Effects of Whole-Body Electromyostimulation on Metabolic Syndrome in Adults at Moderate-to-High Cardiometabolic Risk—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Information Sources and Search Strategy
2.2. Selection Process
2.3. Eligibility Criteria
2.4. Data Items and the Data Collection Process
2.5. Risk of Bias Assessment
2.6. Data Synthesis
2.7. Statistical Analysis
3. Results
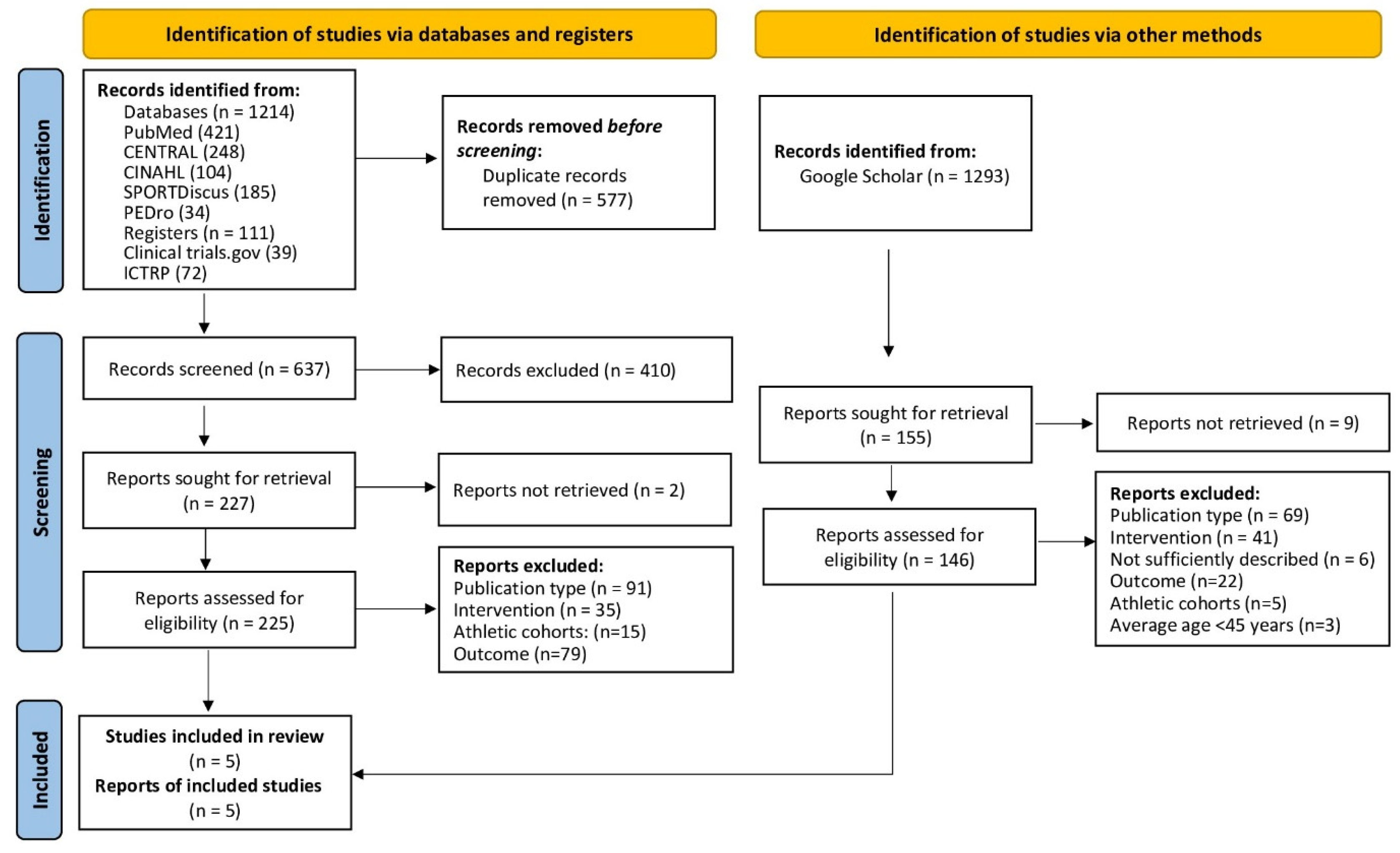
3.1. Study Selection
3.2. Study, Participant, and Exercise Characteristics
3.3. Methodologic Quality of the Trials
3.4. Study Outcomes
3.5. Meta-Analysis Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beier, M.; Schoene, D.; Kohl, M.; von Stengel, S.; Uder, M.; Kemmler, W. Non-athletic cohorts enrolled in longitudinal whole-body electromyostimulation trials—An evidence map. Sensors 2024, 24, 972. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Kleinoder, H.; Fröhlich, M. Editorial: Whole-Body Electromyostimulation: A Training Technology to Improve Health and Performance in Humans? Front. Physiol. 2020, 11, 523. [Google Scholar] [CrossRef]
- Kemmler, W.; Fröhlich, M.; Eifler, C. Whole-Body Electromyostimulation. Effects, Limitations, Perspectives of an Innovative Training Method; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Le, Y.H.; Kohl, M.; von Stengel, S.; Uder, M.; Kemmler, W. Effectiveness and Safety of Whole-Body Electromyostimulation on Musculoskeletal Diseases in Middle Aged-Older Adults—A Systematic Review. Dtsch Z Sportmed. 2024, 75, 41–48. [Google Scholar] [CrossRef]
- Van Buuren, F.; Horstkotte, D.; Mellwig, K.P.; Fründ, A.; Vlachojannis, M.; Bogunovic, N.; Dimitriadis, Z.; Vortherms, J.; Humphrey, R.; Niebauer, J. Electrical Myostimulation (EMS) Improves Glucose Metabolism and Oxygen Uptake in Type 2 Diabetes Mellitus Patients—Results from the EMS Study. Diabetes Technol. Ther. 2015, 17, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, F.; Mellwig, K.P.; Prinz, C.; Körber, B.; Fründ, A.; Fritzsche, D.; Faber, L.; Kottmann, T.; Bogunovic, N.; Dahm, J.; et al. Electrical myostimulation improves left ventricular function and peak oxygen consumption in patients with chronic heart failure: Results from the exEMS study comparing different stimulation strategies. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2013, 102, 523–534. [Google Scholar] [CrossRef]
- Fritzsche, D.; Fruend, A.; Schenk, S.; Mellwig, K.; Keinöder, H.; Gummert, J.; Horstkotte, D. Elektromyostimulation (EMS) bei kardiologischen Patienten. Wird das EMS-Training bedeutsam für die Sekundärprävention? Herz 2010, 35, 34–40. [Google Scholar] [CrossRef]
