Bite Force Mapping Based on Distributed Fiber Sensing Network Approach
Abstract
1. Introduction
2. Materials and Methods
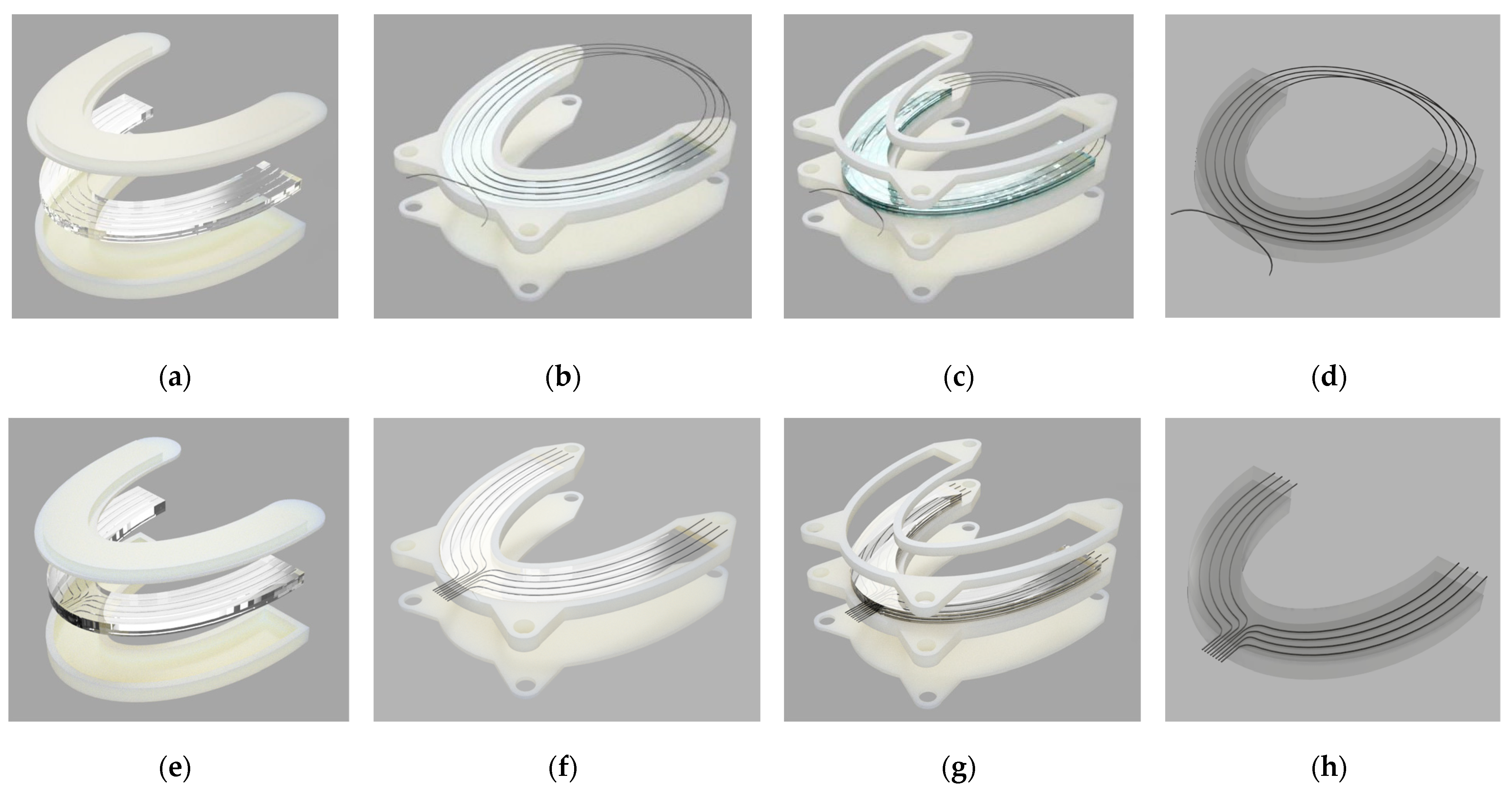
2.1. Silicone Bite Designs
2.1.1. Materials
2.1.2. Methodology
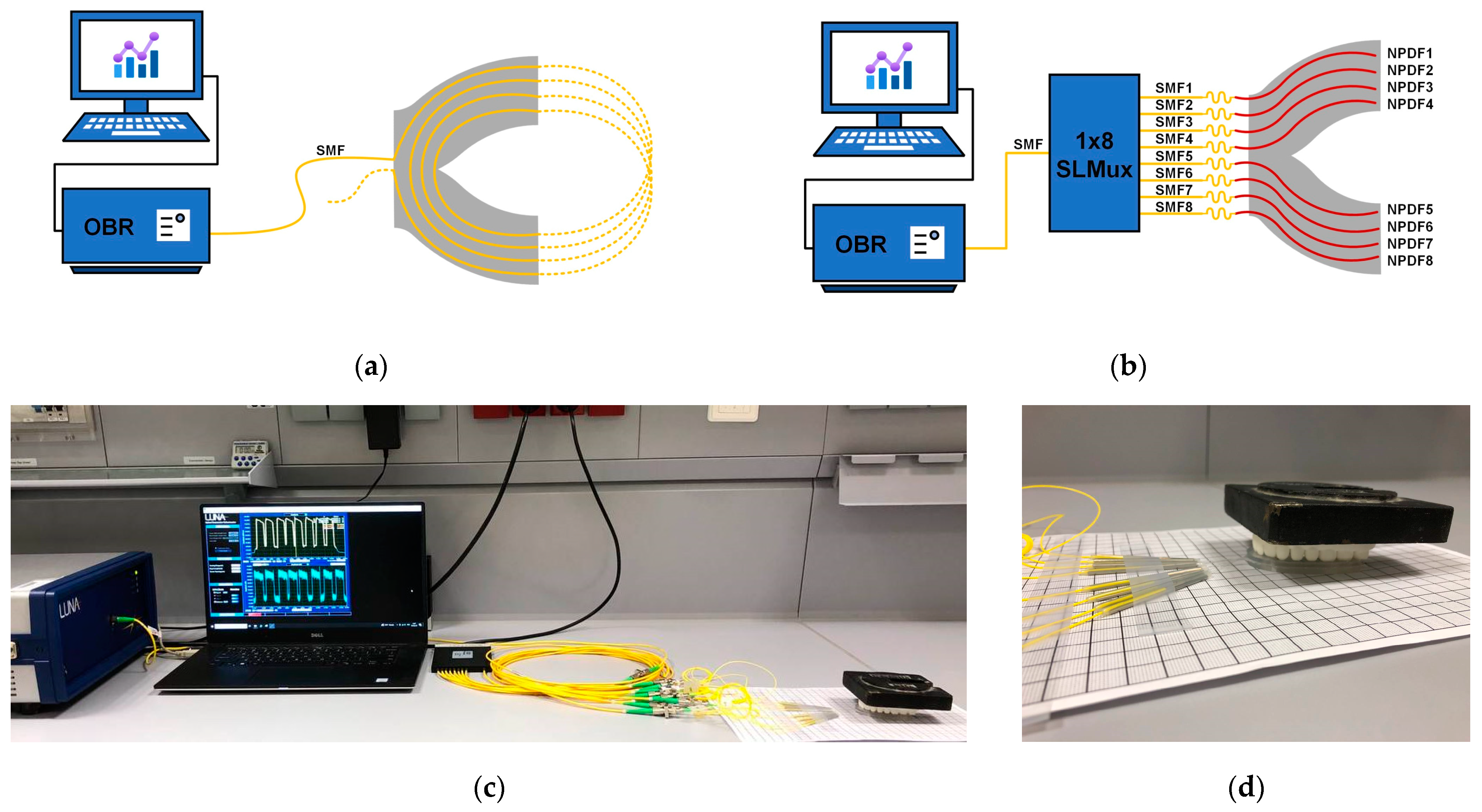
2.2. The Experimental Setup
3. Results and Discussion
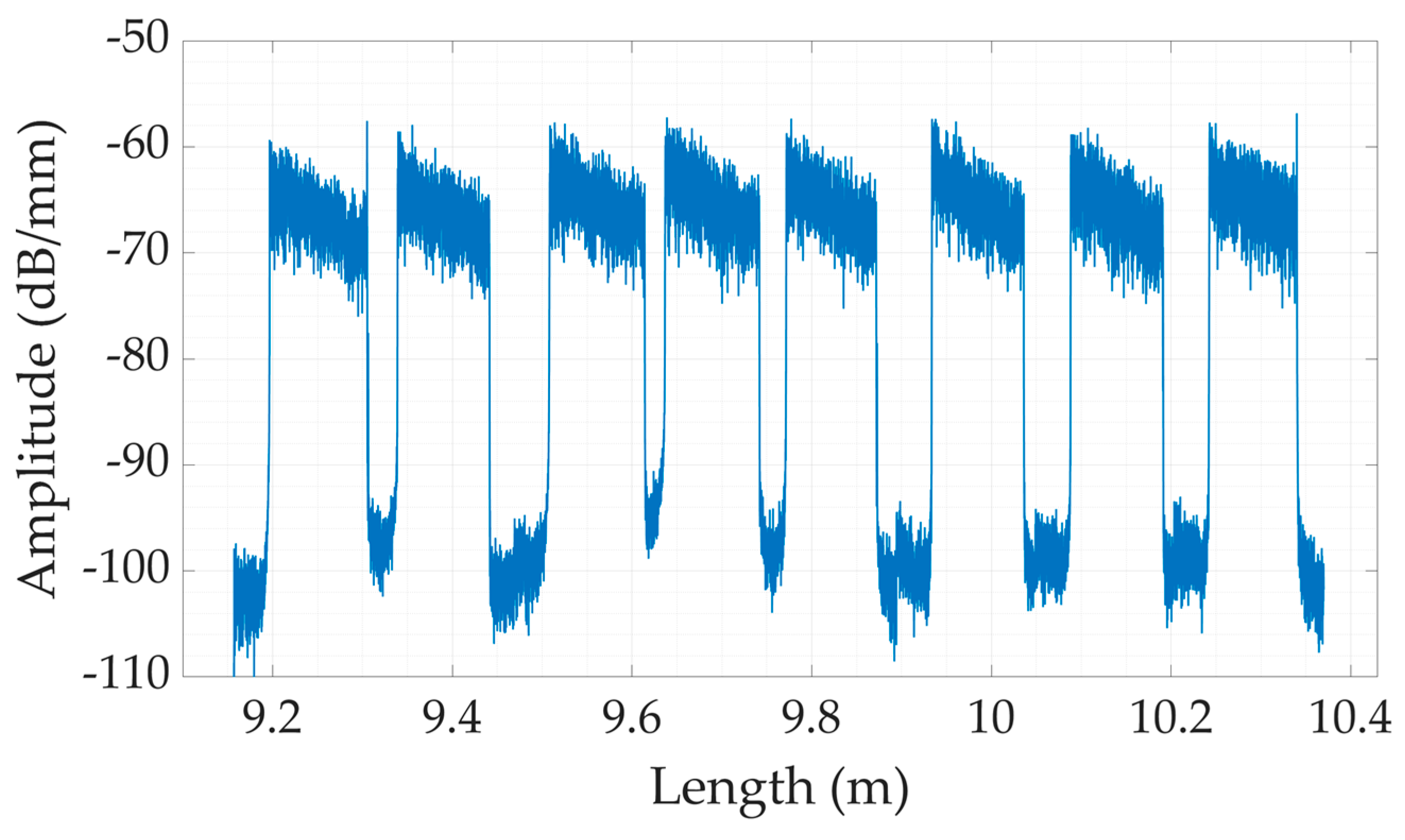
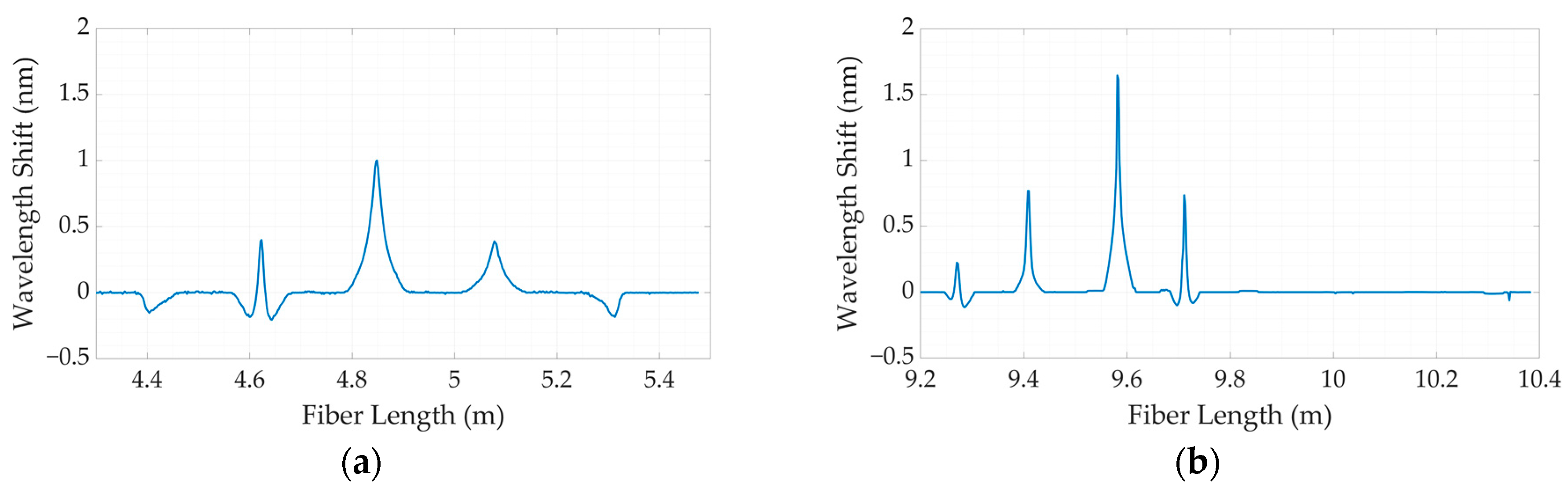
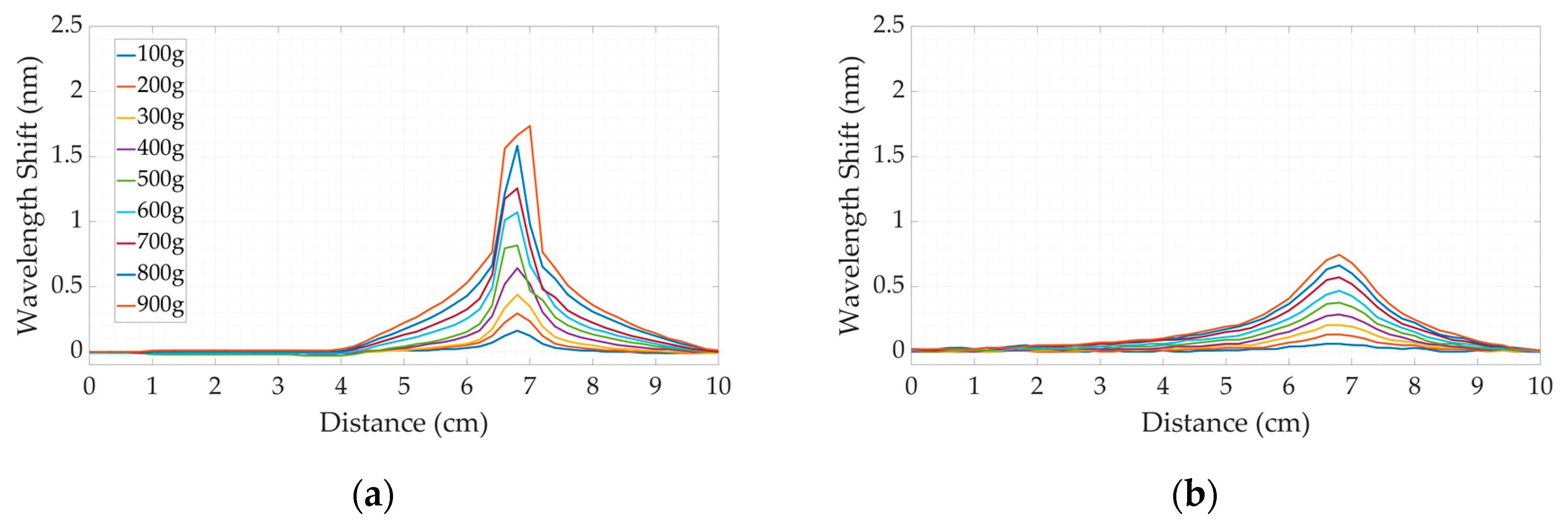
3.1. Calibration
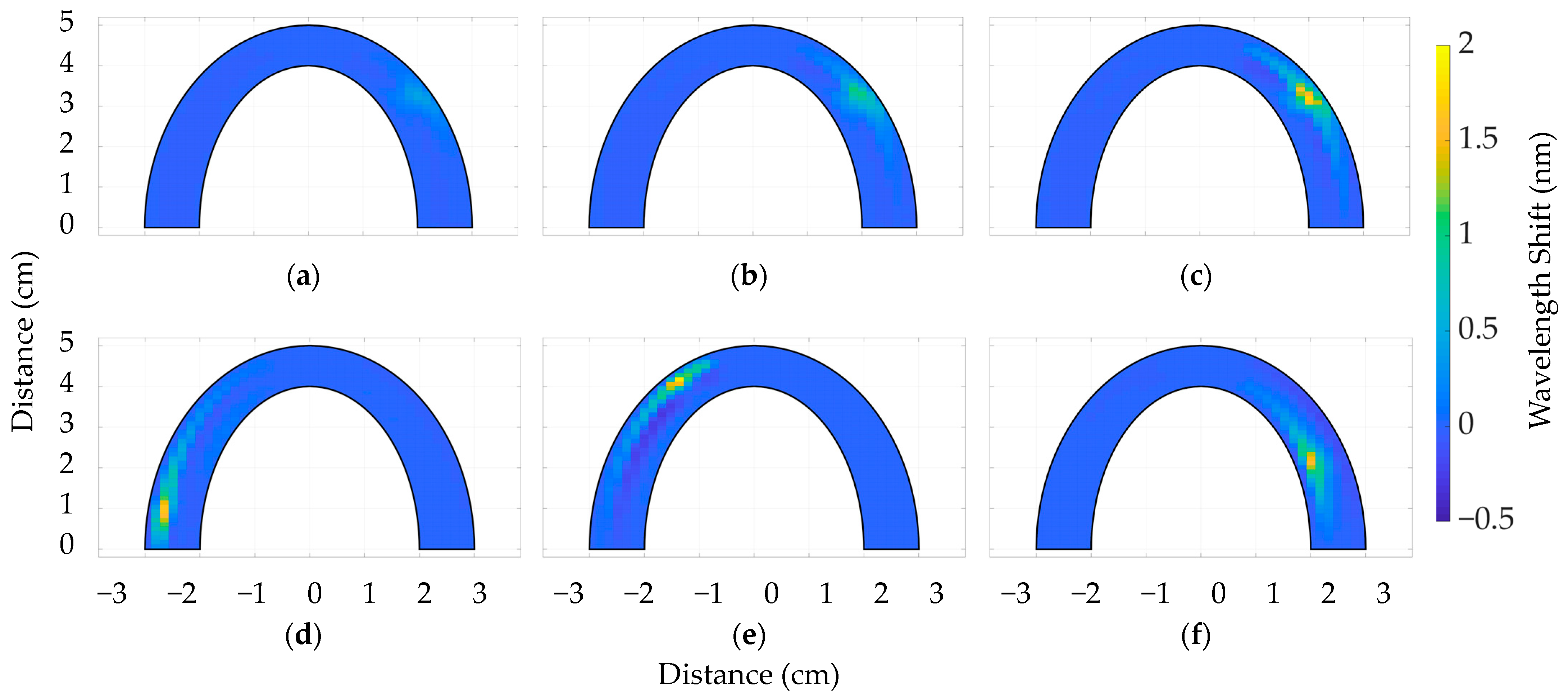
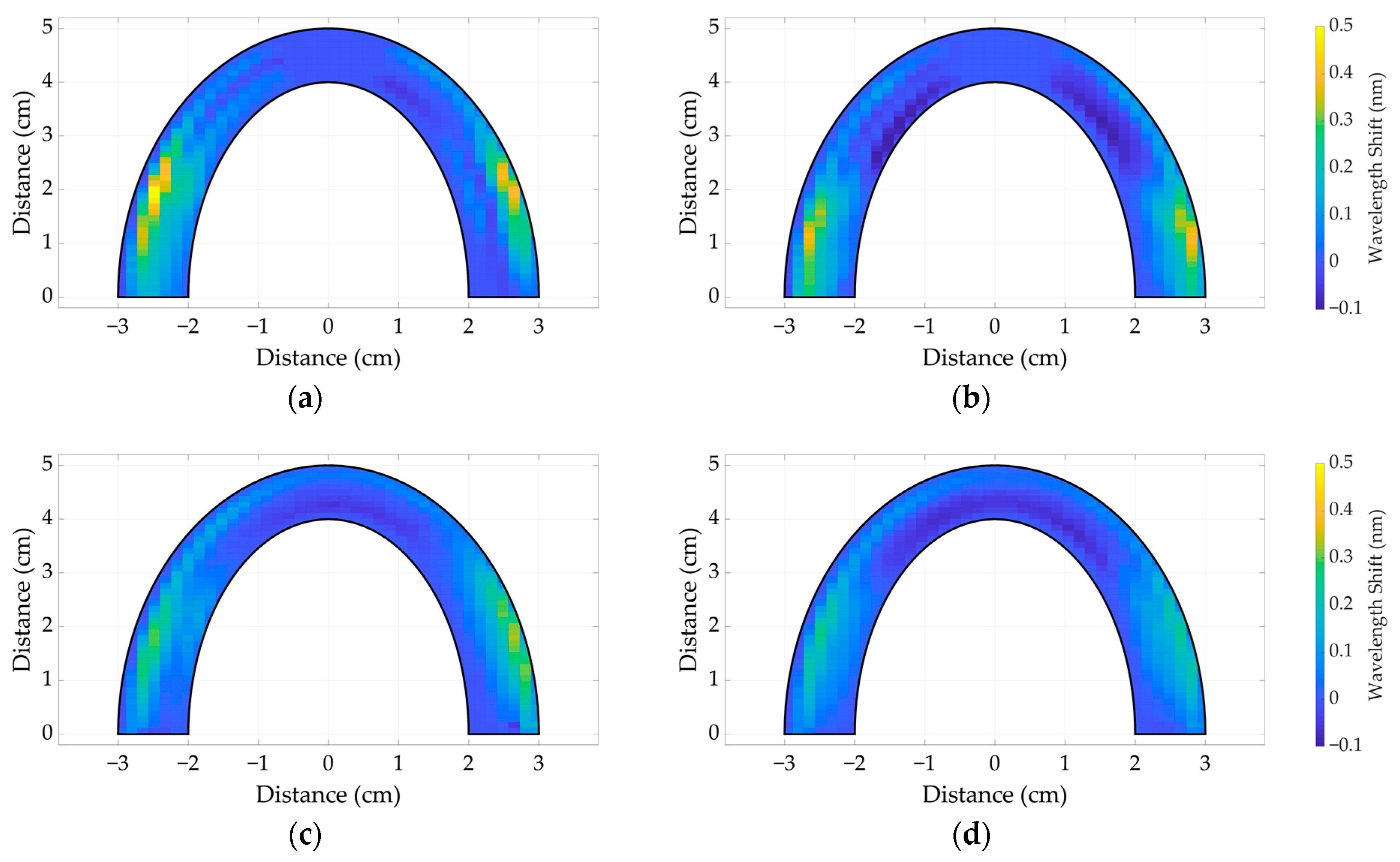
3.2. The Comparative Analysis between Dental Bites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ovando, A.; Ramirez, R.; Steele, S.; Amiri Moghadam, A.A.; Chang, Y.; Voicu, R.C. Biomedical Sensing—A Sensor Fusion Approach for Improved Medical Detection & Monitoring. In Proceedings of the Conference