Abstract
The continuous emergence of new illegal compounds, particularly psychoactive chemicals, poses significant challenges for current drug detection methods. Developing new protocols and kits for each new drug requires substantial time, effort, and dedicated manpower. Whole-cell bacterial bioreporters have been proven capable of detecting diverse hazardous compounds in both laboratory and field settings, identifying not only single compounds but also chemical families. We present the development of a microbial bioreporter for the detection of cocaine, the nervous system stimulant that is the second-most widely used illegal drug in the US. Escherichia coli was transformed with a plasmid containing a bacterial luxCDABEG bioluminescence gene cassette, activated by a cocaine-responsive signaling cascade. The engineered bioreporter is demonstrated to be a sensitive and specific first-generation detection system for cocaine, with detection thresholds of 17 ± 8 μg/L and 130 ± 50 μg/L in a buffer solution and in urine, respectively. Further improvement of the sensor’s performance was achieved by altering the nucleotide sequence of the PBen gene promoter, the construct’s sensing element, using accelerated site-directed evolution. The applicability of ready-to-use paper strips with immobilized bioreporter cells was demonstrated for cocaine detection in aqueous solutions.
1. Introduction
The constant emergence of new illegal psychoactive compounds constitutes a severe hurdle for current drug detection methods. Contemporary methodologies are mostly based on either immunoassays or chromatographic methods, such as LC-MS, and hence target only specific compounds that have been previously identified and characterized. There is thus an acute need for simple and rapid broad-spectrum test kits that will sensitively detect not only the original target but also its potential derivatives and analogs. This need may be at least partially met by the application of synthetic biology approaches to the design of novel drug-targeted whole-cell bioreporters. Such sensors can potentially report not on the chemical nature of the drug molecule, but rather on the biological effects it may exert on living cells; no molecule-based biosensor can provide this level of information nor can it be obtained by chemical analysis [1].
Whole-cell biosensors (bioreporters) are genetically engineered to emit a quantifiable signal in the presence of specific target chemicals, general stress conditions, or deleterious biological effects. They are mostly based on bacterial or yeast cells, the genetic engineering of which is often based on a molecular fusion of two DNA segments: (a) a sensing element, usually a gene promotor that is induced by the target compound(s), and (b) a reporting element, a gene or gene cassette encoding proteins with easily quantifiable activities. Considering the fact that newly developed drug derivatives often activate the same receptors as the parent compound and induce similar biological effects, whole-cell microbial biosensors have the potential to widen the drug detection toolbox. Bacterial sensors have been shown to detect a variety of compounds with accuracy and precision comparable with, and sometimes even surpassing, chemical analysis [2]. They have numerous potential applications, including monitoring of water toxicity [3] and genotoxicity [4], the detection of buried explosives [5], or the quantification of endocrine-disrupting compounds [6]. Here we report on the development of an Escherichia coli-based whole-cell bioreporter for cocaine: a nervous system stimulant and the second-most widely used illegal drug in the US [7,8], the effect of which on the brain, as a dopamine reuptake inhibitor, often leads to dependence and addiction.
The molecular circuit described in this communication is based on the cocaine esterase (CE) gene (cocE), originally identified by Brit et al. [9] in a Pseudomonas maltophilia strain isolated from coca leaves. CE degrades cocaine into ecgonine methyl ester and benzoic acid [9], a reaction similar to that taking place in the human liver [10] and the human blood postmortem [11]. In 2009, Gao et al. developed a variant of the bacterial protein that is stable at human body temperature [12]. This protein shows promise as a long-term protection against cocaine toxicity, potentially reinforcing the liver’s ability to deal with acute cocaine overdoses [13]. CocE has been the subject of multiple studies aimed at its use as a therapeutic intervention for cocaine abuse through intravenous treatment [14,15], in the course of which it has been demonstrated that its activity has not been hindered by body fluids such as blood.
The cocE gene has previously served as the core entity in a cell-free protein expression system described by Voyvodic et al. [16], along with the benzoic acid receptor gene (benR) and the PBen promotor, using the native ribosome binding site (RBS) from Pseudomonas putida. The same components were employed in the sensing circuit reported herein, harboring the following activation cascade in an Escherichia coli host (Figure 1): CocE cleaves cocaine to ecgonine methyl ester and benzoate; the latter forms a complex with BenR, which binds to PBen, thereby activating the Photobacterium leiognathi luxCDABEG bioluminescence gene cassette [17]. The intensity of the elicited bioluminescent response is thus dependent on cocaine concentration. This design lays the foundation for the construction and testing of a cocaine-specific sensor; future generations of this construct will be characterized by an extended capacity for the detection of cocaine derivatives.
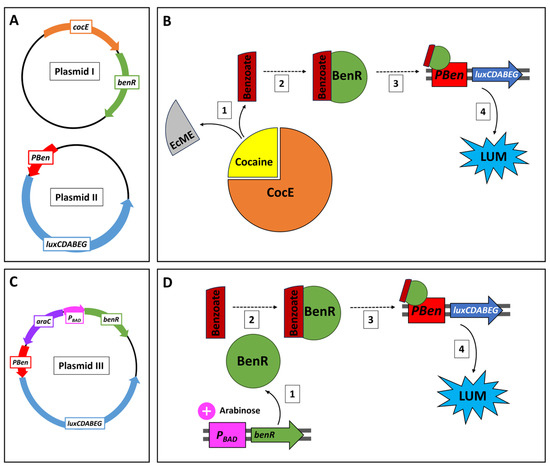
Figure 1.