- Houdijk, A.P.J.; Bos, N.; Verduin, W.M.; Hijdendaal, M.M.; Zwartkruis, M.A.L. Visceral fat loss by whole-body electromyostimulation is attenuated in male and absent in female older Non-Insulin-Dependent diabetes patients. Endocrinol. Diabetes Metab. 2022, 5, e377. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Expert-Panel. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e13–e18. [Google Scholar] [CrossRef]
- Johnson, J.L.; Slentz, C.A.; Houmard, J.A.; Samsa, G.P.; Duscha, B.D.; Aiken, L.B.; McCartney, J.S.; Tanner, C.J.; Kraus, W.E. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am. J. Cardiol. 2007, 100, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Reljic, D.; Dieterich, W.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. “HIIT the Inflammation”: Comparative Effects of Low-Volume Interval Training and Resistance Exercises on Inflammatory Indices in Obese Metabolic Syndrome Patients Undergoing Caloric Restriction. Nutrients 2022, 14, 1996. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; De-la, O.A.; Jurado-Fasoli, L.; Martinez-Tellez, B.; Ruiz, J.R.; Castillo, M.J. Exercise Training as a Treatment for Cardiometabolic Risk in Sedentary Adults: Are Physical Activity Guidelines the Best Way to Improve Cardiometabolic Health? The FIT-AGEING Randomized Controlled Trial. J. Clin. Med. 2019, 8, 2097. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Shojaa, M.; Von Stengel, S.; Schoene, D.; Kohl, M.; Barone, G.; Bragonzoni, L.; Dallolio, L.; Marini, S.; Murphy, M.H.; Stephenson, A.; et al. Effect of exercise training on bone mineral density in postmenopausal women: A systematic review and meta-analysis of intervention studies. Front. Physiol. 2020, 11, 1427–1444. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- R_Development_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Doi, S.A.; Barendregt, J.J.; Khan, S.; Thalib, L.; Williams, G.M. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp. Clin. Trials 2015, 45, 130–138. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; von Stengel, S. Effects of High Intensity Resistance Training versus Whole-body Electromyostimulation on cardiometabolic risk factors in untrained middle-aged males. A randomized controlled trial. J. Sports Res. 2016, 3, 44–55. [Google Scholar] [CrossRef]
