Assessment of Directional–Hemispherical Reflectance of Tablets with Cefuroxime during Storage under Elevated Temperature and Ultraviolet Radiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmaceutical Preparation
2.2. Research Procedure
2.2.1. Hemispherical–Directional Reflectance
2.2.2. Hyperspectral Imaging
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Tablets
3.2. Analysis of THR Values during the Experiment
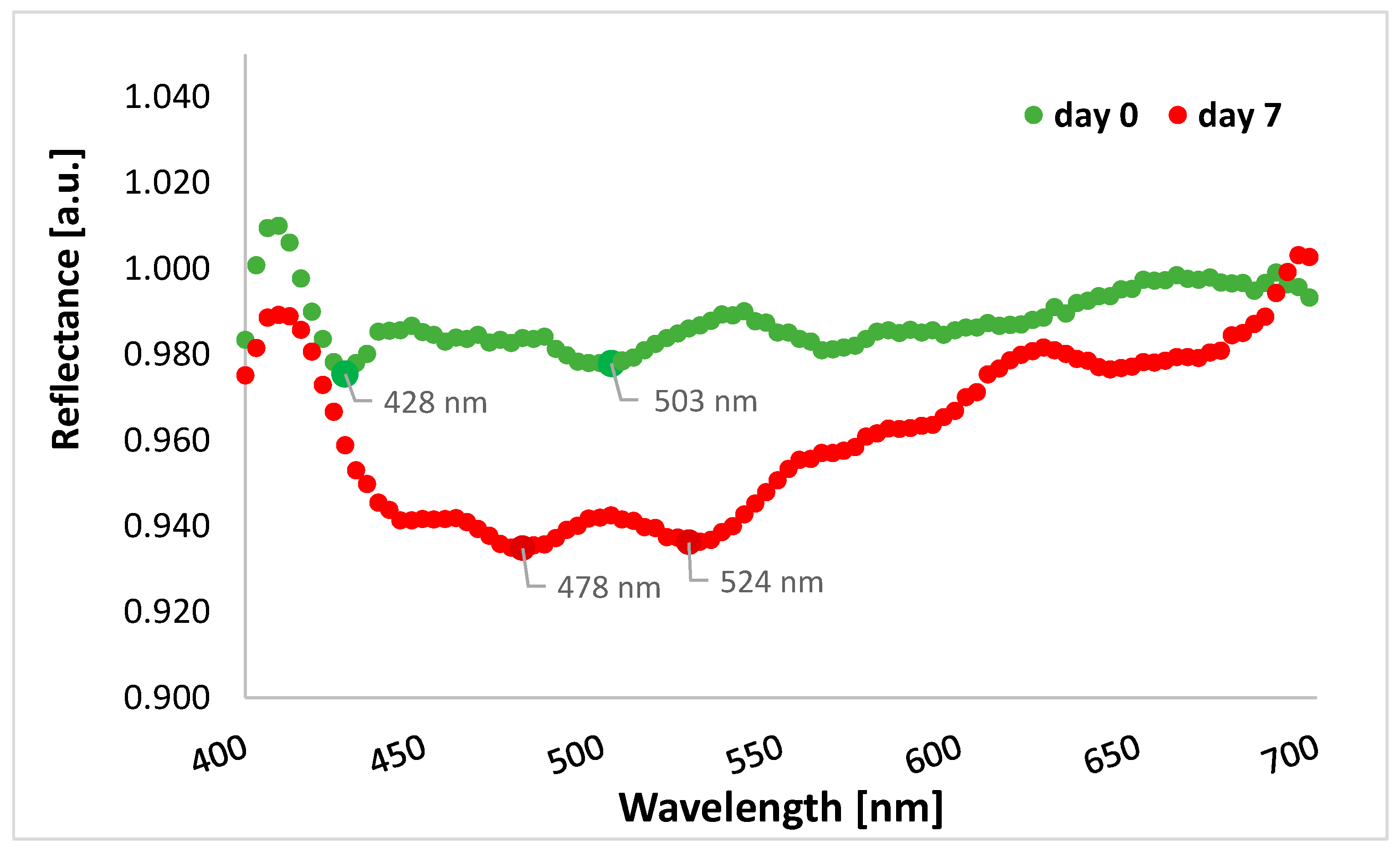
3.3. Analysis of Change in THR Value during the Experiment Depending on the Spectral Range
3.4. Hyperspectral Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaglarz, J.; Duraj, R.; Jaglarz, J.; Duraj, R. Goniometryczny pomiar funkci BRDF dla powierzchni stałych. Czas. Tech. Tech. Trans. 2011, 22, 85–94. (In Polish) [Google Scholar]
- Schaepman-strub, G.; Schaepman, M.E.; Painter, T.H.; Dangel, S.; Martonchik, J. V Reflectance Quantities in Optical Remote Sensing—Definitions and Case Studies. Remote Sens. Environ. 2006, 103, 27–42. [Google Scholar] [CrossRef]
- Wilczyński, S.; Koprowski, R.; Błońska-Fajfrowska, B. Directional Reflectance Analysis for Identifying Counterfeit Drugs: Preliminary Study. J. Pharm. Biomed. Anal. 2016, 124, 341–346. [Google Scholar] [CrossRef]
- Błoński, B.; Wilczyński, S.; Hartman-Petrycka, M.; Michalecki, Ł. The Use of Hemispherical Directional Reflectance to Evaluate the Interaction of Food Products with Radiation in the Solar Spectrum. Foods 2022, 11, 1974. [Google Scholar] [CrossRef]
- Stolecka-Warzecha, A.; Brandys, A.; Bożek, M.; Lebiedowska, A.; Błońska-Fajfrowska, B.; Wilczyński, S. Use Directional-Hemispherical Reflectance to Identify Female Skin Features in Response of Microdermabrasion Treatment. Appl. Sci. 2022, 12, 12087. [Google Scholar] [CrossRef]
- Martonchik, J.V. Retrieval of Surface Directional Reflectance Properties Using Ground Level Multiangle Measurements. Remote Sens. Environ. 1994, 50, 303–316. [Google Scholar] [CrossRef]
