A Novel Wearable Sensor for Measuring Respiration Continuously and in Real Time
Abstract
1. Introduction
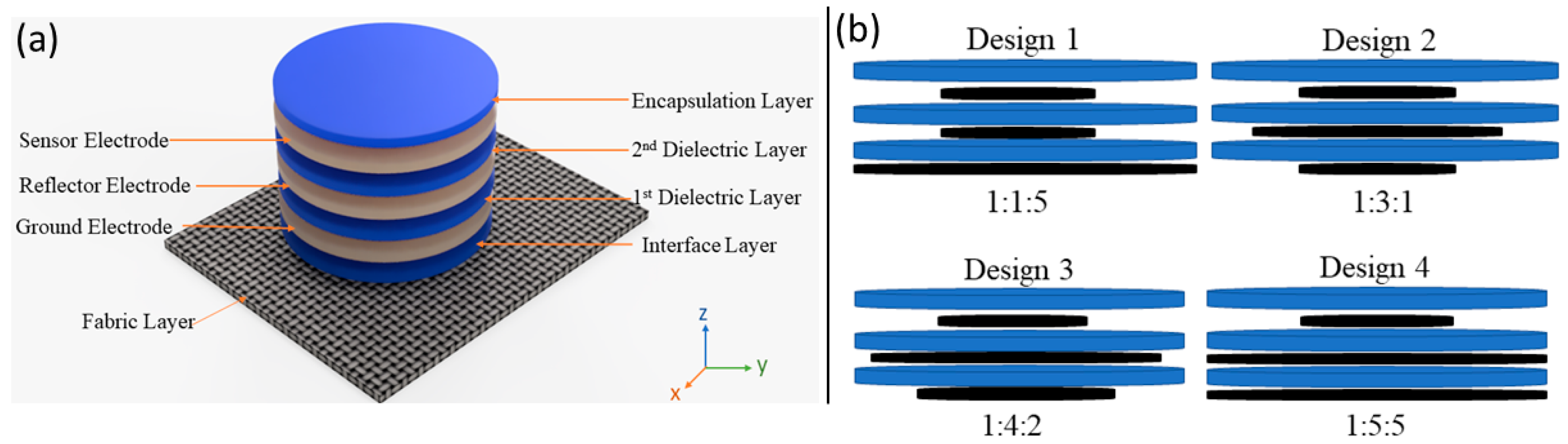
2. Sensor Design, Simulation, and Manufacturing
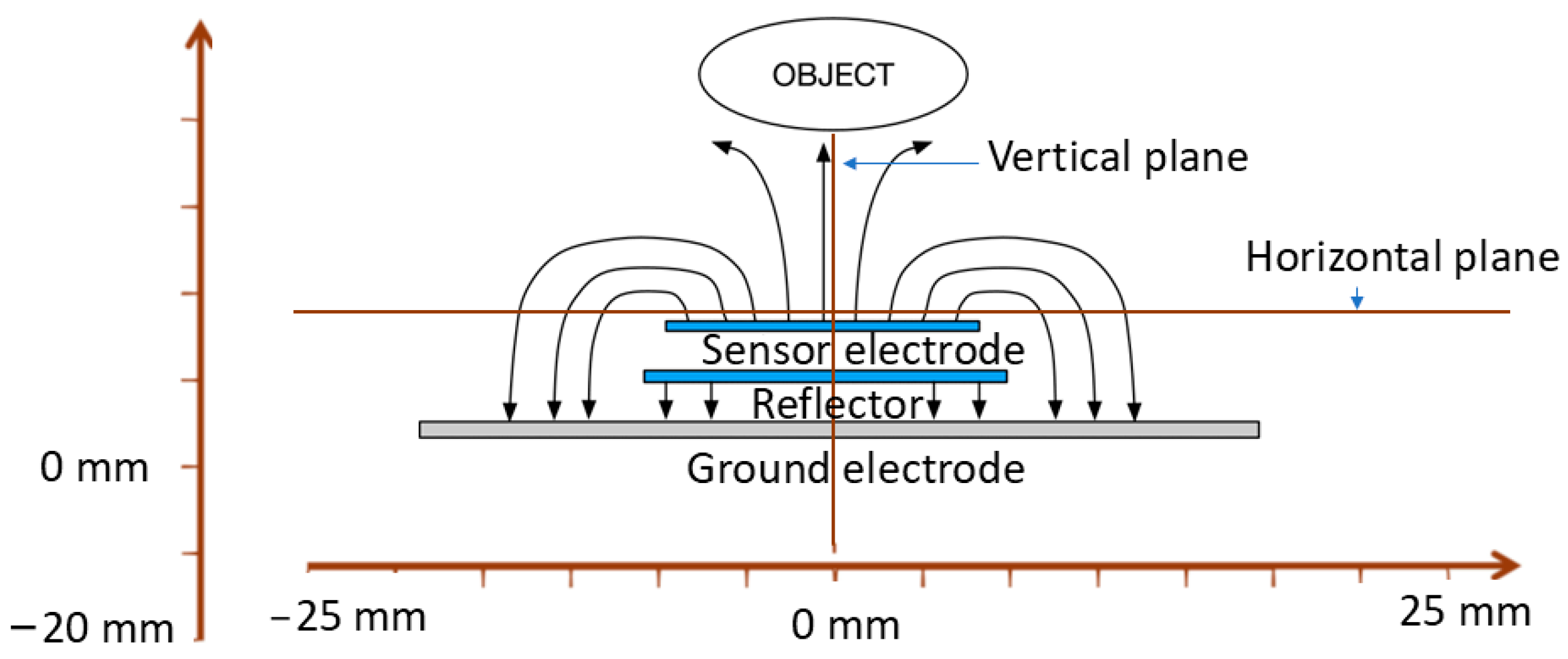
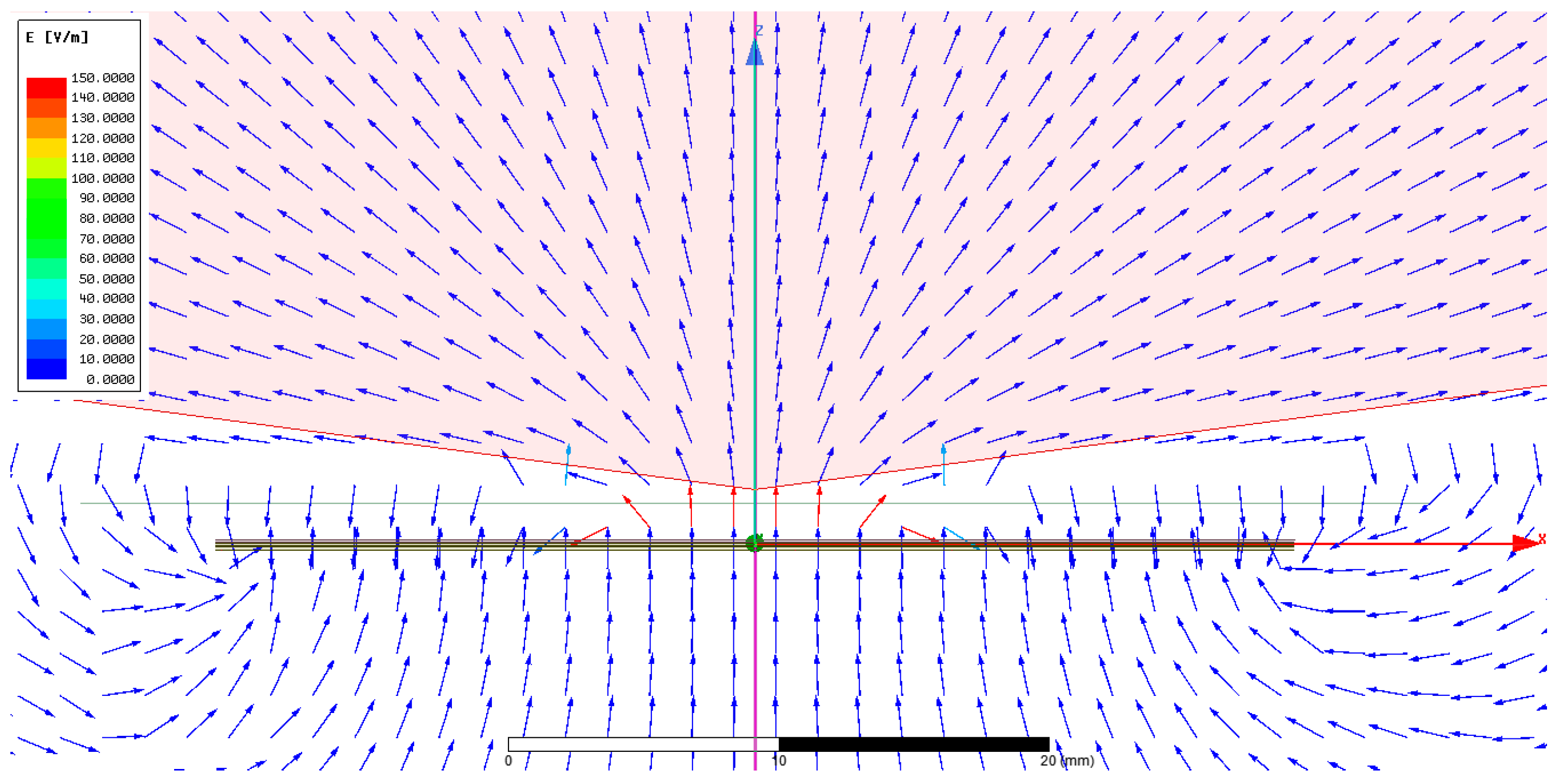
2.1. Ansys Simulation
2.2. Screen-Printing Process
2.2.1. Ink Types
2.2.2. Sensor Screen Printing
3. Results and Discussions
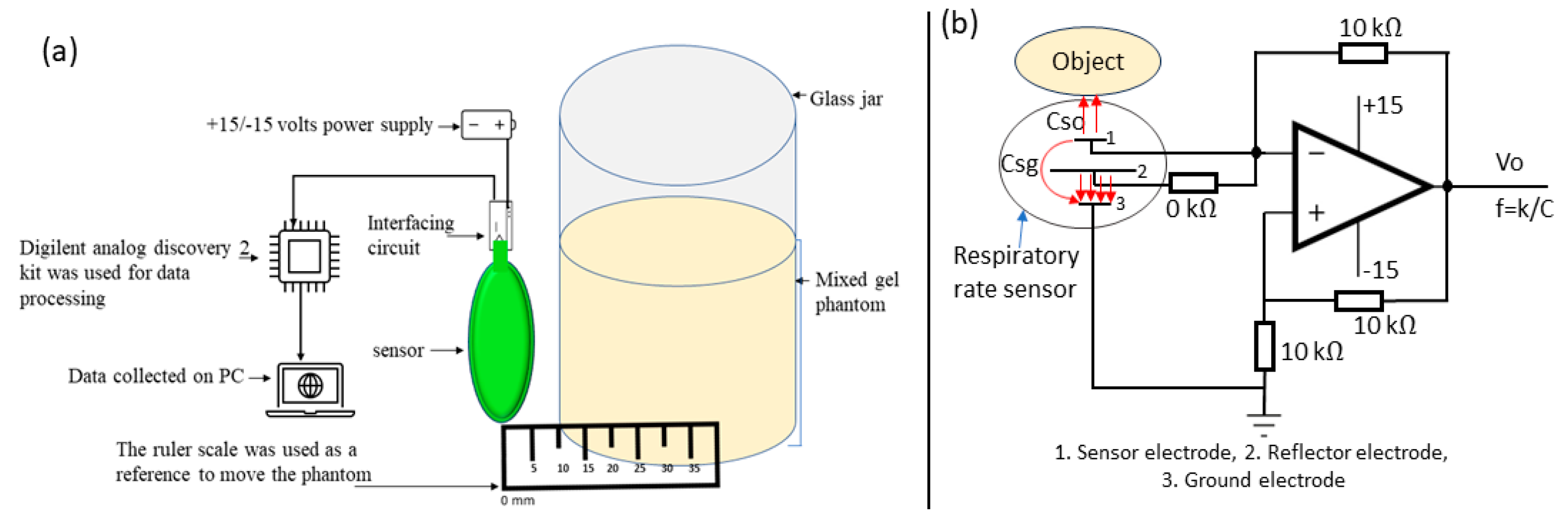
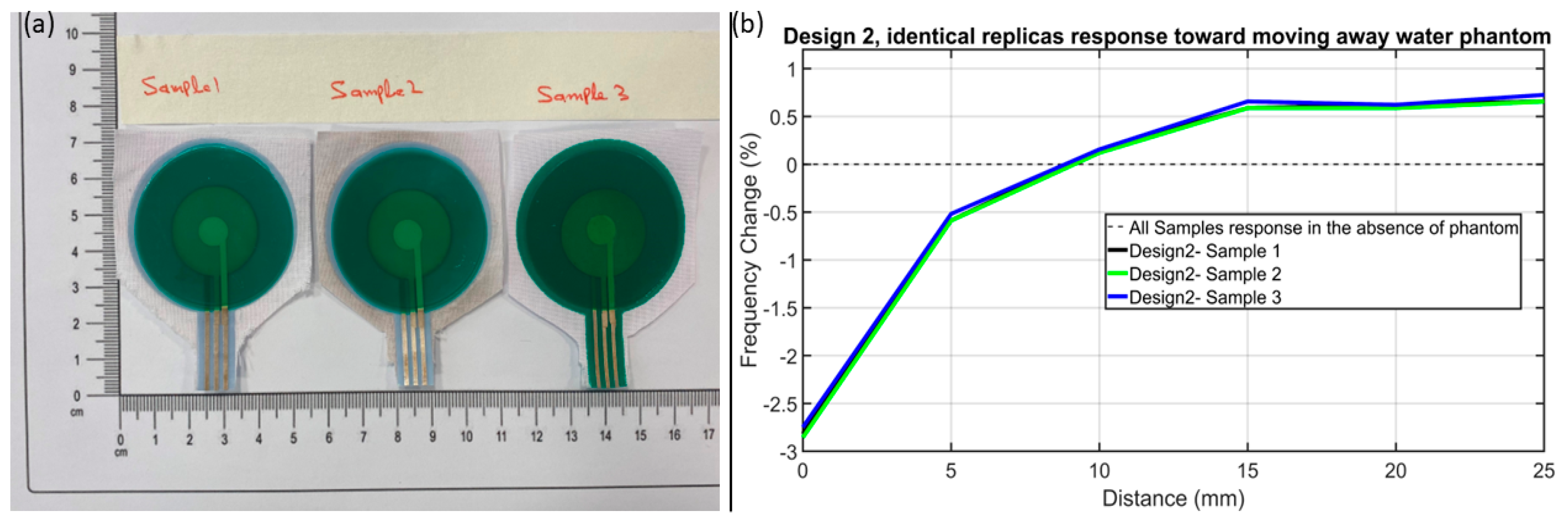
3.1. Characterisation of Screen-Printed Sensor
3.2. Environmental-Induced Drift
3.2.1. Environmental Noise
3.2.2. Humidity Impact
3.2.3. Temperature Impact
3.3. Motion Artifacts
3.3.1. Flexing Durability Impact