(A) Schemes of the two plasmids constituting the cocaine detection circuit. Plasmid I harbors the cocaine esterase gene (cocE) and the transcription factor benR. Plasmid II harbors the P. leiognathi luxCDABEG gene cassette controlled by the BenR-inducible promotor PBen. (B) Cocaine detection signaling cascade. Following cocaine cleavage by cocaine esterase (CocE) to ecgonine methyl ester (EcME) and benzoate (1), the latter complexes with the transcription factor BenR (2) and activates the PBen promotor (3). This drives the expression of the luxCDABEG genes, leading to a dose-dependent luminescence signal (4). (C) Schematic illustration of the benzoate sensor plasmid (plasmid III), which harbors the transcription factor gene benR along with the PBAD promoter and its regulator gene araC. The luxCDABEG gene cassette is under the control of PBen. (D) In the presence of arabinose, benR is expressed (1); the BenR protein forms a complex with a benzoate molecule (2), initiating transcription of luxCDABEG by activation of the PBen promotor (3), leading to quantifiable luminescence (4).
2. Materials and Methods
2.1. Chemicals
Cocaine HCl, fentanyl citrate, and ketamine (Klorketam), kindly provided by Prof. Ami Citri of the Hebrew University of Jerusalem, were dissolved in water and kept at room temperature. Cannabinoids (THC, CBD), kindly provided by Prof. Joseph Tam of the Hebrew University Medical School, were dissolved in ethanol and stored at −20 °C. Norketamine and Na benzoate were purchased from Sigma-Aldrich Israel Ltd (Rehovot, Israel), dissolved in methanol and stored at −20 °C (norketamine) or in water and kept at 4 °C (benzoate). Restriction enzymes SalI, NotI, and XmaI and Phusion High-Fidelity DNA Polymerase were purchased from New England Biolabs (Ipswich, MA, USA) and stored at −20 °C. A 2× Taq master mix from Lambda Biotech (Ballwin, MO, USA) was used for colony PCR and stored at −20 °C.
2.2. Bacterial Strain
E. coli K12 strain DH5α (NEB #C2987H) was used as the host for all of this study’s plasmids and for the whole-cell sensor systems. DH5α cells were grown overnight at 37 °C with 200 rpm shaking in lysogeny broth (LB) containing 5 g/L yeast extract, 10 g/L tryptone, and 5 g/L sodium chloride, supplemented with ampicillin (100 μg/mL) and/or chloramphenicol (30 μg/mL). For long-term preservation, overnight cultures were mixed with glycerol (final concentration 25%) and stored at −80 °C.
2.3. Plasmid Design and Construction
PCR primers employed for the amplification of DNA segments from plasmids included functional sites for restriction enzyme recognition or DNA ligation using Gibson assembly, following the manufacturer’s protocol (NEBuilder HiFi DNA Assembly Cloning kit, New England Biolabs, Ipswich, MA, USA) [18]. Newly constructed plasmids were inserted into competent DH5α cells by a 1 min 42 °C heat shock. Plasmid DNA was purified using NucleoSpin® Plasmid EasyPure (Macherey-Nagel, Dueren, Germany). Primers employed and/or constructed in the course of this study are listed in Table S1. The various molecular components employed in plasmid construction are listed in Table 1.
- Cocaine sensor plasmids
The cocaine sensing circuit spanned two separate plasmids (Figure 1A,B):
- I.
- Plasmid pCocE-benR contained the genes encoding the cocaine esterase enzyme (cocE) and the P. putida transcription factor gene benR. The benR sequence was amplified from plasmid pBEAST-BenR (a gift from Dr. Jerome Bonnet, CNRS, France; Addgene plasmid #114597) and was inserted into plasmid pSB4C5_J23101-CocE (a gift from Dr. Jean-Loup Faulon, INRAE, France; Addgene plasmid #128129).
- II.
- The second plasmid, pBen::luxPleio, harbored a fusion of the P. leiognathi luxCDABEG gene cassette to the BenR-inducible P. putida promotor PBen. It is based on the C55_luxPleio plasmid described by Shemer et al. (2020), the yqjF gene promotor in which was replaced by double restriction enzyme digest with the PBen gene promoter using Gibson assembly.
- Benzoate sensor plasmid
- III.
- A third plasmid (pBen::luxPleio, Figure 1C,D) was constructed for the purpose of improving Pben responses to benzoate by accelerated evolution. The plasmid included the benR gene sequence, along with the E. coli arabinose-inducible expression system, composed of the PBAD promoter and its regulatory gene araC [19]. The benR sequence was amplified from plasmid pCocE-benR (this work), and those of araC and PBAD were amplified from plasmid PBAD-mTagBFP2 (Addgene cat. 34632). The vector backbone was digested with SalI, and the PCR products were assembled in the digested vector pBen::luxPleio using Gibson assembly.

Table 1.
DNA components employed in the construction of cocaine and benzoate detection circuits.
Table 1.
