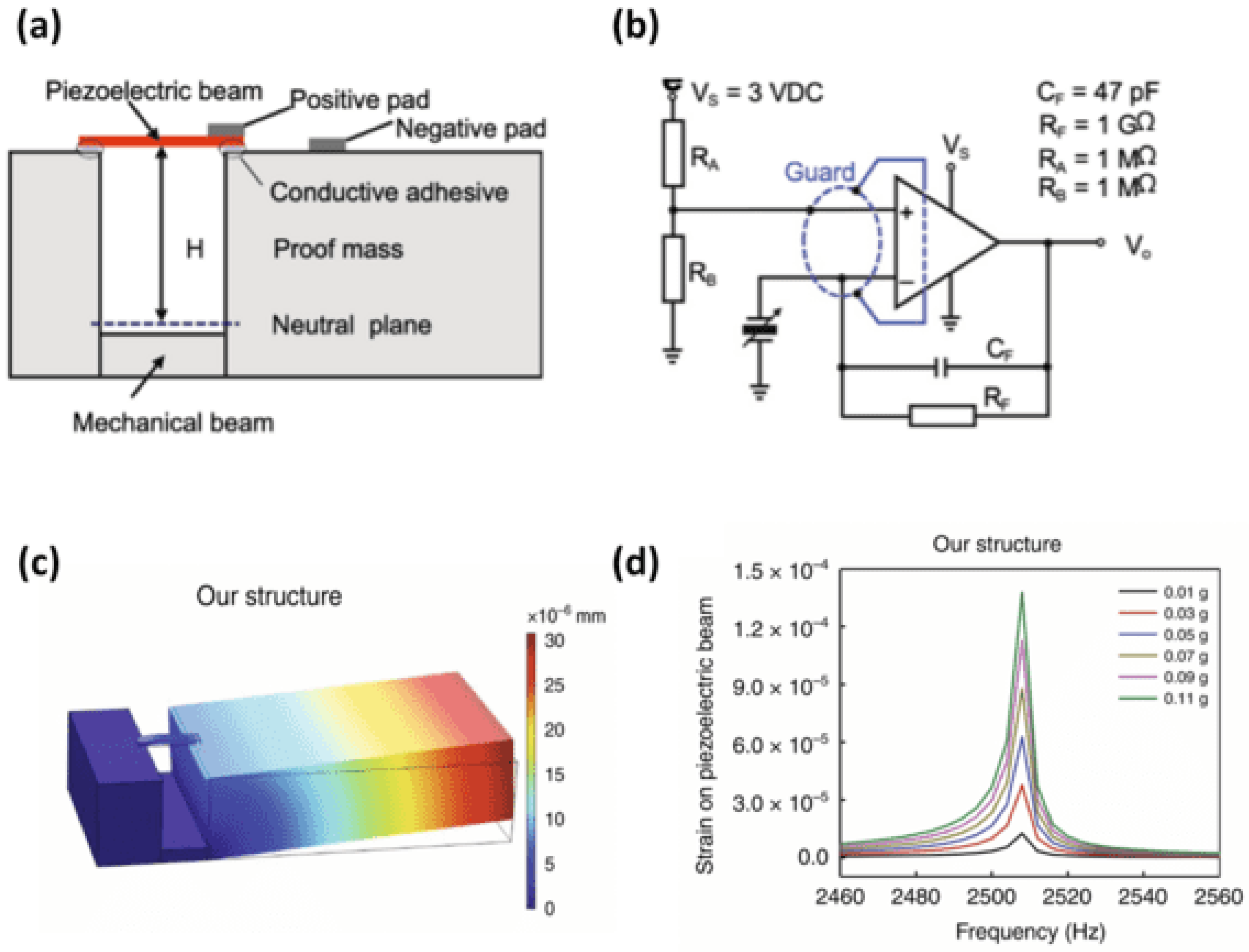
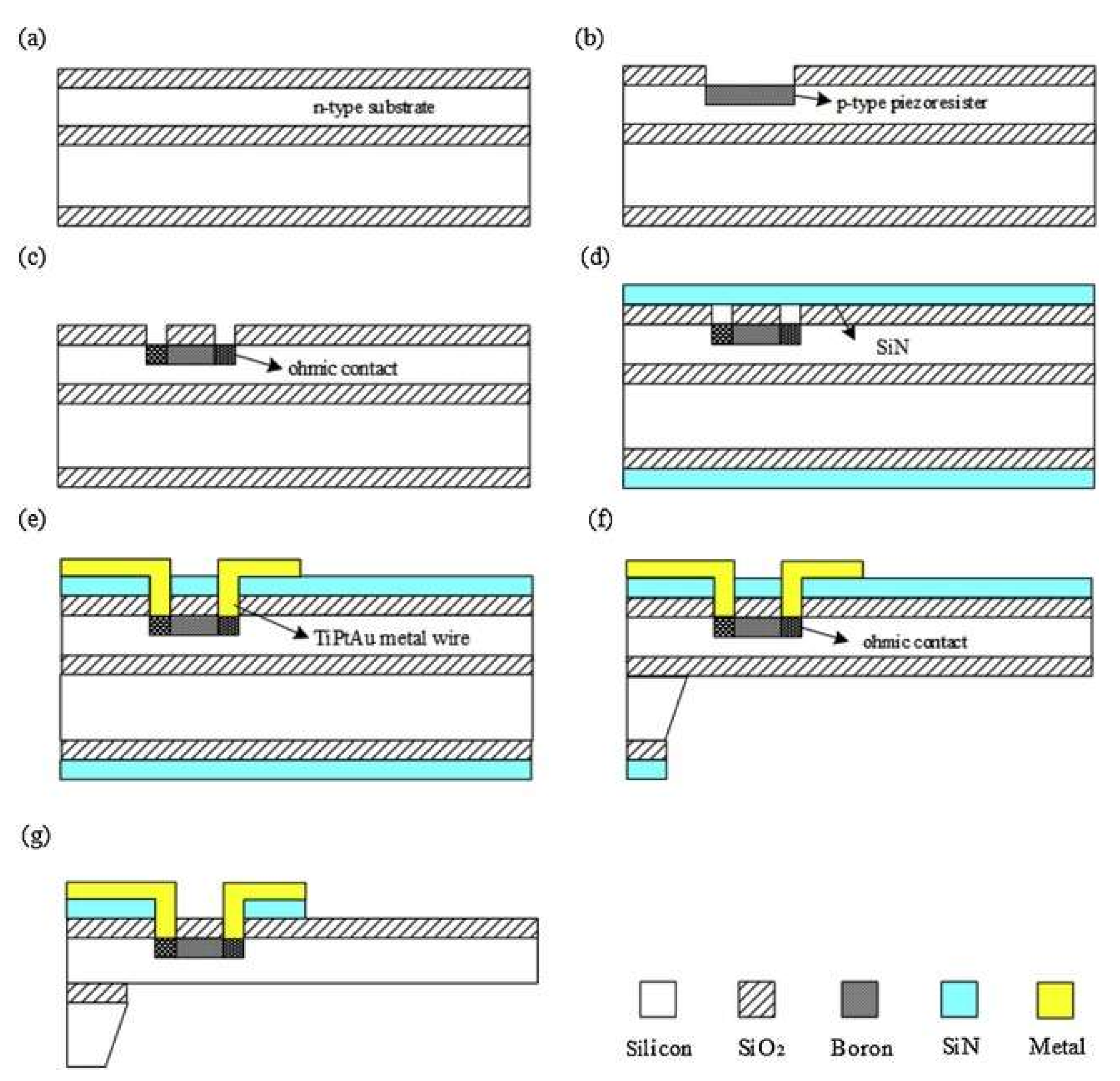
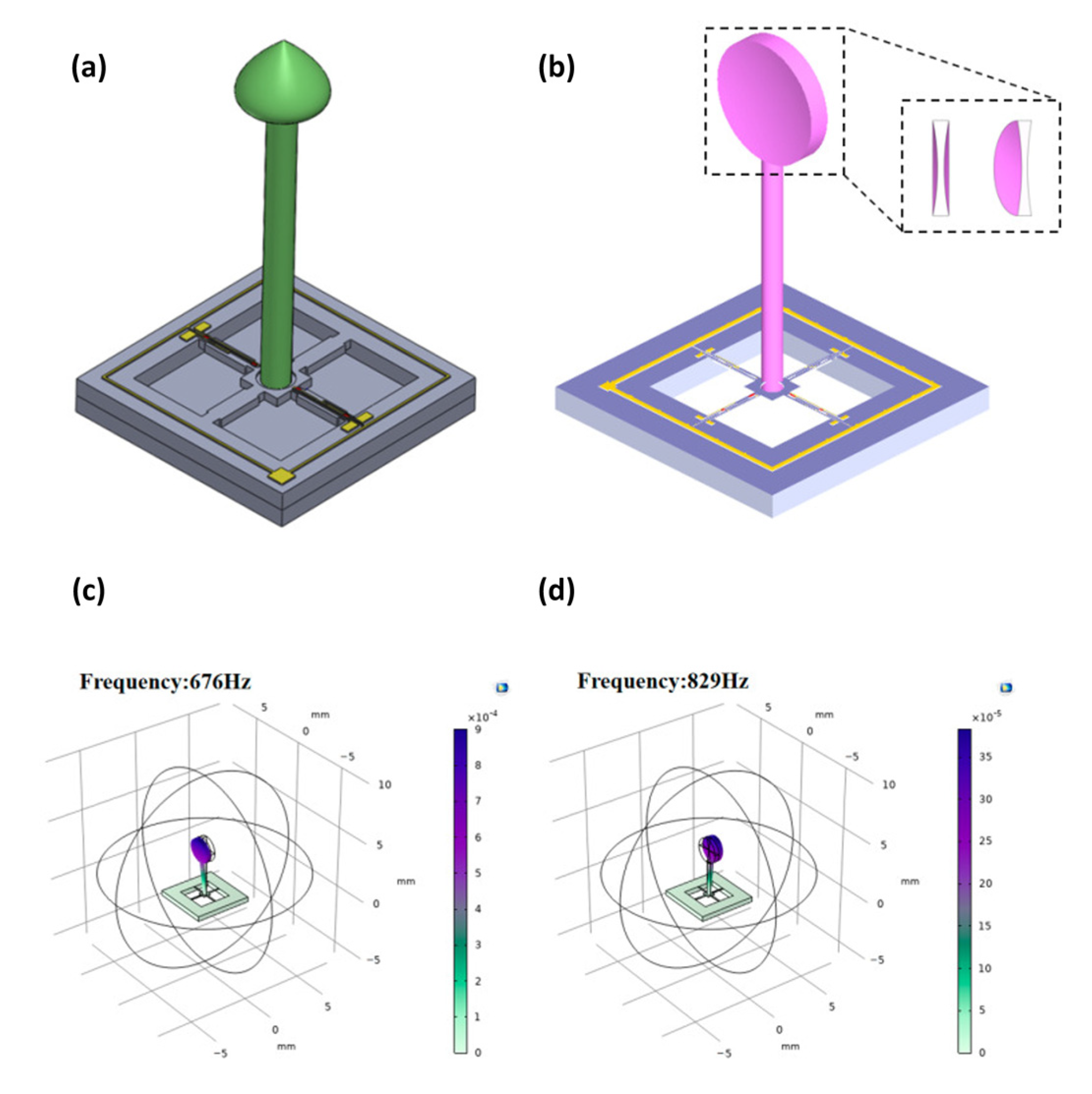
9. MEMSs Technology for Cardiorespiratory Acoustic Application
10. Research Challenges and Future Perspective
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baraeinejad, B.; Shayan, M.F.; Vazifeh, A.R.; Rashidi, D.; Hamedani, M.S.; Tavolinejad, H.; Gorji, P.; Razmara, P.; Vaziri, K.; Vashaee, D.; et al. Design and Implementation of an Ultralow-Power ECG Patch and Smart Cloud-Based Platform. IEEE Trans. Instrum. Meas. 2022, 71, 2506811. [Google Scholar] [CrossRef]
- Cook, J.; Umar, M.; Khalili, F.; Taebi, A. Body Acoustics for the Non-Invasive Diagnosis of Medical Conditions. Bioengineering 2022, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.J. Evolution of the stethoscope. J. R. Soc. Med. 1980, 73, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Tourtier, J.P.; Libert, N.; Clapson, P.; Tazarourte, K.; Borne, M.; Grasser, L.; Debien, B.; Auroy, Y. Auscultation in Flight: Comparison of Conventional and Electronic Stethoscopes. Air Med. J. 2011, 30, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Pereira, D.; Ferreira-Coimbra, J.; Português, J.; Gama, V.; Coimbra, M. A comparative study of electronic stethoscopes for cardiac auscultation. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 2610–2613. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Ge, Y.; Li, M.; Yang, L.; Mao, X. Design Optimization and Fabrication of High-Sensitivity SOI Pressure Sensors with High Signal-to-Noise Ratios Based on Silicon Nanowire Piezo resistors. Micromachines 2017, 7, 187. [Google Scholar] [CrossRef]