3.3.2. Pressure Impact
3.3.3. Rubbing Impact
3.4. Comparisons of Noise and Sensor Response
4. Respiratory Rate Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Radom-Aizik, S.; Jain, R.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. 2019, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferkol, T.; Schraufnagel, D. Schraufnagel The global burden of respiratory disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.B.; Fischer, D.; Lees, M.; Bates, A.; Mann, J.; Arvind, D. Arvind Classifying signals from a wearable accelerometer device to measure respiratory rate. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Brochard, L.; Martin, G.S.; Blanch, L.; Pelosi, P.; Belda, F.J.; Jubran, A.; Gattinoni, L.; Mancebo, J.; Ranieri, V.M.; Richard, J.-C.M.; et al. Clinical review: Respiratory monitoring in the ICU-a consensus of 16. Crit. Care 2012, 16, 219. [Google Scholar] [CrossRef]
- Crapo, R.O. Pulmonary-function testing. N. Engl. J. Med. 1994, 331, 25–30. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Lu, R.; Arifuzzman, A.K.M.; Hossain, K.; Gardner, S.; Eliza, S.A.; Alexander, J.I.D.; Massoud, Y.; Haider, M.R. A Low-Power Sensitive Integrated Sensor System for Thermal Flow Monitoring. IEEE Trans Very Large Scale Integr. VLSI Syst. 2019, 27, 2949–2953. [Google Scholar] [CrossRef]