DNA components employed in the construction of cocaine and benzoate detection circuits.
| Component | Origin | Description |
|---|---|---|
| cocE | Rhococcus sp. | E. coli-optimized cocaine esterase; cleaves cocaine into ecgonine methyl ester and benzoate. |
| benR | P. putida | Regulatory gene of the ben operon, activated by benzoate [20]. |
| PBen | P. putida | The ben operon promoter, induced by a BenR/benzoate complex. |
| luxCDABEG | P. leiognathi | Bioluminescence gene cassette of Photobacterium leiognathi; luxA and luxB encode the heterodimeric luciferase, luxCDE a fatty acid reductase complex, and luxG a flavin reductase [21]. |
| araC | E. coli | Regulatory gene of the arabinose operon [19]. |
| PBAD | E. coli | Promotor of the arabinose operon, activated by arabinose [19]. |
| PBen2 | P. putida | Mutated PBen (this work). |
2.4. Bacterial Sensor Strains
The sensor strains constructed in the course of this study are listed in Table 2. Cocaine sensor (CocS) cells were generated by co-transformation of plasmids pCocE-benR (chloramphenicol resistance) and pBen::luxPleio (ampicillin resistance) into competent DH5α cells. CocE (cocaine esterase) breaks down cocaine into ecgonine methyl ester and benzoic acid. BenR forms a complex with benzoic acid, which activates the PBen promotor [16]. This activation initiates the expression of the lux genes, subsequently leading to dose-dependent luminescence (Figure 1). The benzoate sensor (BenS) was constructed by transformation of plasmid pBR-araBAD:benR-pBen::luxPleio (ampicillin resistance) into DH5α cells. External addition of 6.6 mM arabinose drives benR expression via the PBAD–araC system. Benzoate complexes with BenR, leading to the expression of the lux cassette (Figure 1). Both CocS and BenS sensor strains were stored at −80 °C in 25% glycerol. CocS2 and BenS2 harbor a modified version of the PBen promoter (PBen2), generated by error-prone PCR as described in Section 2.7 below.

Table 2.
E. coli sensor strains constructed in the course of this study.
2.5. Luminescence Assay
Sensor strains were removed from −80 °C storage and grown overnight in 2 mL LB, supplemented with the appropriate antibiotics at 37 °C with shaking (200 rpm). The overnight CocS culture was diluted 100-fold into fresh LB and regrown under the same conditions for ca. 75 min to early exponential growth phase (OD600 ≈ 0.2). The BenS overnight culture was diluted 150-fold with LB containing 6.6 mM arabinose and regrown for 2 h. Aqueous aliquots (100 μL per well) of the target compound (cocaine HCl or Na benzoate, 0 to 2 mg/L) were added to individual wells of an opaque white 96-well plate with a transparent bottom (Greiner Bio-One, Kremsmünster, Austria). For the specificity test of the cocaine sensor strain, 5 mg/L cocaine, 40 mg/L fentanyl, 40 mg/L ketamine, 5 mg/L benzoate, 0.1 mg/L tetrahydrocannabinol (THC) and 2 mg/L cannabidiol (CBD) were used in aqueous solutions (100 μL per well). An aliquot (100 μL) of refreshed bacterial culture was added to each well, the plate was covered with a transparent cover, and luminescence was measured every 10 min for 12 to 16 h at 30 °C in a Tecan Infinite® 200 PRO (Männedorf, Switzerland) plate reader. Luminescence values are depicted in the plate reader’s arbitrary relative luminescence units (RLUs) or as the ratio of the maximum luminescence intensity of the induced sample to that of the uninduced control (response ratio).
2.6. Cocaine Detection by CocS Paper Strips
Paper strips imbued with lyophilized CocS cells were designed for testing a simplified “dipstick”-type concept for rapid cocaine testing. CocS cells were grown overnight in 25 mL LB containing 100 μg/mL ampicillin and 30 μg/mL chloramphenicol to an optical density at 600 nm of ca. 2.5. Cells were pelleted by a 6 min centrifugation (3220 x g, 20 °C, Eppendorf 5810R) and resuspended in a prewarmed (37 °C) sterile protectant medium adapted from Stocker et al. [22] containing (w/v) 0.5% peptone, 0.25% yeast extract, 10% gelatin, 1% sodium ascorbate, 5% raffinose, and 5% glutamate. A 5 μL drop of cell suspension was placed on a marked spot of a 0.5 cm × 2 cm filter paper strip (Whatman #3). The strips were incubated for 5 min at room temperature and then transferred to a lyophilizer (Epson 1–4 LSCplus-CHRIST) and freeze-dried to −20 °C using the following program: 5 min at 1000 mbar, 2 h at 4 mbar, 2 h at 0.4 mbar, and 2 h at 0.04 mbar. Following the 6 h freeze-drying program, the lyophilizer was reset to 1000 mbar, and the paper strips were removed and stored at 4 °C.
For cocaine testing, paper strips were removed from refrigeration and, following equilibration to room temperature, were dipped into a cocaine solution (0 to 2 mg/L) for 30 min at 30 °C. The strips were removed from the samples, and luminescence was imaged in the dark for up to 5 h at 30 °C using a Sony Alpha 7sII camera with an exposure time ranging from 5 to 15 s. To prevent drying of the cells during storage, paper strips were either wrapped in a plastic foil or placed in a sealed plastic test tube.