- Saveliev, A.; Pshchelko, N.; Krestovnikov, K. Method of Sensitivity Calculation for Electret Diaphragm Capacitive Sensors. In Proceedings of the 2019 12th International Conference on Developments in eSystems Engineering (DeSE), Kazan, Russia, 7–10 October 2019; pp. 721–725. [Google Scholar] [CrossRef]
- Zawawi, S.A.; Hamzah, A.A.; Majlis, B.Y.; Mohd-Yasin, F. A Review of MEMS Capacitive Microphones. Micromachines 2020, 11, 484. [Google Scholar] [CrossRef]
- Yoshinobu, Y.; Kenzo, M. Sensitivity changes with long-term preservation and practical use of electret condenser microphone. Acoust. Sci. Technol. 2006, 27, 302–304. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Microelectromechanical Systems (MEMS) for Biomedical Applications. Micromachines 2022, 13, 164. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Zhang, G.; Xue, C. Package Optimization of the Cilium-Type MEMS Bionic Vector Hydrophone. IEEE Sens. J. 2014, 14, 1185–1192. [Google Scholar] [CrossRef]
- MEMS Journal. MEMS Microphones: Emerging Technology and Application Trends. Available online: https://www.memsjournal.com/2015/07/mems-microphones-emerging-technology-and-application-trends (accessed on 2 December 2023).
- Tilkian, A.G.; Conover, M.B. Understanding Heart Sounds and Murmurs: With an Introduction to Lung Sounds, 4th ed.; W.B. Saunders: Philadelphia, PA, USA, 2001. [Google Scholar]
- Pollock, J.D.; Makaryus, A.N. Physiology, Cardiac Cycle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459327/ (accessed on 3 October 2022).
- Fukuta, H.; Little, W.C. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail. Clin. 2008, 4, 1–11. [Google Scholar] [CrossRef]
- McDonald, I.G. The shape and movements of the human left ventricle during systole: A study by cineangiography and by cineradiography of epicardial markers. Am. J. Cardiol. 1970, 26, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Rehman, I.; Rehman, A. Anatomy, Thorax, Heart. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470256/ (accessed on 28 August 2023).