- Lu, R.; Haider, M.R.; Gardner, S.; Alexander, J.I.D.; Massoud, Y. A Paper-Based Inkjet-Printed Graphene Sensor for Breathing-Flow Monitoring. IEEE Sens. Lett. 2019, 3, 6000104. [Google Scholar] [CrossRef]
- Mahbub, I.; Wang, H.; Islam, S.K.; Pullano, S.A.; Fiorillo, A.S. A low power wireless breathing monitoring system using piezoelectric transducer. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; IEEE: New York, NY, USA, 2016; pp. 1–5. [Google Scholar]
- Sackner, M.A.; Watson, H.; Belsito, A.S.; Feinerman, D.; Suarez, M.; Gonzalez, G.; Bizousky, F.; Krieger, B. Calibration of respiratory inductive plethysmograph during natural breathing. J. Appl. Physiol. 1989, 66, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Wijesiriwardana, R. Inductive fiber-meshed strain and displacement transducers for respiratory measuring systems and motion capturing systems. IEEE Sens. J. 2006, 6, 571–579. [Google Scholar] [CrossRef]
- Carpi, F.; De Rossi, D. Electroactive polymer-based devices for e-textiles in biomedicine. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 295–318. [Google Scholar] [CrossRef]
- Brady, S.; Dunne, L.E.; Tynan, R.; Diamond, D.; Smyth, B.; O’hare, G.M.P. Garment-Based Monitoring of Respiration Rate Using a Foam Pressure Sensor. In Proceedings of the Ninth IEEE International Symposium on Wearable Computers (ISWC’05), Osaka, Japan, 18–21 October 2005; Available online: https://ieeexplore.ieee.org/abstract/document/1550817 (accessed on 12 February 2024).