2.7. Random Mutagenesis of the PBen Gene Promoter
To generate a library of PBen promoter mutants, the PBen region within the pBR-araBAD:benR-pBen::luxPleio plasmid was subjected to error-prone PCR using primers listed in Table S1. Taq polymerase was induced to introduce sequence errors into the amplification products by supplementing the PCR mixture with Mn2+ ions (250–375 μM as MnCl2), known to interfere with polymerase activity and to reduce its fidelity [23]. Fragment ends were functionalized by PCR primers for re-legation with the original plasmid. Plasmid pBR-araBAD:benR-pBen::luxPleio was pre-digested with NotI and XmaI restriction enzymes to remove the PBen promotor, generating a promotor-less, linearized plasmid. Purified mutated PBen PCR fragments were re-ligated into the promotor-less plasmid using Gibson assembly and transformed into E. coli strain DH5α to generate a library of approximately 1000 BenS variants. The library was screened for improved clones by inducing the cells with 0.5 mg/L benzoate and comparing the ensuing luminescence intensity with that of the benzoate-free control. Opaque white 384-well microtiter plates with a transparent bottom were prepared to contain 25 μL of either 0 mg/L or 0.5 mg/L benzoate in water per well, and 20 μL of a pre-grown and arabinose-activated BenS variant was added to each well. Luminescence and optical density at 600 nm were determined every 30 min for 15 h at room temperature. Promotor variants exhibiting an enhanced signal intensity and an increased response ratio compared with the parental plasmid were selected for further testing by exposure to a broader range of benzoate concentrations.
2.8. Calculations
The response ratio was determined by dividing the maximal luminescence (RLU) of the specific compound concentrations within the experimental course of 16 h with the maximal luminescence (RLU) of the compound-free control.
where i is the respective concentration of the compound of interest, and CTRL describes the noise. Values are presented in the mean and the standard deviation of the mean (SEM). Error propagation according to Gauss was included in these calculations. Response data were analyzed by using the non-linear, four-parameter fit function of Origin (Academic) software (Version 2022b) with the Levenberg-Marquardt iteration logarithm. The chi-square tolerance value was set to 10−9.
The limit of detection (LOD) of the generated sensor strains was calculated as the average signal intensity of the noise (the luminescence signal of the uninduced controls) plus three standard deviations of the noise.
where is the mean of the maximal luminescence of the noise, and is the standard deviation of the noise.
Data from independent experiments were combined by calculating the weighted average. The best estimate was set as
and the uncertainty of the weighted average
with the weighting factor as
3. Results
3.1. Cocaine Detection by CocS
CocS sensor strains, harboring plasmids pCocE-BenR and pBen::luxPleio, were exposed to a concentration series of cocaine as described under Materials and Methods, and luminescence was recorded every 10 min for 16 h. For concentrations above 0.25 mg/L, luminescence peaked after 8 h, with an apparent small shoulder after 4 h (Figure 2A). Figure 2B displays the maximal signal obtained in the course of the experiment, depicted both as the response ratio and as luminescence intensity, as a function of cocaine concentration. From photographic images of individual wells from a 96-well microtiter plate where lower concentrations were used (Figure 2C) and the pixel intensity of these images (Figure 2D), it may be observed that already at a concentration of 0.015 mg/L the luminescent signal appeared to be visually discernable from the cocaine-free control, displaying an apparent increase in detection sensitivity compared with the plate reader data. The calculated cocaine limits of detection (LODs) for both assays were indeed different—0.1 ± 0.1 mg/L from the plate reader data (Figure 2B) and 0.01 ± 0.02 mg/L from the image-derived data (Figure 2D). This 10-fold difference may be explained by the long exposure (4 sec) of the photographic image compared with the much shorter integration time (0.1 sec) of the plate reader. As shown in panel E, CocS cells luminesced only in the presence of cocaine and benzoate; no luminescence was observed in the presence of fentanyl (40 mg/L), ketamine (40 mg/L), THC (0.1 mg/L), and CBD (2 mg/L), highlighting the cocaine specificity of the assay. The tested concentrations of these compounds were selected following preliminary experiments, which determined them to be the highest non-toxic concentrations for the E. coli strain used. To test the sensor’s potential functionality in human body fluids, the same analytical protocol was employed on cocaine dissolved in urine. The data in Figure 2F exhibit dose dependency very similar to that observed in Figure 2B in an aqueous buffer, and a similarly low LOD (0.13 ± 0.05 mg/L). The reason for the higher error values at the lower cocaine concentrations is unclear; it may be due to differences in the urine composition in samples from different individuals.

Figure 2.