- Hinton, R.B.; Yutzey, K.E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Sharma, M. Detection of pathological heart murmurs by feature extraction of phonocardiogram signals. J. Appl. Adv. Res. 2017, 2, 200–205. [Google Scholar] [CrossRef]
- Tseng, Y.L.; Ko, P.Y.; Jaw, F.S. Detection of the third and fourth heart sounds using Hilbert-Huang transform. Biomed. Eng. Online 2012, 11, 8. [Google Scholar] [CrossRef]
- Spencer, C.S.; Pennington, K. Nurses with Undiagnosed Hearing Loss: Implications for Practice. Online J. Issues Nurs. 2015, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.P.; Lindsell, C.J.; Peacock, W.F.; Storrow, A.B. Prevalence of S3 and S4 in emergency department patients with decompensated heart failure. Ann. Emerg. Med. 2004, 44, S98–S99. [Google Scholar] [CrossRef]
- Randhawa, S.K.; Singh, M. Classification of Heart Sound Signals Using Multi-modal Features. Procedia Comput. Sci. 2015, 58, 165–171. [Google Scholar] [CrossRef]
- Rangayyan, R.M.; Lehner, R.J. Phonocardiogram signal analysis: A review. Crit. Rev. Biomed. Eng. 1987, 15, 211–236. [Google Scholar]
- Li, S.; Park, W.H.; Borg, A. Phase-dependent respiratory-motor interactions in reaction time tasks during rhythmic voluntary breathing. Mot. Control 2012, 16, 493–505. [Google Scholar] [CrossRef][Green Version]
- De Troyer, A.; Kirkwood, P.A.; Wilson, T.A. Respiratory Action of the Intercostal Muscles. Physiol. Rev. 2005, 85, 717–756. [Google Scholar] [CrossRef]
- Registered Nursing Staff Writer. Respiratory System: TEAS. 2022. Available online: https://www.registerednursing.org/teas/respiratory-system/ (accessed on 3 January 2023).