- Mira, J.J.; Lorenzo, S.; Guilabert, M.; Navarro, I.; Pérez-Jover, V. A Systematic Review of Patient Medication Error on Self-Administering Medication at Home. Expert Opin. Drug Saf. 2015, 14, 815–838. [Google Scholar] [CrossRef]
- Ramos, P.; Broncel, M. Influence of Storage Conditions on the Stability of Gum Arabic and Tragacanth. Molecules 2022, 27, 1510. [Google Scholar] [CrossRef]
- Gul, W.; Basheer, S.; Karim, F.; Ayub, S. Effect of Acid, Base, Temperature and U.V Light on Amlodipine Besylate. Int. J. Adv. Res. Chem. Sci. 2015, 2, 21–24. [Google Scholar]
- Lyon, R.C.; Taylor, J.S.; Porter, D.A.; Prasanna, H.R.; Hussain, A.S. Stability Profiles of Drug Products Extended beyond Labeled Expiration Dates. J. Pharm. Sci. 2006, 95, 1549–1560. [Google Scholar] [CrossRef]
- Kawabata, K.; Sakaue, M.; Akimoto, S.; Miyara, M.; Kotake, Y.; Nishi, H. Evaluation of Photostability of Azelnidipine Tablets and Structure Determination of Its Photoproducts. J. Pharm. Biomed. Anal. 2023, 233, 115471. [Google Scholar] [CrossRef]
- Kawabata, K.; Kohashi, M.; Akimoto, S.; Nishi, H. Structure Determination of Felodipine Photoproducts in UV-Irradiated Medicines Using ESI-LC/MS/MS. Pharmaceutics 2023, 15, 697. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, H.; Zhou, Y.; Xiao, J.; Shi, Y.; Cao, H. Mechanism and Kinetics of Prothioconazole Photodegradation in Aqueous Solution. J. Agric. Food Chem. 2023, 71, 6594–6602. [Google Scholar] [CrossRef]
- Cacciari, R.D.; Reynoso, E.; Montejano, H.A.; Biasutti, M.A. Photodegradation of Prednisolone under UVB Solar Irradiation. Role of Photogenerated ROS in the Degradation Mechanism. Photochem. Photobiol. Sci. 2017, 16, 1717–1726. [Google Scholar] [CrossRef]
- Summary of Product Characteristics. Available online: www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PPA1562-163-001_04092015135053.pdf (accessed on 12 January 2024).