Luminescent cocaine detection by CocS cells in liquid medium. (A) Time course of luminescence development following exposure of CocS cells to the indicated cocaine concentrations. (B) Dose dependency of the luminescent signal (maximal values over a 16 h exposure), displayed both as signal intensity (◊) and as the response ratio (♦). Luminescence intensity values (mean ± SEM, n = 6) in both panels are presented in the plate reader’s arbitrary relative luminescence units (RLUs). LOD was determined using the response ratio plus three times the standard deviation of the background. (C) Luminescence of CocS cells 5.5 h post exposure to different cocaine concentrations. Image was captured by a Sony Alpha 7sII camera (4 s exposure, f-number 2, ISO 40,000). (D) Increase in pixel intensity of images shown in C compared with the cocaine-free control. Intensities were determined using the mean gray value measurement in ImageJ. Data shown represent mean ± SD (area = 3268 pixels) with incorporation of error propagation. LOD was set to be three standard deviations above the background. (E) Response specificity: luminescence development over time during exposure of CocS cells in the presence of different compounds (mean ± SEM, n = 2)—5 mg/L cocaine, 5 mg/L benzoate, 40 mg/L fentanyl, 40 mg/L ketamine, 0.1 mg/L THC, and 2 mg/L CBD. (F) Functionality in human urine: maximal luminescence presented both as signal intensity (◊) and as the response ratio (♦) of two individual samples from different donors (mean ± SEM, n = 2) with cocaine concentrations ranging from 0.152 to 5 mg/L.
3.2. Cocaine Detection by CocS Paper Strips
CocS cells were lyophilized on paper strips to evaluate the potential applicability as a “dipstick”-type test. The bacteria-containing section of the strips was immersed in varying concentrations of cocaine (in H2O) for 30 min, air-dried, and imaged in the dark by a digital camera following a 5 h incubation at 30 °C. A clear luminescent signal was observed on the paper spots where CocS cells had been immobilized, indicating the presence of cocaine. Paper strips were stored at 4 °C and tested for cocaine detection functionality after 1, 2, and 3 weeks. Images for the first two weeks are shown in Figure 3A and the pixel intensities for the entire test period in Figure 3B. As demonstrated in Figure 3B, luminescence intensity decreased with incubation time but was nevertheless apparent even after 3 weeks of storage.

Figure 3.
Induction of lyophilized CocS cells on paper strips by cocaine (30 min exposure, 30 °C). (A) Luminescence, imaged 5 h after induction using a Sony Alpha 7sII camera (15 s exposure, f-number = 2, ISO 40,000). (B) Increase in pixel intensity (compared with the cocaine-free control) of the images in panel A, analyzed using ImageJ software (version 1.54i, March 2024) which provided both the mean gray value and standard deviation within the bacterial spot region. The figure presents data from two independent experiments after storage at 4 °C. Values are presented in mean ± SEM.
3.3. Benzoate Detection by BenS
The single-plasmid benzoate sensor strain constructed as described under Materials and Methods was employed as a chassis for screening the PBen variants emerging from its directed evolution process. The benzoate detection sensitivity of the parental BenS sensor was studied in both the presence and absence of arabinose. In the latter case, the expression of benR was not anticipated, and, consequently, no luminescence was expected.
As shown in Figure 4, the BenS strain responded in the presence of arabinose to all benzoate concentrations tested, from 31 μg/L to 2 mg/L, with luminescence peaking approximately 3 h post induction (Figure 4A). This response was earlier than that of the CocS sensor to cocaine (Figure 2A), as expected due to the lack of dependency on cocaine cleavage by cocaine esterase. The clear dose response (Figure 4B) allowed the calculation of the LOD, determined to be 0.016 ± 0.01 mg/L. Interestingly, there was a strong luminescent response to benzoate also without benR induction by arabinose (Figure 4C); this is likely due to a certain leakiness of the PBAD promoter, as has been previously shown for this element [19,24,25]. Dose response validation (Figure 4D) revealed a clear luminescence signal for benzoate concentrations higher than 0.5 mg/L but not for the lower concentration range. LOD was determined to be ca. 20 times higher (0.3 ± 0.12 mg/L) compared with the arabinose-induced experimental setup.

Figure 4.
Induction of BenS sensor strain by benzoate. Bacteria were grown overnight at 37 °C and 200 rpm and diluted ×1/150 2 h prior to benzoate induction in the presence (A,B) or absence (C) of 6.6 mM arabinose. Luminescence (mean ± SEM, n = 3) was measured every 10 min at 30 °C. Luminescence values are in the plate reader’s arbitrary relative light units (RLUs). Panels (B,D) present the maximal luminescence (RLU) and calculated response ratio as a function of benzoate concentration in the presence or absence of arabinose, respectively.
3.4. Directed Evolution of the PBen Promotor
A single cycle of error-prone PCR amplification of the promotor region of the benzoate sensor yielded ca. 1,000 variants of the BenS sensor, which were then screened for their response to benzoate (0 and 0.5 mg/L). A small number of clones that displayed an improved performance compared with the parent construct, as evidenced by both the signal intensity and the response ratio, were analyzed for their response to a benzoate concentration series. The best performer out of these stood out by demonstrating a 7-fold increase in signal intensity (Figure 5A) and a remarkable 64-fold increase in the response ratio (in the presence of 2 mg/L benzoate; Figure 5B) when compared with the original BenS sensor. The new promoter variant was termed PBen2, and the sensor harboring it was termed BenS2. Results of the sequence analysis of this mutant revealed seven distinct point mutations at positions (Figure 5C,D).

Figure 5.