- Dou, F.; Ren, A.; Zhao, N.; Yang, X.; Zhang, Z.; Shah, S.A. Breathing Rhythm Analysis in Body Centric Networks. IEEE Access 2018, 6, 2846605. [Google Scholar] [CrossRef]
- Dugdale, D.C. Breath Sounds. 2021. Available online: https://www.mountsinai.org/health-library/symptoms/breath-sounds (accessed on 3 January 2021).
- Zimmerman, B.; Williams, D. Lung Sounds. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537253/ (accessed on 28 August 2023).
- Lowe, R. Lung Sounds. Available online: https://www.physio-pedia.com/Lung_Sounds (accessed on 3 January 2023).
- Marques, A.; Oliveira, A. Breath Sounds; Normal versus Adventitious Respiratory Sounds; Springer International Publishing: Cham, Switzerland, 2018; pp. 181–206, Chapter 10. [Google Scholar]
- Abbas, A.; Fahim, A. An automated computerized auscultation and diagnostic system for pulmonary diseases. J. Med. Syst. 2010, 34, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.M.; Pessoa, D.; Marques, A.; Carvalho, P.; Paiva, R.P. Automatic Classification of Adventitious Respiratory Sounds: A (Un)Solved Problem? Sensors 2020, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Bohadana, A.; Izbicki, G.; Kraman, S.S. Fundamentals of Lung Auscultation. N. Engl. J. Med. 2014, 370, 744–751. [Google Scholar] [CrossRef]
- Sarkar, M.; Madabhavi, I. Vocal resonance: A narrative review. Monaldi Arch. Chest Dis. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Ganesan, P. Characterization of Cardiac Electrogram Signals during Atrial Fibrillation. Master’s Thesis, RIT, Henrietta, NY, USA, 2015. [Google Scholar]
- Arslan, A.; Yildiz, O. Automated Auscultative Diagnosis System for Evaluation of Phonocardiogram Signals Associated with Heart Murmur Diseases. Gazi Univ. J. Sci. 2018, 31, 112–124. [Google Scholar]
- Doyle, D.J. Acoustical Respiratory Monitoring in the Time Domain. Open Anesth. J. 2019, 13, 144–151. [Google Scholar] [CrossRef]
- Alemán-Soler, N.M.; Travieso, C.M.; Guerra-Segura, E.; Alonso, J.B.; Dutta, M.K.; Singh, A. Biometric approach based on physiological human signals. In Proceedings of the 2016 3rd International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 11–12 February 2016; pp. 681–686. [Google Scholar] [CrossRef]
- Nussbaumer, M.; Agarwal, A. Stethoscope acoustics. J. Sound Vib. 2022, 539, 117194. [Google Scholar] [CrossRef]
- Strutt, J.W. The theory of the Helmholtz resonator. R. Soc. Lond. Ser. A 1916, 92, 265–275. [Google Scholar] [CrossRef]
- Bohadana, A.; Azulai, H.; Jarjoui, A.; Kalak, G.; Izbicki, G. Influence of observer preferences and auscultatory skill on the choice of terms to describe lung sounds: A survey of staff physicians, residents, and medical students. BMJ Open Respir. Res. 2020, 7, e000564. [Google Scholar] [CrossRef]
- Lella, K.K.; Jagadeesh, M.S.; Alphonse, P.J.A. Artificial intelligence-based framework to identify the abnormalities in the COVID-19 disease and other common respiratory diseases from digital stethoscope data using deep CNN. Health Inf. Sci. Syst. 2024, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, W.; Kang, W.; Shin, E.; Ryu, J.; Choi, H. Review of piezoelectric micromachined ultrasonic transducers and their applications. J. Micromech. Microeng. 2017, 27, 113001. [Google Scholar] [CrossRef]
- Liu, J.; Tian, G.; Yang, W.; Deng, W. Recent progress in flexible piezoelectric devices toward human-machine interactions. Soft Sci. 2022, 2, 22. [Google Scholar] [CrossRef]
- Almeida, V.G.; Pereira, H.C.; Pereira, T.; Figueiras, E.; Borges, E.; Cardoso, J.M.R.; Correia, C. Piezoelectric probe for pressure waveform estimation in flexible tubes and its application to the cardiovascular system. Sens. Actuators A Phys. 2011, 169, 217–226. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, C.; Zhang, Z.; Yang, S.; Peng, J.; Wu, B. Vector High-Resolution Marine Turbulence Sensor Based on a MEMS Bionic Cilium-Shaped Structure. IEEE Sens. J. 2021, 21, 8741–8750. [Google Scholar] [CrossRef]
- Guan, L. Advancements in technology and design of NEMS vector hydrophone. Microsyst. Technol. 2011, 17, 459. [Google Scholar] [CrossRef]
- Zhang, G.J.; Liu, L.X.; Zhang, W.D.; Xue, C.Y. Design of a monolithic integrated three-dimensional MEMS bionic vector hydrophone. Microsyst. Technol. 2015, 21, 1697–1708. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Guan, L.; Xiong, J.; Zhang, W. Improvement of the MEMS bionic vector hydrophone. Microelectron. J. 2017, 42, 815–819. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Zhang, G.; Du, J.; Zhao, L.; Xue, C.; Zhang, W.; Liu, J. Lollipop-shaped high-sensitivity Microelectromechanical Systems vector hydrophone based on Parylene encapsulation. J. Appl. Phys. 2017, 118, 44501. [Google Scholar] [CrossRef]
- Li, H.; Ren, Y.; Zhang, G.; Wang, R.; Zhang, X.; Zhang, T.; Zhang, L.; Cui, J.; Xu, Q.; Duan, S. Design of a high SNR electronic heart sound sensor based on a MEMS bionic hydrophone. AIP Adv. 2019, 9, 015005. [Google Scholar] [CrossRef]