- Reljic, D.; Konturek, P.C.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Effects of whole-body electromyostimulation exercise and caloric restriction on cardiometabolic risk profile and muscle strength in obese women with the metabolic syndrome: A pilot study. J. Physiol. Pharmacol. 2020, 71, 89–98. [Google Scholar] [CrossRef]
- Wittmann, K.; Sieber, C.; von Stengel, S.; Kohl, M.; Freiberger, E.; Jakob, F.; Lell, M.; Engelke, K.; Kemmler, W. Impact of whole body electromyostimulation on cardiometabolic risk factors in older women with sarcopenic obesity: The randomized controlled FORMOsA-sarcopenic obesity study. Clin. Interv. Aging 2016, 11, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.; Borg, E. Borg CR Scales® Folder; Borg Perception: Hasselby, Sweden, 2010. [Google Scholar]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; Frohlich, M.; Kohl, M.; von Stengel, S. Effects of Whole-Body Electromyostimulation versus High-Intensity Resistance Exercise on Body Composition and Strength: A randomized controlled study. Evid. Based Complement. Altern. Med. 2016, 2016, 9236809. [Google Scholar] [CrossRef]
- Ribeiro de Avila, V.; Bento, T.; Gomes, W.; Leitao, J.; Fortuna de Sousa, N. Functional Outcomes and Quality of Life After Ankle Fracture Surgically Treated: A Systematic Review. J. Sport Rehabil. 2018, 27, 274–283. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; Batrakoulis, A.; Ghazali, W.S.W.; Mohamed, M.; Aldayel, A.; Alhussain, M.H.; Afolabi, H.A.; Wada, Y.; Gülü, M.; Elkholi, S.; et al. Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: A systematic review and meta-analysis. PeerJ 2024, 12, e17525. [Google Scholar] [CrossRef]
- Liang, M.; Pan, Y.; Zhong, T.; Zeng, Y.; Cheng, A.S.K. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: A systematic review and network meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1523–1533. [Google Scholar] [CrossRef]
- Wewege, M.A.; Thom, J.M.; Rye, K.A.; Parmenter, B.J. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Després, J.-P. Visceral obesity with excess ectopic fat: A prevalent and high-risk condition requiring concerted clinical and public health actions. CardioMetabolic Syndr. J. 2021, 1, 1–17. [Google Scholar] [CrossRef]
- Doi, S.A.R.; Furuya-Kanamori, L. Selecting the best meta-analytic estimator for evidence-based practice: A simulation study. Int. J. Evid. Based Healthc. 2020, 18, 86–94. [Google Scholar] [CrossRef]