SoutheastCon 2023, Orlando, FL, USA, 1–16 April 2023; pp. 450–455. [Google Scholar] [CrossRef]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef] [PubMed]
- Koc, D.; Dogan, A.; Bek, B. Bite Force and Influential Factors on Bite Force Measurements: A Literature Review. Eur. J. Dent. 2010, 4, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M. Bite Force and Occlusion. Semin. Orthod. 2006, 12, 120–126. [Google Scholar] [CrossRef]
- Bulanda, S.; Ilczuk-Rypuła, D.; Nitecka-Buchta, A.; Nowak, Z.; Baron, S.; Postek-Stefańska, L. Sleep Bruxism in Children: Etiology, Diagnosis, and Treatment—A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 9544. [Google Scholar] [CrossRef] [PubMed]
- Kuninori, T.; Tomonari, H.; Uehara, S.; Kitashima, F.; Yagi, T.; Miyawaki, S. Miyawaki Influence of Maximum Bite Force on Jaw Movement during Gummy Jelly Mastication. J. Oral Rehabil. 2014, 41, 338–345. [Google Scholar] [CrossRef]
- Kogawa, E.M.; Calderon, P.S.; Lauris, J.R.P.; Araujo, C.R.P.; Conti, P.C.R. Evaluation of Maximal Bite Force in Temporomandibular Disorders Patients. J. Oral Rehabil. 2006, 33, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, H.M.; Glowacka, H. The Ontogeny of Maximum Bite Force in Humans. J. Anat. 2020, 237, 529–542. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kosaka, T.; Nakai, M.; Kida, M.; Fushida, S.; Kokubo, Y.; Watanabe, M.; Higashiyama, A.; Ikebe, K.; Ono, T.; et al. A Lower Maximum Bite Force Is a Risk Factor for Developing Cardiovascular Disease: The Suita Study. Sci. Rep. 2021, 11, 7671. [Google Scholar] [CrossRef]
- Gu, Y.; Bai, Y.; Xie, X. Bite Force Transducers and Measurement Devices. Front. Bioeng. Biotechnol. 2021, 9, 665081. [Google Scholar] [CrossRef]