- Duan, S.; Wang, W.; Zhang, S.; Yang, X.; Zhang, Y.; Zhang, G. A Bionic MEMS Electronic Stethoscope with Double-Sided Diaphragm Packaging. IEEE Access 2021, 9, 27122–27129. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Yang, Y.; Shi, P.; Wang, B.; Wang, S.; Zhang, G.; Zhang, W. Design and optimisation of MEMS heart sound sensor based on bionic structure. Sens. Actuators A Phys. 2021, 333, 113188. [Google Scholar] [CrossRef]
- Zhou, C.; Zang, J.; Xue, C.; Ma, Y.; Hua, X.; Gao, R.; Zhang, Z.; Li, B.; Zhang, Z. Design of a Novel Medical Acoustic Sensor Based on MEMS Bionic Fish Ear Structure. Micromachines 2022, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Ke, M.; Chen, G.; Horng, R.; Chang, C.; Tsai, J.; Lai, C.; Chen, J. Design And Fabrication of Condenser Microphone Using Wafer Transfer and Micro-electroplating Technique. In Proceedings of the DTIP of MEMS & MOEMS, Nice, France, 9–11 April 2008. [Google Scholar]
- Li, X.; Lin, R.; Kek, H.; Miao, J.; Zou, Q. Sensitivity-improved silicon condenser microphone with a novel single deeply corrugated diaphragm. Sens. Actuators A Phys. 2001, 92, 257–262. [Google Scholar] [CrossRef]
- Printezis, G.; Aage, N.; Lucklum, F. A non-dimensional time-domain lumped model for externally DC biased capacitive microphones with two electrodes. Appl. Acoust. 2024, 216, 109758. [Google Scholar] [CrossRef]
- Fraden, J. Handbook of Modern Sensors; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Lardies, J.; Arbey, O.; Berthillier, M. Analysis of the pull-in voltage in capacitive mechanical sensors. In Proceedings of the International Conference on Multidisciplinary Design Optimization and Applications, Paris, France, 21–23 June 2010. [Google Scholar]
- Gerhard, R. Dielectric materials for electro-active (electret) and/or electro-passive (insulation) applications. In Proceedings of the 2019 2nd International Conference on Electrical Materials and Power Equipment (ICEMPE), Guangzhou, China, 7–10 April 2019; pp. 91–96. [Google Scholar] [CrossRef]
- Audio-Technica. What Are the Differences Between the Microphones That Audio-Technica Offers? (Part 3). Available online: https://www.audio-technica.com/en-us/support (accessed on 2 January 2024).
- Turnhout, J. The use of polymers for electrets. J. Electrost. 1975, 1, 147–163. [Google Scholar] [CrossRef]
- Józef, P.; Maciej, K. Issues of Data Acquisition and Interpretation of Paraseismic Measuring Signals Triggered by the Detonation of Explosive Charges. Sensors 2021, 21, 1290. [Google Scholar] [CrossRef]
- Xu, J.; Dapino, M.; Gallego-Perez, D.; Hansford, D. Microphone based on Polyvinylidene Fluoride (PVDF) micro-pillars and patterned electrodes. Sens. Actuators A Phys. 2009, 153, 24–32. [Google Scholar] [CrossRef]
- Zuckerwar, A.J. Acoustical Measurement. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2003; pp. 91–115. ISBN 9780122274107. [Google Scholar] [CrossRef]
- PUI Audio, Inc. AOM-4544P-2-R Datasheet. Available online: https://api.puiaudio.com/file/53873b5a-a40b-45c9-87e9-40627beb81b2.pdf (accessed on 5 January 2024).
- Sedra, A.S.; Smith, K.C. Microelectronic Circuits; Oxford University Press: Oxford, UK, 2004; Chapter 5. [Google Scholar]
- Arrow Division. MEMS vs. Electret Condenser: Which Microphone Technology Should You Use? 2019. Available online: https://www.arrow.com/en/research-and-events/articles/mems-vs-electret-condenser-which-microphone-technology-should-you-use (accessed on 3 January 2023).
- CUI Devices. CMA-4544PF-W Datasheet. Available online: https://cuidevices.com/product/resource/cma-4544pf-w.pdf (accessed on 5 January 2024).
- Soberton Inc. EM-6050P Datasheet. Available online: https://www.soberton.com/wp-content/uploads/2019/02/EM-6050P-14-Feb-2019.pdf (accessed on 5 January 2024).
- Raltron Electronics. RMIC-110-10-6027-NS1 Datasheet. Available online: https://www.raltron.com/webproducts/specs/MICROPHONES/RMIC-110-10-6027-NS1.pdf (accessed on 5 January 2024).
- Electronics Tutorials. JFET. Available online: https://www.electronics-tutorials.ws/transistor/tran_5.html (accessed on 3 January 2023).
- Leach, W.M., Jr.; The JFET. Georgia Institute of Technology, School of Electrical and Computer Engineering. 2008. Available online: https://www.leachlegacy.ece.gatech.edu/ece3050/notes/jfet/thejfet.pdf (accessed on 20 July 2024).
- Van Rhijn, A. Integrated Circuits for High Performance Electret Microphones. In Proceedings of the 114th Convention of the Audio Engineering Society, Amsterdam, The Netherlands, 22–25 March 2003. [Google Scholar]
- Texas Instruments. Integrated Circuits for High Performance Electret Microphones. Literature Number: SNAA114. Available online: https://www.ti.com/lit/wp/snaa114/snaa114.pdf (accessed on 2 January 2024).