- Dunne, L.E.; Brady, S.; Smyth, B.; Diamond, D. Initial development and testing of a novel foam-based pressure sensor for wearable sensing. J. Neuroeng. Rehabil. 2005, 2, 4–7. [Google Scholar] [CrossRef][Green Version]
- Elgeziry, M.; Costa, F.; Tognetti, A.; Genovesi, S. Wearable textile-based sensor tag for breath rate measurement. IEEE Sens. J. 2022, 22, 22610–22619. [Google Scholar] [CrossRef]
- Atalay, O.; Kennon, W.R.; Demirok, E. Weft-knitted strain sensor for monitoring respiratory rate and its electro-mechanical modeling. IEEE Sens. J. 2014, 15, 110–122. [Google Scholar] [CrossRef]
- Huang, C.-T.; Shen, C.-L.; Tang, C.-F.; Chang, S.-H. A wearable yarn-based piezo-resistive sensor. Sens. Actuators A Phys. 2008, 141, 396–403. [Google Scholar] [CrossRef]
- Min, S.D.; Yun, Y.; Shin, H. Simplified structural textile respiration sensor based on capacitive pressure sensing method. IEEE Sens. J. 2014, 14, 3245–3251. [Google Scholar]
- Hoffmann, T.; Eilebrecht, B.; Leonhardt, S. Respiratory monitoring system on the basis of capacitive textile force sensors. IEEE Sens. J. 2010, 11, 1112–1119. [Google Scholar] [CrossRef]
- Kusche, R.; John, F.; Cimdins, M.; Hellbruck, H. Contact-Free Biosignal Acquisition via Capacitive and Ultrasonic Sensors. IEEE Access 2020, 8, 95629–95641. [Google Scholar] [CrossRef]
- Rohit, A.; Kaya, S. A Systematic Study of Wearable Multi-Modal Capacitive Textile Patches. IEEE Sens. J. 2021, 21, 26215–26225. [Google Scholar] [CrossRef]
- White, N.M.; Ash, J.; Wei, Y.; Akerman, H. A Planar Respiration Sensor Based on a Capaciflector Structure. IEEE Sens. Lett. 2017, 1, 6000604. [Google Scholar] [CrossRef]
- Hayward, N.; Shaban, M.; Badger, J.; Jones, I.; Wei, Y.; Spencer, D.; Isichei, S.; Knight, M.; Otto, J.; Rayat, G.; et al. A capaciflector provides continuous and accurate respiratory rate monitoring for patients at rest and during exercise. J. Clin. Monit. Comput. 2022, 36, 1535–1546. [Google Scholar] [CrossRef]
- Vranish, J.M. Commercial Capaciflector. In Technology 2001, Proceedings of the Second National Technology Transfer Conference and Exposition, San Jose, CA, USA, 3–5 December 1991; NASA: Washington, DC, USA, 1991; Volume 2, pp. 423–434. [Google Scholar]
- Bowers, C.A.; Chantrey, G. Factors controlling the soiling of white polyester cotton fabrics: Part I: Laboratory studies. Text. Res. J. 1969, 39, 1–11. [Google Scholar] [CrossRef]
- Kilinc, F.S. A review of isolation gowns in healthcare: Fabric and gown properties. J. Eng. Fiber Fabr. 2015, 10, 155892501501000320. [Google Scholar] [CrossRef]
- Ali, A.; Jafri, S.I.; Habib, A.; Amin, Y.; Tenhunen, H. RFID Humidity Sensor Tag for Low-cost Applications. Appl. Comput. Electromagn. Soc. J. 2017, 32, 1083–1088. [Google Scholar]
- Gabriel, C.; Gabriel, S.; Corthout, Y.E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231. [Google Scholar] [CrossRef]
- White, G.H. Basics of Estimating Measurement Uncertainty. 2008. Available online: http://www.bipm.org/en/publications/guides/vim.html (accessed on 13 January 2024).
- Humidity Levels: How Humidity Can Affect Your Health. Available online: https://www.total-environmental.co.uk/humidity-levels-how-humidity-can-affect-your-health/ (accessed on 13 November 2023).