- Al-Gunaid, T.H. Bite Force—What We Should Know: A Literature Review. Int. J. Orthod. Rehabil. 2019, 10, 168. [Google Scholar] [CrossRef]
- Verma, T.P. Bite Force Recording Devices—A Review. J. Clin. Diagn. Res. 2017, 11, ZE01–ZE05. [Google Scholar] [CrossRef] [PubMed]
- Krohn, D.A.; MacDougall, T.W.; Mendez, A. Fiber Optic Sensors: Fundamentals and Applications; Society of Photo-Optical Instrumentation Engineers (SPIE): Bellingham, WA, USA, 2014; ISBN 9781628411805. [Google Scholar]
- Khan, F.; Donder, A.; Galvan, S.; Baena, F.R.Y.; Misra, S. Pose Measurement of Flexible Medical Instruments Using Fiber Bragg Gratings in Multi-Core Fiber. IEEE Sens. J. 2020, 20, 10955–10962. [Google Scholar] [CrossRef]
- Udd, E. The Emergence of Fiber Optic Sensor Technology. In Fiber Optic Sensors: An Introduction for Engineers and Scientists, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Padma, S.; Umesh, S.; Asokan, S.; Srinivas, T.; Padma, S.; Asokan, S.; Srinivas, T. Bite Force Measurement Based on Fiber Bragg Grating Sensor. J. Biomed. Opt. 2017, 22, 107002. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Milczewski, M.S.; Souza, M.A.; Kalinowski, H.J.; MacHuca, O.F.; Marin, G.C.; Camargo, E.S. The Force Magnitude of a Human Bite Measured at the Molar Intercuspidation Using Fiber Bragg Gratings. J. Microw. Optoelectron. Electromagn. Appl. 2017, 16, 434–444. [Google Scholar] [CrossRef]
- Erdogan, T. Fiber Grating Spectra. J. Light. Technol. 1997, 15, 1277–1294. [Google Scholar] [CrossRef]
- Umesh, S.; Padma, S.; Asokan, S.; Srinivas, T. Fiber Bragg Grating Based Bite Force Measurement. J. Biomech. 2016, 49, 2877–2881. [Google Scholar] [CrossRef]