- Akerib, D.S.; Barnes, P.D., Jr.; Brink, P.L.; Cabrera, B.; Clarke, R.M.; Gaitskell, R.J.; Golwala, S.R.; Huber, M.E.; Kurylowicz, M.; Mandic, V.; et al. Design and performance of a modular low-radioactivity readout system for cryogenic detectors in the CDMS experiment. Nucl. Instrum. Methods Phys. Res. A 2008, 591, 476–489. [Google Scholar] [CrossRef]
- Wang, T.; Gong, M.; Yu, X.; Lan, G.; Shi, Y. Acoustic-pressure sensor array system for cardiac-sound acquisition. Biomed. Signal Process. Control 2021, 69, 102836. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Zaferani, S.H.; Rafiefard, N.; Baraeinejad, B.; Vazifeh, A.R.; Mohammadpour, R.; Ghomashchi, R.; Dillersberger, H.; Tham, D.; Vashaee, D.; et al. Advancing personalized healthcare and entertainment: Progress in energy harvesting materials and techniques of self-powered wearable devices. Prog. Mater. Sci. 2023, 139, 101184. [Google Scholar] [CrossRef]
- Hamid, M.A.A.; Abdullah, M.; Khan, N.A.; AL-Zoom, Y.M.A. Biotechnical system for recording phonocardiography. Int. J. Adv. Comput. Sci. Appl. 2019, 10, 493–497. [Google Scholar] [CrossRef]
- Daniel, A.-A.; Josely, V.; Eder, M. Detecting the heart and wrist sounds with electret microphones. Acad. Lett. 2021, 677. [Google Scholar] [CrossRef]
- Shervegar, M.V.; Bhat, G.V.; Shetty, R.M.K. Phonocardiography—The future of cardiac auscultation. Int. J. Sci. Eng. Res. 2011, 2, 1–6. [Google Scholar]
- Song, P.; Ma, Z.; Ma, J.; Yang, L.; Wei, J.; Zhao, Y.; Zhang, M.; Yang, F.; Wang, X. Recent Progress of Miniature MEMS Pressure Sensors. Micromachines 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- CUI Devices. CMM-3526DB-37165-TR Datasheet. Available online: https://www.cuidevices.com/product/resource/cmm-3526db-37165-tr.pdf (accessed on 5 January 2024).
- Infineon Technologies. IM66D130AXTMA1 Datasheet. Available online: https://www.infineon.com/dgdl/Infineon-IM66D130A-DataSheet-v01_00-EN.pdf (accessed on 5 January 2024).
- TDK InvenSense. ICS-52000 Datasheet. Available online: https://www.invensense.tdk.com/wp-content/uploads/2016/05/DS-000121-ICS-52000-v1.3.pdf (accessed on 5 January 2024).
- Knowles Corporation. SPH0645LM4H-B Datasheet. Available online: https://mm.digikey.com/Volume0/opasdata/d220001/medias/docus/908/SPH0645LM4H-B.pdf (accessed on 5 January 2024).
- PUI Audio, Inc. DMM-4026-B-I2S-R Datasheet. Available online: https://www.api.puiaudio.com/file/3d9a865c-fddb-4f42-b619-1b96c3a17c74.pdf (accessed on 5 January 2024).
- Leng, S.; Tan, R.S.; Chai, K.T.C.; Wang, C.; Ghista, D.; Zhong, L. The electronic stethoscope. BioMed. Eng. OnLine 2015, 14, 66. [Google Scholar] [CrossRef]
- Chandramohan, G. Electrical Characterization of MEMS Microphones. Master Thesis, Tu Delft, Delft, The Netherlands, 2010. [Google Scholar]
- Arnau, A.; Soares, D. Fundamentals of Piezoelectricity. Piezoelectric Transducers and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- American Piezo Company. What Is a Transducer. Available online: https://www.americanpiezo.com/knowledge-center/piezo-theory/whats-a-transducer/ (accessed on 3 January 2023).
- Seo, Y.; Corona, D.; Hall, N.A. On the theoretical maximum achievable signal-to-noise ratio (SNR) of piezoelectric microphones. Sens. Actuators A Phys. 2017, 264, 341–346. [Google Scholar] [CrossRef]
- Chen, H.; Yu, S.; Liu, H.; Liu, J.; Xiao, Y.; Wu, D.; Pan, X.; Zhou, C.; Lei, Y.; Liu, S.; et al. A two-stage amplified PZT sensor for monitoring lung and heart sounds in discharged pneumonia patients. Microsyst. Nanoeng. 2021, 7, 55. [Google Scholar] [CrossRef]
- DB Unlimited. MM023802-1 Datasheet. Available online: https://www.dbunlimitedco.com/images/product_images/2D-Drawings/MM023802-1.pdf (accessed on 5 January 2024).
- 3S (Solid State System). 3SM121PZB1MB Datasheet. Available online: https://mm.digikey.com/Volume0/opasdata/d220001/medias/docus/5854/3SM121PZB1MB.pdf (accessed on 5 January 2024).
- Hu, Y.; Xu, Y. An ultra-sensitive wearable accelerometer for continuous heart and lung sound monitoring. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 694–697. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, M.; Guo, N.; Zhang, W. Design of the MEMS Piezoresistive Electronic Heart Sound Sensor. Sensors 2016, 16, 1728. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Q.; Zhang, G.; Lian, Y.; Zhang, L.; Zhang, X.; Shi, Y.; Duan, S.; Wang, R. A bat-shape piezoresistor electronic stethoscope based on MEMS technology. Measurement 2019, 147, 106850. [Google Scholar] [CrossRef]
- Riley, C. Understanding the Use and Function of MEMS Piezoresistive Pressure Sensors. Available online: https://www.meritsensor.com/understanding-the-use-and-function-of-mems-piezoresistive-pressure-sensors/ (accessed on 16 June 2024).