| First Author, Year | Study Design | Sample Size/Group [n] | Gender (Men/Women) | Age [Years] | Body Mass Index [kg/m2] | Waist Circum- Ference (cm) | Dietary Intervention/Energy Restriction | Cardio- Vascular Health Status | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Amaro-Gahete et al. 2019 [14] | RCT | WB-EMS: 19 CG: 18 | WB-EMS:10/9 CG: 9/9 | WB-EMS: 53.5 ± 5.3 CG: 53.1 ± 5.6 | 28.6 ± 4.6 26.4 ± 3.2 | 99.3 ± 13.7 97.5 ± 10.9 | no | MR MR |
| 2 | Kemmler et al. 2016 [22] | RCT | WB-EMS: 23 CG: 23 | Only men | WB-EMS: 43.7 ± 6.1 CG: 41.9 ± 6.4 | 28.5 ± 4.1 26.9 ± 3.3 | 102.6 ± 9.4 100.5 ± 9.6 | no | MR |
| 3 | Reljic et al. 2020 [23] | RCT | WB-EMS: 15 CG: 14 | Only women | 56.0 ± 10.9 Details n.g. | 36.1 ± 4.5 37.4 ± 4.8 | 107.2 ± 7.3 109.6 ± 8.6 | −500 kcal/d + Protein ≥ 1 g/d −500 kcal/d+ Protein ≥ 1 g/d | HR |
| 4 | Reljic et al. 2022 [13] | RCT | WB-EMS: 26 CG: 26 | WB-EMS: 8/18 CG: 8/18 | WB-EMS: 52.7 ± 12.5 CG: 49.0 ± 15.1 | 37.2 ± 4.0 38.0 ± 6.3 | 114 ± 10 109 ± 11 | −500 kcal/d/ −500 kcal/d | HR |
| 5 | Wittmann et al. 2016 [24] | RCT | WB-EMS: 25 CG: 25 | Only women | WB-EMS: 77.3 ± 4.9 CG: 77.4 ± 4.9 | 24.2 ± 2.0 23.9 ± 1.4 | 93.5 ± 4.8 91.4 ± 6.4 | no | MR |
| First Author, Year | Superimposed WB-EMS | Intervention Length [Weeks] | Sessions/Week [n] | Length of Session [min] | Impulse Frequency [Hz] | Impulse Intensity | Duty Cycle [%] Impulse- Rest Phase | Control Physical Intervention | Loss to Follow-Up [%] | Attendance [%] | Adverse Effects | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Amaro-Gahete et al. 2019 [14] | HIIT AE + RT and WB-EMS | 12 | 2 | 20, 33 | 10–20, 35–75 | moderate–high | AE: 99 RT: 50–63 | HIIT AE + RT | HIIT + WB-EMS: 17 HIIT: 30 | HIIT+WB-EMS: 99 HIIT: 99 | no |
| 2 | Kemmler et al. 2016 [22] | no | 16 | 1.5 | 20 | 85 | high | 60 6–4 s | HIT-RT | WB-EMS: 9 HIT-RT: 13 | WB-EMS: 90 ± 11 HIT-RT: 93 ± 7 | no |
| 3 | Reljic et al. 2020 [23] | no | 12 | 2 | 20 | 85 | moderate | 60 6–4 s | none | 25 | 93 ± 8 | no |
| 4 | Reljic et al. 2022 [13] | no | 12 | 2 | 20 | 85 | moderate | 60 6–4 s | none | 23 | 93 ± 8 | no |
| 5 | Wittmann et al. [24] | no | 26 | 1 | 20 | 85 | low–moderate | 50 4–4 s | none | 4 | 89 ± 6 | no |
| First Author, Year | Eligibility Criteria | Random Allocation | Allocation Concealment | Inter Group Homogeneity | Blinding Subjects | Blinding Personnel | Blinding Assessors | Participation ≥ 85% Allocation | Intention to Treat Analysis a | Between Group Comparison | Measure of Variability | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amaro-Gahete et al. 2019 [14] | Y | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Kemmler et al. 2016 [22] | Y | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Reljic et al. 2020 [23] | Y | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Reljic et al. 2022 [13] | Y | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Wittmann et al. 2016 [24] | Y | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Amaro-Gahete et al. 2019 [14] 1 | Kemmler et al. 2016 [22] | Reljic et al. 2020 [23] 2 | Reljic et al. 2022 [13] 1,2 | Wittmann et al. 2016 [24] | |
|---|---|---|---|---|---|
| Δ Waist circumference WB-EMS (cm) | −4.0 ± 2.4 | −3.4 ± 4.5 | −2.3 | −3.0 | −1.4 ± 2.1 |
| Δ Waist circumference Control (cm) | −4.5 ± 2.5 | −2.1 ± 4.1 | −1.0 | −2.0 | −0.0 ± 2.3 |
| Δ MAP WB-EMS (mmHg) | −5.4 ± 3.1 | −4.9 ± 7.3 | −7.0 | 2.0 | −8.8 ± 11.0 |
| Δ MAP Control (mmHg) | −1.6 ± 1.8 | −3.6 ± 5.6 | 1.0 | −1.0 | −2.2 ± 9.5 |
| Δ Triglycerides WB-EMS (mg/dL) | −30 ± 41 | 9.5 ± 55.5 | −6.0 | −15.0 | 2.8 ± 28.5 |
| Δ Triglycerides Control (mg/dL) | −15 ± 60 | −10.1 ± 47.9 | −30.0 | −18.0 | 9.8 ± 39.2 |
| Δ HDL-C WB-EMS (mg/dL) | 5.1 ± 12.9 | n.g. 3 | −1.0 | −1.0 | −1.3 ± 6.35 |
| Δ HDL-C Control (mg/dL) | 2.2 ± 12.8 | n.g. | 0 | −2.0 | −4.6 ± 6.6 |
| Δ Fasting Glucose WB-EMS (mg/dL) | 0.6 ± 5.9 | −4.3 ± 9.0 | −2.0 | −2.0 | −3.0 ± 10.3 |
| Δ Fasting Glucose Control (mg/dL) | −4.1 ± 6.1 | 1.7 ± 8.5 | −5.0 | −3.0 | −3.6 ± 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guretzki, E.; Kohl, M.; von Stengel, S.; Uder, M.; Kemmler, W. Effects of Whole-Body Electromyostimulation on Metabolic Syndrome in Adults at Moderate-to-High Cardiometabolic Risk—A Systematic Review and Meta-Analysis. Sensors 2024, 24, 6788. https://doi.org/10.3390/s24216788
Guretzki E, Kohl M, von Stengel S, Uder M, Kemmler W. Effects of Whole-Body Electromyostimulation on Metabolic Syndrome in Adults at Moderate-to-High Cardiometabolic Risk—A Systematic Review and Meta-Analysis. Sensors. 2024; 24(21):6788. https://doi.org/10.3390/s24216788
Chicago/Turabian StyleGuretzki, Ellen, Matthias Kohl, Simon von Stengel, Michael Uder, and Wolfgang Kemmler. 2024. "Effects of Whole-Body Electromyostimulation on Metabolic Syndrome in Adults at Moderate-to-High Cardiometabolic Risk—A Systematic Review and Meta-Analysis" Sensors 24, no. 21: 6788. https://doi.org/10.3390/s24216788
APA StyleGuretzki, E., Kohl, M., von Stengel, S., Uder, M., & Kemmler, W. (2024). Effects of Whole-Body Electromyostimulation on Metabolic Syndrome in Adults at Moderate-to-High Cardiometabolic Risk—A Systematic Review and Meta-Analysis. Sensors, 24(21), 6788. https://doi.org/10.3390/s24216788