- Milczewski, M.S.; Cardozo Da Silva, J.C.; Martelli, C.; Grabarski, L.; Abe, I.; Kalinowski, H.J. Force Monitoring in a Maxilla Model and Dentition Using Optical Fiber Bragg Gratings. Sensors 2012, 12, 11957–11965. [Google Scholar] [CrossRef]
- Tosi, D.; Schena, E.; Molardi, C.; Korganbayev, S. Fiber Optic Sensors for Sub-Centimeter Spatially Resolved Measurements: Review and Biomedical Applications. Opt. Fiber Technol. 2018, 43, 6–19. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Aitkulov, A.; Jelbuldina, M.; Issatayeva, A.; Beisenova, A.; Molardi, C.; Saccomandi, P.; Blanc, W.; Inglezakis, V.J.; Tosi, D. Distributed 2D Temperature Sensing during Nanoparticles Assisted Laser Ablation by Means of High-Scattering Fiber Sensors. Sci. Rep. 2020, 10, 12593. [Google Scholar] [CrossRef]
- Issatayeva, A.; Amantayeva, A.; Blanc, W.; Tosi, D.; Molardi, C. Design and Analysis of a Fiber-Optic Sensing System for Shape Reconstruction of a Minimally Invasive Surgical Needle. Sci. Rep. 2021, 11, 8609. [Google Scholar] [CrossRef]
- Palmieri, L.; Schenato, L.; Santagiustina, M.; Galtarossa, A. Rayleigh-Based Distributed Optical Fiber Sensing. Sensors 2022, 22, 6811. [Google Scholar] [CrossRef] [PubMed]
- OBR 4600 Optical Backscatter ReflectometerTM. Available online: https://lunainc.com/product/obr-4600 (accessed on 12 July 2023).
- Bao, X.; Chen, L. Recent Progress in Distributed Fiber Optic Sensors. Sensors 2012, 12, 8601–8639. [Google Scholar] [CrossRef] [PubMed]
- Hartog, A. An Introduction to Distributed Optical Fibre Sensors; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1482259575. [Google Scholar]