- Yilmaz, G.; Rapin, M.; Pessoa, D.; Rocha, B.M.; de Sousa, A.M.; Rusconi, R.; Carvalho, P.; Wacker, J.; Paiva, R.P.; Chételat, O. A Wearable Stethoscope for Long-Term Ambulatory Respiratory Health Monitoring. Sensors 2020, 20, 5124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Wen, H.; Di Francesco, L.; Ayazi, F. Detection of pathological mechano-acoustic signatures using precision accelerometer contact microphones in patients with pulmonary disorders. Sci. Rep. 2021, 11, 13427. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, P.; Yang, Y.; Cui, J.; Zhang, G.; Duan, S. Design and verification of magnetic-induction electronic stethoscope based on MEMS technology. Sens. Actuators A Phys. 2021, 331, 112951. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Cui, J.; Zhang, G.; Wang, R.; Zhang, W.; He, C.; Li, Y.; Shi, P.; Wang, S. Design and Realization of MEMS Heart Sound Sensor with Concave, Racket-Shaped Cilium. Biosensors 2022, 12, 534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Shi, P.; Yang, Y.; Cui, J.; Zhang, G.; Wang, R.; Zhang, W.; He, C.; Li, Y.; Wang, S. Design and Fabrication of an Integrated Hollow Concave Cilium MEMS Cardiac Sound Sensor. Micromachines 2022, 13, 2174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.H.; Kim, Y.S.; Yeo, M.K.; Mahmood, M.; Zavanelli, N.; Chung, C.; Heo, J.Y.; Kim, Y.; Jung, S.S.; Yeo, W.H.; et al. Fully portable continuous real-time auscultation with a soft wearable stethoscope designed for automated disease diagnosis. Sci. Adv. 2022, 8, eabo5867. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.R.; Kim, T.; Im, S.; Lee, Y.J.; Jeong, S.; Shin, H.; Kim, M.; Lee, J.; Kim, D.; et al. A Wearable Stethoscope for Accurate Real-Time Lung Sound Monitoring and Automatic Wheezing Detection Based on an AI Algorithm. Res. Sq. 2023. Preprint (Version 1). [Google Scholar] [CrossRef]
- Baraeinejad, B.; Shams, M.; Hamedani, M.S.; Nasiri-Valikboni, A.; Forouzesh, M.; Fakhraeelotfabadi, S.; Karimian, R.; Babaei, S.; Rashidi, D.; Germchi, D.; et al. Clinical IoT in Practice: A Novel Design and Implementation of a Multi-functional Digital Stethoscope for Remote Health Monitoring. TechRxiv 2023. [Google Scholar] [CrossRef]
- Mallegni, N.; Molinari, G.; Ricci, C.; Lazzeri, A.; La Rosa, D.; Crivello, A.; Milazzo, M. Sensing Devices for Detecting and Processing Acoustic Signals in Healthcare. Biosensors 2022, 12, 835. [Google Scholar] [CrossRef]
- Messner, E.; Fediuk, M.; Swatek, P.; Scheidl, S.; Smolle-Juttner, F.-M.; Olschewski, H. Crackle and Breathing Phase Detection in Lung Sounds with Deep Bidirectional Gated Recurrent Neural Networks. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 356–359. [Google Scholar] [CrossRef]







| Model | Manufacturer | Size (Diameter Øx Height) (mm) | Frequency Response (Hz) | Sensitivity (dB) | SNR (dB) | Voltage Range (V) |
|---|---|---|---|---|---|---|
| AOM-4544P-2-R [68] | PUI Audio Inc., Fairborn, OH, USA | 9.70 Ø × 4.70 mm | 50 Hz~16 kHz | −44 dB ± 2 dB | 60 dB | 1.5~10 V |
| CMA-4544PF-W [71] | CUI Devices, Portland, OR, USA | 9.70 Ø × 4.65 mm | 20 Hz~20 kHz | −44 dB ± 2 dB | 60 dB | 3~10 V |
| EM-6050P [72] | Soberton Inc., Minneapolis, MN, USA | 6.00 Ø × 5.44 mm | 100 Hz~15 kHz | −42 dB ± 3 dB @ 94 dB SPL | 58 dB | 1~10 V |
| RMIC-110-10-6027-NS1 [73] | Raltron Electronics, Doral, FL, USA | 6.00 Ø × 2.90 mm | 50 Hz~10 kHz | −42 dB ± 3 dB @ 94 dB SPL | 58 dB | 1~10 V |
| Parameter | Value |
|---|---|
| Diameter of the ECM sensor | 5 mm |
| Thickness of the ECM sensor | 2 mm |
| Sensor Array Arrangement | 4 × 4 rectangular arrays |
| Dimensions of the Printed Circuit Board | 85 × 70 × 4 mm3 |
| Oversampling Frequency | 48 kHz |
| File Saving Sampling Rate and Format | 8-kHz WAV files |
| Ref | Technology | Dimension (mm) | Bandwidth (Hz) | Sensitivity (dB) | SNR (dB) |
|---|---|---|---|---|---|
| [79] | Electret Sensor Array FPGA | 5 Ø × 2 mm (Microphone) 85 × 70 × 4 mm3 (PCB) | 1 Hz~1 kHz | N/A | 29.36 dB |
| [81] | CZN-15E ECM Microphone NE5534P Amplifier | 9.7 Ø × 6.7 mm (Microphone) | 20 Hz~16 kHz | −58 ± 2 dB (0 dB = 1 V/pa,1 kHz) | 60 dB |
| [82] | ECM Microphone Micropore® tape FFT | 3 Ø mm (Microphone) | 1~10 Hz | N/A | N/A |