- Huang, Y.; Wang, L.; Zhong, C.; Huang, S. Factors influencing the attention to home storage of medicines in China. BMC Public Health 2019, 19, 833. [Google Scholar] [CrossRef]
- Vlieland, N.D.; van den Bemt, B.J.F.; Bekker, C.L.; Bouvy, M.L.; Egberts, T.C.G.; Gardarsdottir, H. Older Patients’ Compliance with Drug Storage Recommendations. Drugs Aging 2018, 35, 233–241. [Google Scholar] [CrossRef]
- Maclean, N.; Khadra, I.; Mann, J.; Abbott, A.; Mead, H.; Markl, D. Formulation-dependent stability mechanisms affecting dissolution performance of directly compressed griseofulvin tablets. Int. J. Pharm. 2023, 631, 122473. [Google Scholar] [CrossRef]
- Zhang, Q.; Gladden, L.F.; Avalle, P.; Axel Zeitler, J.; Mantle, M.D. Terahertz Pulsed Imaging and Magnetic Resonance Imaging as Tools to Probe Formulation Stability. Pharmaceutics 2013, 5, 591–608. [Google Scholar] [CrossRef]
- Ramos, P.; Pepliński, P.; Pilawa, B. Impact of UVC Radiation on Interaction of Selected Antifungal Drugs (Azole Derivatives) with Model DPPH Free Radical. Ann. Acad. Med. Silesiensis 2020, 74, 77–90. [Google Scholar] [CrossRef]
- Fabre, H.; Ibork, H.; Lerner, D.A. Photoisomerization Kinetics of Cefuroxime Axetil and Related Compounds. J. Pharm. Sci. 1994, 83, 553–558. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, L.; Ji, R. Biotic and Abiotic Degradation of Four Cephalosporin Antibiotics in a Lake Surface Water and Sediment. Chemosphere 2010, 80, 1399–1405. [Google Scholar] [CrossRef]
- Al Ktash, M.; Stefanakis, M.; Boldrini, B.; Ostertag, E. Characterization of Pharmaceutical Tablets Using UV Hyperspectral Imaging as a Rapid In-Line Analysis Tool. Sensors 2021, 21, 4436. [Google Scholar] [CrossRef]