Responses to benzoate of the BenS2 sensor, harboring the mutated version of the PBen promoter (PBen2). (A) Maximal luminescence response (mean ± SEM, n = 3) of BenS2 and BenS to benzoate. (B) Response ratios of the two sensors. (C) Gene sequence of the PBen and PBen2 promoters, with the seven point mutations highlighted. (D) Mutations in PBen2 and their positions.
3.5. Cocaine Detection with PBen2
The PBen2 promoter sequence was cloned into the pBen::luxPleio plasmid, which was then transformed into DH5α cells along with the second plasmid (pCocE-benR), to generate a new cocaine sensor strain termed CocS2. Cocaine detection by this strain is summarized in Figure 6 along with those of the original CocS, demonstrating a notable enhancement in the response ratio. However, detection sensitivity remained similar, with calculated cocaine LODs of 12 ± 9 μg/L and 13 ± 9 μg/L for CocS and CocS2, respectively.

Figure 6.
Cocaine detection (as response ratio) by CocS and CocS2 sensor strains.
4. Discussion
The design and construction of microbial whole-cell biosensors (bioreporters) depends upon the identification of a genetic element (most often a gene promoter) inducible by the target compound and its fusion to reporter genes, the expression of which can be quantitatively monitored [1]. For the design of a cocaine bioreporter, we drew upon the report of Voyvodic et al. [16], who have used the production of benzoate by cocaine esterase (CocE) as a measure of cocaine concentration in a cell-free system. Based upon this report, we constructed a two-plasmid system that together generated a cascade involving a breakdown of cocaine to benzoic acid, the complexation of the latter with BenR, induction by this complex of the PBen gene promoter, and the luminescent expression of the luxCDABEG gene cassette. The sensor’s performance was further improved by modifying the sequence of the benzoate sensing element, significantly improving signal intensity but with no effect on detection sensitivity. The ability to distinguish cocaine from other substances was validated by testing it against a panel of commonly encountered drugs. Detection was demonstrated in liquid media as well as in urine, and its reproducibility was verified in several independent experiments. Table 3 summarizes the cocaine detection performance of the original (CocS) and mutated (CocS2) sensor strains. The CocS sensors displayed an averaged maximal response ratio of 7.3 ± 0.3, with luminescence values of (4.6 ± 1.8) × 104 RLU in four independent experiments using cocaine dissolved in a buffer. The luminescent response peaked after ca. 8 h, but a discernable divergence from the background signal was apparent already after 2 h (Figure 2E). Measurable response in the paper strip assay was also obtained 1–2 h after exposure. Dose response evaluation revealed LOD values between 0.012 and 0.1 mg/L, with the camera achieving a LOD as low as 0.01 ± 0.02 mg/L cocaine using a long exposure time. The sensor was effective in human urine, though with a slightly higher LOD of 0.13 ± 0.05 mg/L cocaine. The mutated CocS2 strain exhibited a 30-fold increase in maximal luminescence signal intensity, a reduced background signal, and a 150-fold higher response ratio.

Table 3.
Cocaine detection performance of sensor strains CocS and CocS2. Maximal luminescence and response ratio data are presented in mean ± SEM (n = 4) unless noted differently.
Furthermore, a proof of concept was presented for CocS application in a “dipstick”-type paper strip, which could be stored at 4 °C for 3 weeks without loss of functionality. The process of cocaine testing using CocS paper strips was as simple as using commercially available home-use point-of-care tests. For evaluation, a simple consumer-quality camera was required to assess the level of luminescence.
As mentioned in the Introduction, a major advantage of live cell-based sensors is their capacity to detect not only the original target molecule but also potential derivatives that share its biological activity. In the present communication, however, we concentrated on a cocaine-specific sensor and did not attempt to examine its ability to detect other molecules that share the same basic chemical structure. Such expended capacity will be the design objective of future generations of this bioreporter.
The cocaine concentration in the blood of addicts ranges between 0.5 and 1 mg/L, and concentrations around 4 mg/L were measured in cases of comatose-fatal doses [26,27]. The best limit of detection achieved with the optimized CocS2 (13 μg/L) not only is much lower than these values but can also compete with commercially available, point-of-care drug tests with cutoff levels between 10 and 20 μg/L cocaine [28]. Much lower concentrations of ca. 0.5 μg/L can be detected by laboratory-based ELISA or radioimmunoassays [29]. Similarly, detection limits of GC-MS and LC-MS techniques are in the range of 1 μg/L [30]. The cutoff level for cocaine detection in oral fluid, as defined by the U.S. Nuclear Regulatory Commission (NRC), is 15 μg/L [31]. This value is in the range of the CocS2 bioassay described herein, which does not require high-end laboratory equipment or specially trained personnel. The performance envelope presented in this study is thus in the detection range required by medical needs and is close to that characteristic of sophisticated bioanalytical methodologies. Additional molecular improvements in the construction of future generations of the CocS sensor are likely to push this envelope further.