| [83] | SONY ECM Microphone LT1115CN8 Amplifier Wavelet | 16.7552 cm3 | 20 Hz~1 kHz | N/A | 90.84 dB |
| Sensor Type | Purpose of Power Usage | Operating Voltage | Power Consumption |
|---|---|---|---|
| ECM | Powering the JFET amplifier for signal amplification | 1.5–9 V | Moderate |
| MEMS | Powering the sensor element and integrated circuits | 1.8–3.6 V | Low |
| Materials | Density (kg/m3) | Young’s Modulus (GPa) | Size (mm) | |
|---|---|---|---|---|
| Piezoelectric beam | Lead Zirconate Titanate (PZT) | 7.8 × 103 | 66 | 3 × 1 × 0.127 |
| Mechanical beam | Aluminum | 2.7 × 103 | 69 | 3 × 12 × 0.38 |
| Proof mass | Aluminum | 2.7 × 103 | 69 | 20 × 12 × 1.5 |
| Model | Output Type | Manufacturer | Size (L × W × H) (mm3) | Frequency Response (Hz) | Sensitivity (dB) | SNR (dB) |
|---|---|---|---|---|---|---|
| SPH0645LM4H-B [88] | Digital, I2S | Knowles, Itasca, IL, USA | 3.50 × 2.65 × 1.10 mm3 | 20 Hz~10 kHz | −26 dB ± 3 dB @ 94 dB SPL | 65 dB |
| ICS-52000 [87] | Digital, TDM | TDK InvenSense, San Jose, CA, USA | 4.00 × 3.00 × 1.10 mm3 | 50 Hz~20 kHz | −26 dB ± 1 dB @ 94 dB SPL | 65 dB |
| MM023802-1 [96] | Analog | DB Unlimited, Dayton, OH, USA | 2.75 × 1.85 × 1.05 mm3 | 30 Hz~10 kHz | −38 dB ± 1 dB | 65 dB |
| DMM-4026-B-I2S-R [89] | Digital, I2S | PUI Audio Inc., Fairborn, OH, USA | 4.00 × 3.00 × 1.10 mm3 | 20 Hz~20 kHz | −26 dB ± 1 dB | 64 dB |
| CMM-3526DB-37165-TR [85] | Digital, PDM | CUI Devices, Portland, OR, USA | 3.50 × 2.65 × 0.98 mm3 | 100 Hz~10 kHz | −37 dB ± 1 dB @ 94 dB SPL | 65 dB |
| 3SM121PZB1MB [97] | Analog | 3S (Solid State System), Shenzen, China | 4.72 × 3.76 × 1.30 mm3 | 100 Hz~10 kHz | −38 dB ± 1 dB @ 94 dB SPL | 68 dB |
| IM66D130AXTMA1 [86] | Digital, PDM | Infineon Technologies, Austin, TX, USA | 3.50 × 2.65 × 0.99 mm3 | 10 Hz~10 kHz | −36 dB ± 1 dB @ 94 dB SPL | 66 dB |
| Technique | Purpose | Application |
|---|---|---|
| DRIE | Precise etching of nano-scale structures | Creation of micro-cantilever structures |
| LPCVD | Deposition of silicon dioxide for gap filling | Sensing element formation |
| Thermal Oxidation | Formation of nano-gaps in SOI wafers | Definition of sensor structures |
| TSVs | Provides electrical connections through the sensor | Electrical integration in MEMS sensors |
| Ref. | Size (L × W × H) | Bandwidth | Sensitivity | SNR |
|---|---|---|---|---|
| [98] | 35 × 18 × 7.8 mm3 | 20~1 kHz | N/A 1 | 65 dB |
| [99] | 2 × 2 × 0.02 mm3 | N/A | N/A | 27 dB |
| [100] | 1.2 × 1.2 × 0.02 mm3 | 20~1 kHz | −180.7 dB@500 Hz | 38 dB |
| [102] | 37 × 30 × 7.6 mm3 | 100~1.6 kHz | N/A | N/A |
| [103] | 20 × 20 mm2 | ~10 kHz | N/A | N/A |
| [104] | 2500 × 12 × 20 μm3 | 20~600 Hz | −189 dB@500 Hz | 27.38 dB |
| [105] | 0.1 × 0.34 × 5.7 mm3 | 20~600 Hz | −180.6 dB@500 Hz | 27.05 dB |
| [106] | 0.12 × 0.34 × 4.9 mm3 | 20~800 Hz | −206.9 dB @200 Hz | 26.471 dB |
| [107] | 20 × 20 mm2 | 20~1.35 kHz | N/A | 14.8 dB |
| [108] | 40 × 40 mm2 | 100~2 kHz | N/A | N/A |
| [109] | 41 mm diameter | 20~1 kHz | N/A | N/A |
| Characteristic | MEMS Sensor | ECM Sensor |
|---|---|---|
| Volume (mm3) | 11.90 mm3 ± 5.20 mm3 | 231.69 mm3 ± 116.59 mm3 |
| Frequency Response (Hz) | 10 Hz to 20 kHz | 20 Hz to 20 kHz |
| Sensitivity (dB) | −32.43 dB ± 5.60 dB | −43.00 dB ± 1.00 dB |
| SNR (dB) | 65.43 dB ± 1.18 dB | 59.00 dB ± 1.00 dB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torabi, Y.; Shirani, S.; Reilly, J.P.; Gauvreau, G.M. MEMS and ECM Sensor Technologies for Cardiorespiratory Sound Monitoring—A Comprehensive Review. Sensors 2024, 24, 7036. https://doi.org/10.3390/s24217036
Torabi Y, Shirani S, Reilly JP, Gauvreau GM. MEMS and ECM Sensor Technologies for Cardiorespiratory Sound Monitoring—A Comprehensive Review. Sensors. 2024; 24(21):7036. https://doi.org/10.3390/s24217036
Chicago/Turabian StyleTorabi, Yasaman, Shahram Shirani, James P. Reilly, and Gail M. Gauvreau. 2024. "MEMS and ECM Sensor Technologies for Cardiorespiratory Sound Monitoring—A Comprehensive Review" Sensors 24, no. 21: 7036. https://doi.org/10.3390/s24217036
APA StyleTorabi, Y., Shirani, S., Reilly, J. P., & Gauvreau, G. M. (2024). MEMS and ECM Sensor Technologies for Cardiorespiratory Sound Monitoring—A Comprehensive Review. Sensors, 24(21), 7036. https://doi.org/10.3390/s24217036