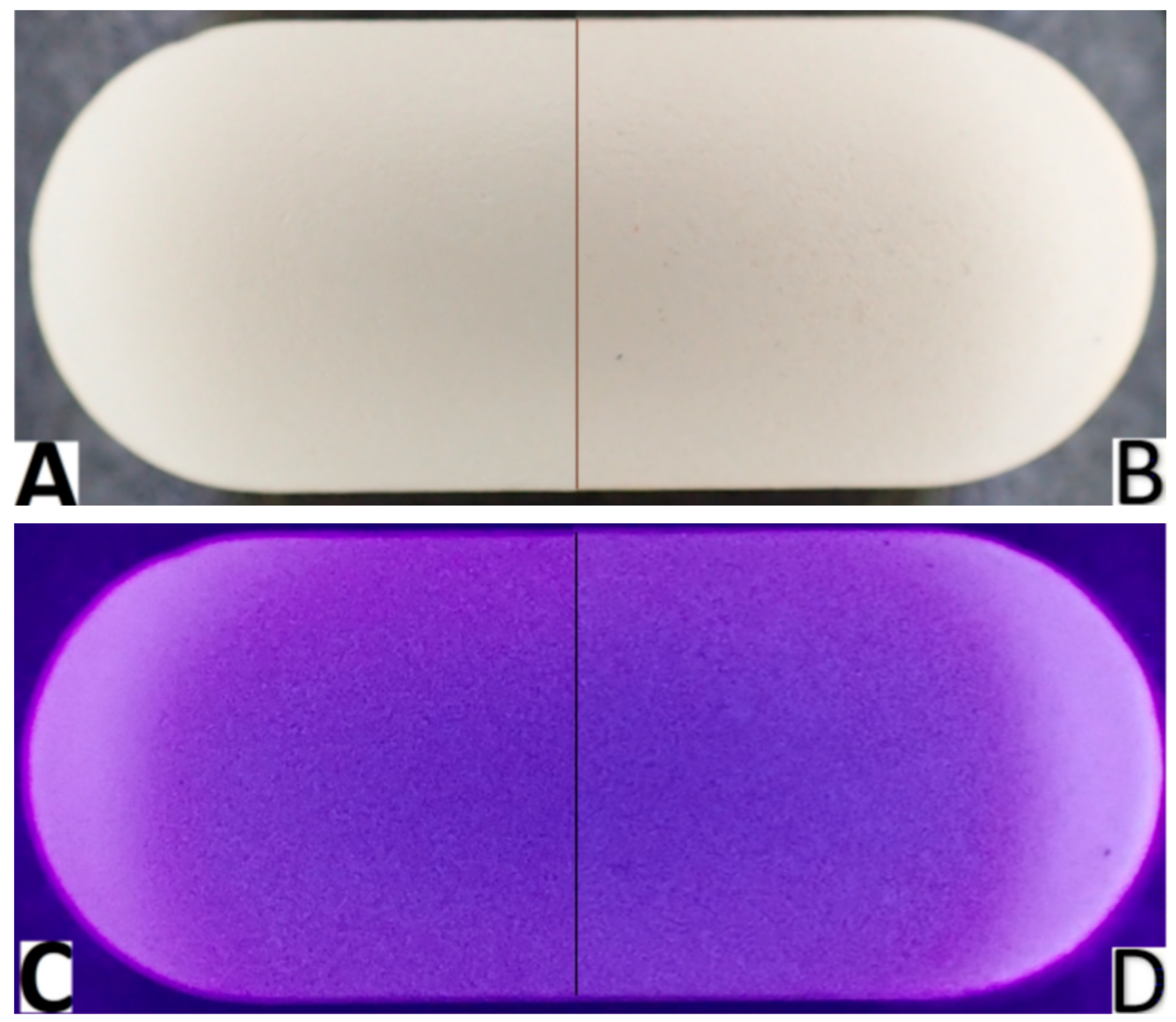
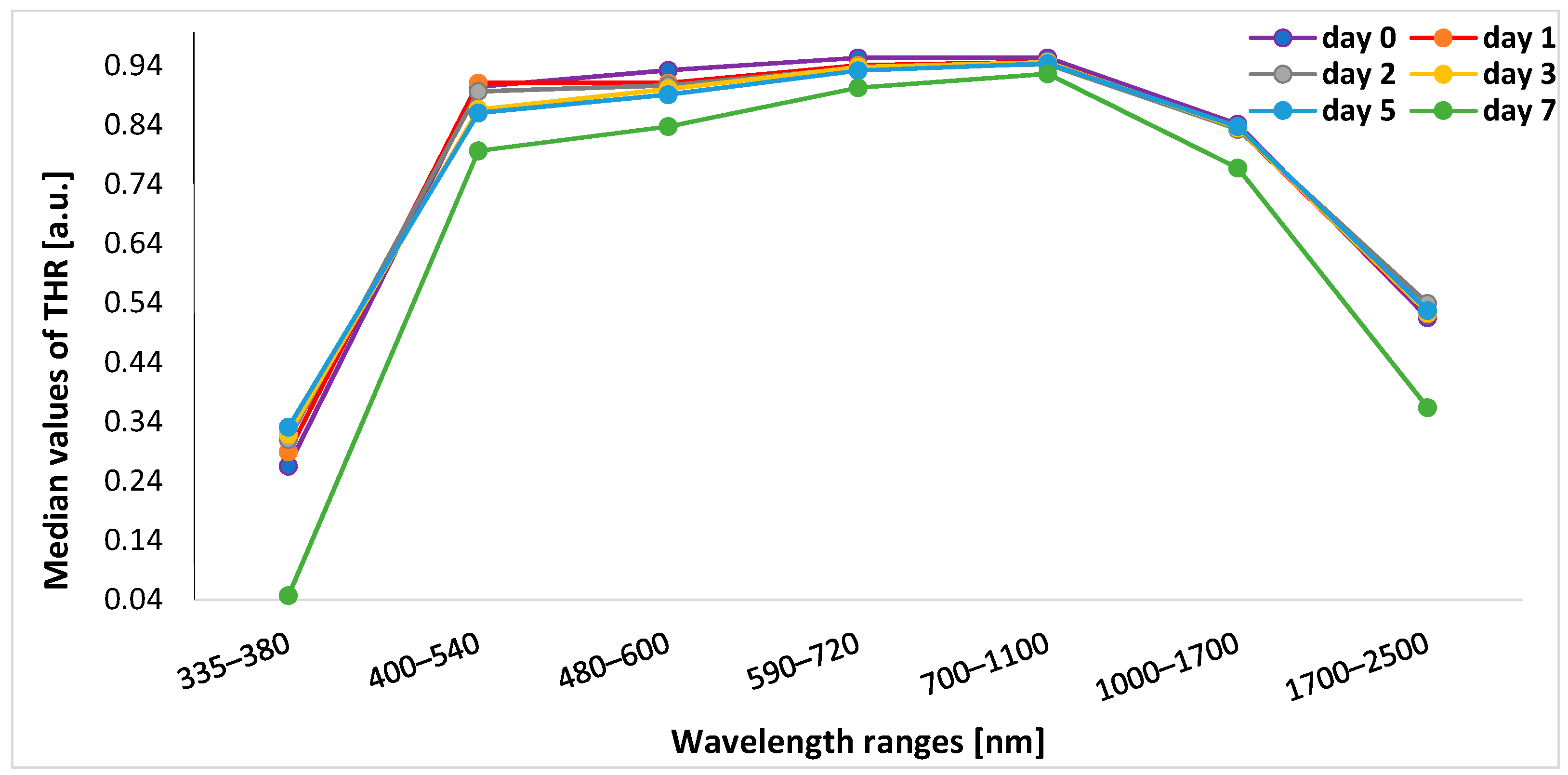

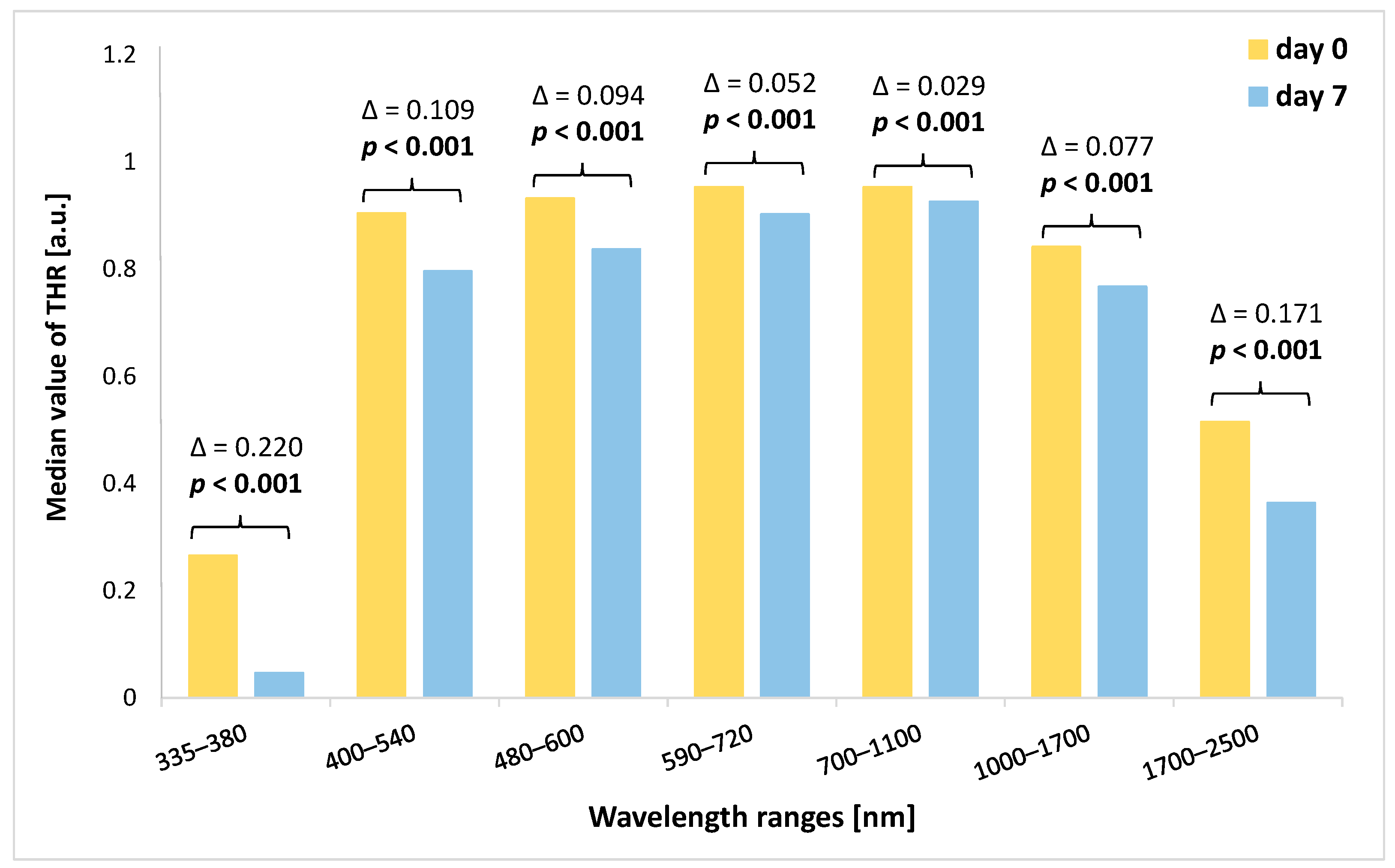
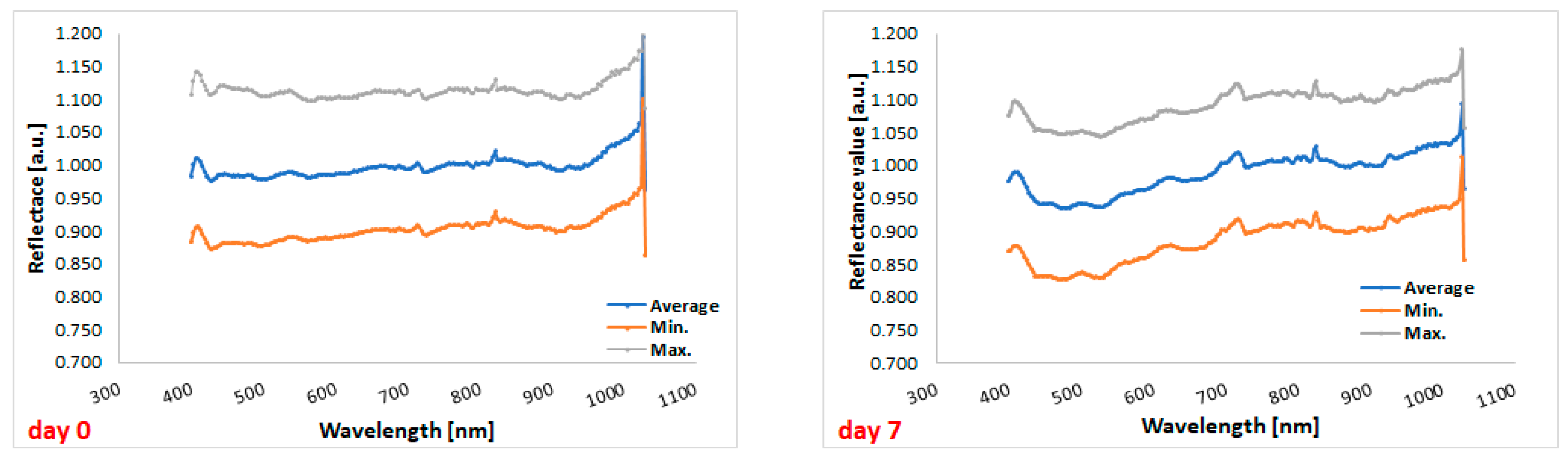
| Spectral Ranges (nm) | Day 0 | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 | p 1 |
|---|---|---|---|---|---|---|---|
| THR (a.u.) Median (Min.–Max.) | |||||||
| 335–380 | 0.265 (0.259–0.271) | 0.289 (0.279–0.297) | 0.310 (0.302–0.315) | 0.319 (0.312–0.329) | 0.331 (0.321–0.340) | 0.047 (0.029–0.053) | <0.001 |
| 400–540 | 0.904 (0.899–0.909) | 0.910 (0.871–0.918) | 0.896 (0.854–0.883) | 0.866 (0.849–0.883) | 0.860 (0.838–0.887) | 0.796 (0.766–0.824) | <0.001 |
| 480–600 | 0.932 (0.926–0.935) | 0.910 (0.871–0.918) | 0.906 (0.888–0.909) | 0.900 (0.882–0.909) | 0.891 (0.872–0.908) | 0.837 (0.810–0.859) | <0.001 |