- Lee, S.; Franklin, S.; Hassani, F.A.; Yokota, T.; Nayeem, O.G.; Wang, Y.; Leib, R.; Cheng, G.; Franklin, D.W.; Someya, T. Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science (1979) 2020, 370, 966–970. [Google Scholar] [CrossRef]
- Dean, E.; Frownfelter, D.L. Cardiovascular and Pulmonary Physical Therapy: Evidence and Practice; Mosby: St. Louis, MO, USA, 2006. [Google Scholar]
- Price, K.; Schartz, P.; Watson, A.H.D. The effect of standing and sitting postures on breathing in brass players. SpringerPlus 2014, 3, 210. [Google Scholar] [CrossRef]







| Tissue | Permittivity | Phantom Material | Permittivity |
|---|---|---|---|
| Fat | 5.28 | Glass | 5 |
| Deflated lung | 20.5 | Acetone | 20.4 |
| Inflated lung | 48.4 | Lab-prepared gel mix | 50.6 |
| Body fluid | 78.2 | DI water | 80 |
| Theoretical | Simulated | Measured |
|---|---|---|
| 21.285 pF | 21.675 pF | 19.8 pF |
| Design | Water | Acetone | Gel | Cumulative %f-c |
|---|---|---|---|---|
| %f-c | %f-c | %f-c | ||
| 1 | 0.4 | 1 | 0.5 | 1.9 |
| 2 | 2.8 | 1.8 | 1.6 | 6.2 |
| 3 | 0.6 | 0.4 | 0.4 | 1.4 |
| 4 | 0.4 | 0.2 | 0.1 | 0.7 |
| Design # | Environmental Noise | Motion Artefact | Cumulative %f-c due to All Noises | Cumulative %f-c with Phantoms (Given in Table 3) | Net %f-c | ||
|---|---|---|---|---|---|---|---|
| Humidity | Flexing Durability | Pressure | Rubbing | ||||
| 1 | 0.129 | 0.8 | 0.5 | 0.07 | 1.5 | 1.9 | 0.4 |
| 2 | 0.19 | 0.61 | 1.1 | 0.09 | 1.99 | 6.2 | 4.21 |
| 3 | 0.129 | 0.4 | 0.658 | 0.04 | 1.26 | 1.4 | 0.14 |
| 4 | 0.25 | 0.5 | 0.25 | 0.04 | 0.92 | 0.7 | −0.22 |
| Ref. | PT a | Sensor Mounting Position | Monitoring Parameter | RRM b | Sensing Mechanism | IEMN c | MC d |
|---|---|---|---|---|---|---|---|
| [7,8] | No | Mouthpiece | Airflow | Yes | Resistive based | Not given | Not given |
| [9] | No | Mounted on torso | Chest vibrations | Yes | Resistive based | Not given | Not given |
| [13,14] | No | Mounted on torso | Chest applied pressure | Yes | Resistive based | Not given | Not given |
| [11] | No | Wrap around chest | Chest expansion | Yes | Strain based | Not given | Not given |
| [20] | No | Distant monitoring | Thorax movement | Yes | Capacitive based | Not given | Not given |
| [21] | No | Wrap around chest | Thorax movement | Yes | Capacitive based | Not given | Not given |
| This work | Yes | Anywhere on torso | Lung inflation and deflation | Yes | Capacitive sensing (capaciflector) | Sensor response 2.4-fold > sum of all noise | 98.68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Wei, Y.; Elsaboni, Y.; Tyson, J.; Akerman, H.; Jackson, A.I.R.; Lane, R.; Spencer, D.; White, N.M. A Novel Wearable Sensor for Measuring Respiration Continuously and in Real Time. Sensors 2024, 24, 6513. https://doi.org/10.3390/s24206513
Ali A, Wei Y, Elsaboni Y, Tyson J, Akerman H, Jackson AIR, Lane R, Spencer D, White NM. A Novel Wearable Sensor for Measuring Respiration Continuously and in Real Time. Sensors. 2024; 24(20):6513. https://doi.org/10.3390/s24206513
Chicago/Turabian StyleAli, Amjad, Yang Wei, Yomna Elsaboni, Jack Tyson, Harry Akerman, Alexander I. R. Jackson, Rod Lane, Daniel Spencer, and Neil M. White. 2024. "A Novel Wearable Sensor for Measuring Respiration Continuously and in Real Time" Sensors 24, no. 20: 6513. https://doi.org/10.3390/s24206513
APA StyleAli, A., Wei, Y., Elsaboni, Y., Tyson, J., Akerman, H., Jackson, A. I. R., Lane, R., Spencer, D., & White, N. M. (2024). A Novel Wearable Sensor for Measuring Respiration Continuously and in Real Time. Sensors, 24(20), 6513. https://doi.org/10.3390/s24206513