In contrast to home-targeted drug testing kits, which are antibody-based immunological assays and thus invariably target specific chemicals, cell-based sensors offer a more versatile approach. A cell-based assay, which targets the biological effect of a drug rather than its chemical identity, is likely to detect additional chemicals with similar activities, such as derivatives of the same molecule. Such assays may thus be expected to provide at least a partial answer to the detection of new drug derivatives constantly appearing on the streets. Furthermore, as previously reported by Yagur-Kroll et al. [32], the response characteristics of a microbial sensor, such as signal intensity, response time, and detection threshold, can be significantly improved by directed evolution. We took a first step in this direction by the construction of the CocS2 sensor by a single round of error-prone PCR; while the process did not affect detection sensitivity, it nevertheless generated much stronger luminescence intensities, thus potentially reducing the need for high-performance optics. This directed evolution process will be repeated in the future for the construction of new and improved sensor strains, with an ability to also sensitively detect cocaine derivatives and analogs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s24206549/s1, Table S1: Plasmids and PCR primers constructed or employed in the course of this study [33,34,35].
Author Contributions
Conceptualization, D.R., A.-K.G. and S.B.; methodology, D.R., A.-K.G., E.S. and L.M.; resources, L.M.; writing—original draft preparation A.-K.G. and D.R.; writing S.B., A.-K.G. and E.S.; supervision, S.B. and E.S.; project administration, L.M.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
Research was partially funded by the Minerva Center for Biohybrid Complex Systems.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data not included in the body of this article will be made freely available upon a request to the corresponding author.
Acknowledgments
A.-K.G. is grateful to the Minerva Foundation for a Minerva Short-Term Research Grant. The generous gifts of materials by Ami Citri and by Joseph Tam from the Hebrew University of Jerusalem are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- van der Meer, J.R.; Belkin, S. Where microbiology meets microengineering: Design and applications of reporter bacteria. Nat. Rev. Microbiol. 2010, 8, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Applications of whole-cell bacterial sensors in biotechnology and environmental science. Appl. Microbiol. Biotechnol. 2007, 3, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Almog, R.; Yagur-Kroll, S.; Levkov, K.; Melamed, S.; Shacham-Diamand, Y.; Belkin, S. Online monitoring of water toxicity by use of bioluminescent reporter bacterial biochips. Environ. Sci. Technol. 2011, 45, 8536–8544. [Google Scholar] [CrossRef] [PubMed]
- Reifferscheid, G.; Buchinger, S. Cell-based genotoxicity testing: Genetically modified and genetically engineered bacteria in environmental genotoxicology. Adv. Biochem. Eng. Biotechnol. 2010, 118, 85–111. [Google Scholar]
- Belkin, S.; Yagur-Kroll, S.; Kabessa, Y.; Korouma, V.; Septon, T.; Anati, Y.; Zohar-Perez, C.; Rabinovitz, Z.; Nussinovitch, A.; Agranat, A.J. Remote detection of buried landmines using a bacterial sensor. Nat. Biotechnol. 2017, 35, 308–310. [Google Scholar] [CrossRef]
- Finckh, S.; Buchinger, S.; Escher, B.I.; Hollert, H.; König, M.; Krauss, M.; Leekitratanapisan, W.; Schiwy, S.; Schlichting, R.; Shuliakevich, A.; et al. Endocrine disrupting chemicals entering European rivers: Occurrence and adverse mixture effects in treated wastewater. Environ. Int. 2022, 170, 107608. [Google Scholar] [CrossRef]
- National Survey on Drug Use and Health, 2022 NSDUH National Releases. Available online: https://www.samhsa.gov/data/data-we-collect/nsduh-national-survey-drug-use-and-health (accessed on 28 August 2024).
- National Center for Drug Abuse Statistics, Drug Abuse Statistics 2023. Available online: https://drugabusestatistics.org (accessed on 28 August 2024).
- Britt, A.J.; Bruce, N.C.; Lowe, C.R. Identification of a cocaine esterase in a strain of Pseudomonas maltophilia. J. Bacteriol. 1992, 174, 2087–2094. [Google Scholar] [CrossRef][Green Version]
- Shimomura, E.T.; Jackson, G.F.; Paul, B.D. Cocaine, Crack Cocaine, and Ethanol. In Critical Issues in Alcohol and Drugs of Abuse Testing; Dasgupta, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 215–224. [Google Scholar]
- Isenschmid, D.S.; Levine, B.S.; Caplan, Y.H. A comprehensive study of the stability of cocaine and its metabolites. J. Anal. Toxicol. 1989, 13, 250–256. [Google Scholar] [CrossRef]
- Gao, D.; Narasimhan, D.L.; Macdonald, J.; Brim, R.; Ko, M.-C.; Landry, D.W.; Woods, J.H.; Sunahara, R.K.; Zhan, C.-G. Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol. Pharmacol. 2009, 75, 318–323. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Bi, G.-H.; Yang, H.-J.; He, Y.; Xue, G.; Cao, J.; Tanda, G.; Gardner, E.L.; Newman, A.H.; Xi, Z.-X. The Novel Modafinil Analog, JJC8-016, as a Potential Cocaine Abuse Pharmacotherapeutic. Neuropsychopharmacology 2017, 42, 1871–1883. [Google Scholar] [CrossRef]