| 590–720 | 0.953 (0.946–0.956) | 0.940 (0.902–0.946) | 0.936 (0.922–0.944) | 0.937 (0.920–0.940) | 0.931 (0.916–0.941) | 0.902 (0.810–0.859) | <0.001 |
| 700–1100 | 0.953 (0.947–0.956) | 0.946 (0.915–0.951) | 0.942 (0.932–0.951) | 0.945 (0.932–0.950) | 0.943 (0.929–0.949) | 0.926 (0.902–0.928) | <0.001 |
| 1000–1700 | 0.841 (0.835–0.847) | 0.834 (0.811–0.838) | 0.832 (0.825–0.837) | 0.835 (0.826–0.839) | 0.837 (0.825–0.843) | 0.767 (0.749–0.771) | <0.001 |
| 1700–2500 | 0.515 (0.505–0.517) | 0.522 (0.513–0.529) | 0.539 (0.527–0.547) | 0.523 (0.508–0.527) | 0.527 (0.511–0.540) | 0.364 (0.360–0.379) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meisner, M.; Sarecka-Hujar, B. Assessment of Directional–Hemispherical Reflectance of Tablets with Cefuroxime during Storage under Elevated Temperature and Ultraviolet Radiation. Sensors 2024, 24, 630. https://doi.org/10.3390/s24020630
Meisner M, Sarecka-Hujar B. Assessment of Directional–Hemispherical Reflectance of Tablets with Cefuroxime during Storage under Elevated Temperature and Ultraviolet Radiation. Sensors. 2024; 24(2):630. https://doi.org/10.3390/s24020630
Chicago/Turabian StyleMeisner, Michał, and Beata Sarecka-Hujar. 2024. "Assessment of Directional–Hemispherical Reflectance of Tablets with Cefuroxime during Storage under Elevated Temperature and Ultraviolet Radiation" Sensors 24, no. 2: 630. https://doi.org/10.3390/s24020630
APA StyleMeisner, M., & Sarecka-Hujar, B. (2024). Assessment of Directional–Hemispherical Reflectance of Tablets with Cefuroxime during Storage under Elevated Temperature and Ultraviolet Radiation. Sensors, 24(2), 630. https://doi.org/10.3390/s24020630