- Brim, R.L.; Nance, M.R.; Youngstrom, D.W.; Narasimhan, D.; Zhan, C.-J.; Tesmer, J.J.G.; Sunahara, R.K.; Woods, J.H. A thermally stable form of bacterial cocaine esterase: A potential therapeutic agent for treatment of cocaine abuse. Mol. Pharmacol. 2010, 77, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, D.; Woods, J.H.; Sunahara, R.K. Bacterial cocaine esterase: A protein-based therapy for cocaine overdose and addiction. Future Med. Chem. 2012, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Voyvodic, P.L.; Pandi, A.; Koch, M.; Conejero, I.; Valjent, E.; Courtet, P.; Renard, E.; Faulon, J.-L.; Bonnet, J. Plug-and-play metabolic transducers expand the chemical detection space of cell-free biosensors. Nat. Commun. 2019, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Shemer, B.; Shpigel, E.; Hazan, C.; Kabessa, Y.; Agranat, A.J.; Belkin, S. Detection of buried explosives with immobilized bacterial bioreporters. Microb. Biotechnol. 2021, 14, 251–261. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1996, 177, 4121–4130. [Google Scholar] [CrossRef]
- Silva-Rocha, R.; de Lorenzo, V. A GFP-lacZ bicistronic reporter system for promoter analysis in environmental gram-negative bacteria. PLoS ONE 2012, 7, e34675. [Google Scholar] [CrossRef]
- Brodl, E.; Winkler, A.; Macheroux, P. Molecular Mechanisms of Bacterial Bioluminescence. Comput. Struct. Biotechnol. J. 2018, 16, 551–564. [Google Scholar] [CrossRef]
- Stocker, J.; Balluch, D.; Gsell, M.; Harms, H.; Feliciano, J.; Daunert, S.; Malik, K.A.; van der Meer, J.R. Development of a set of simple bacterial biosensors for quantitative and rapid measurements of arsenite and arsenate in potable water. Environ. Sci. Technol. 2003, 37, 4743–4750. [Google Scholar] [CrossRef]
- Dymond, J.S. PCR-based random mutagenesis. Methods Enzymol. 2013, 529, 249–258. [Google Scholar]
- Banerjee, S.; Salunkhe, S.S.; Apte-Deshpande, A.D.; Mandi, N.S.; Mandal, G.; Padmanabhan, S. Over-expression of proteins using a modified pBAD24 vector in E. coli expression system. Biotechnol. Lett. 2009, 31, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Lozano Terol, G.; Gallego-Jara, J.; Sola Martínez, R.A.; Martínez Vivancos, A.; Cánovas Díaz, M.; de Diego Puente, T. Impact of the expression system on recombinant protein production in Escherichia coli BL21. Front. Microbiol. 2021, 12, 682001. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Pierigè, F.; Agostini, M.; Bigini, N.; Termopoli, V.; Cai, Y.; Zheng, F.; Zhan, C.-G.; Landry, D.W.; Magnani, M. Efficient Cocaine Degradation by Cocaine Esterase-Loaded Red Blood Cells. Front. Physiol. 2020, 11, 573492. [Google Scholar] [CrossRef]
- Schulz, M.; Iwersen-Bergmann, S.; Andresen, H.; Schmoldt, A. Therapeutic and toxic blood concentrations of nearly 1000 drugs and other xenobiotics. Crit. Care 2012, 16, R136. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Hokamp, E.G.; Bott, U.; Madea, B. Performance evaluation of on-site oral fluid drug screening devices in normal police procedure in Germany. Forensic Sci. Int. 2014, 238, 120–124. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bertolín, J.R.; Bonel, L.; Asturias, L.; Arcos-Martínez, M.J.; Castillo, J.R. Rapid determination of recent cocaine use with magnetic particles-based enzyme immunoassays in serum, saliva, and urine fluids. J. Pharm. Biomed. Anal. 2016, 125, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Roque Bravo, R.; Faria, A.C.; Brito-da-Costa, A.M.; Carmo, H.; Mladěnka, P.; Da Dias Silva, D.; Remião, F. Cocaine: An updated overview on chemistry, detection, biokinetics, and pharmacotoxicological aspects including abuse pattern. Toxins 2022, 14, 278. [Google Scholar] [CrossRef]
- United States Nuclear Regulatory Commission. Available online: https://www.nrc.gov/about-nrc.html (accessed on 30 September 2024).
- Yagur-Kroll, S.; Amiel, E.; Rosen, R.; Belkin, S. Detection of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene by an Escherichia coli bioreporter: Performance enhancement by directed evolution. Appl. Microbiol. Biotechnol. 2015, 99, 7177–7188. [Google Scholar] [CrossRef]
- Pandi, A.; Koch, M.; Voyvodic, P.L.; Soudier, P.; Bonnet, J.; Kushwaha, M.; Faulon, J.-L. Metabolic perceptrons for neural computing in biological systems. Nat. Commun. 2019, 10, 3880. [Google Scholar] [CrossRef]
- Shemer, B.; Shpigel, E.; Glozman, A.; Yagur-Kroll, S.; Kabessa, Y.; Agranat, A.J.; Belkin, S. Genome-wide gene-deletion screening identifies mutations that significantly enhance explosives vapor detection by a microbial sensor. N. Biotechnol. 2020, 59, 65–73. [Google Scholar] [CrossRef]
- Subach, O.M.; Cranfill, P.J.; Davidson, M.W.; Verkhusha, V.V. An enhanced monomeric blue fluorescent protein with the high chemical stability of the chromophore. PLoS ONE 2011, 6, e28674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).