- Beisenova, A.; Issatayeva, A.; Sovetov, S.; Korganbayev, S.; Jelbuldina, M.; Ashikbayeva, Z.; Blanc, W.; Schena, E.; Sales, S.; Molardi, C.; et al. Multi-Fiber Distributed Thermal Profiling of Minimally Invasive Thermal Ablation with Scattering-Level Multiplexing in MgO-Doped Fibers. Biomed. Opt. Express 2019, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Tosi, D.; Molardi, C.; Blanc, W.; Paixão, T.; Antunes, P.; Marques, C. Performance Analysis of Scattering-Level Multiplexing (SLMux) in Distributed Fiber-Optic Backscatter Reflectometry Physical Sensors. Sensors 2020, 20, 2595. [Google Scholar] [CrossRef] [PubMed]
- Blanc, W.; Mauroy, V.; Nguyen, L.; Shivakiran Bhaktha, B.N.; Sebbah, P.; Pal, B.P.; Dussardier, B. Fabrication of Rare Earth-Doped Transparent Glass Ceramic Optical Fibers by Modified Chemical Vapor Deposition. J. Am. Ceram. Soc. 2011, 94, 2315–2318. [Google Scholar] [CrossRef]
- Katrenova, Z.; Alisherov, S.; Abdol, T.; Yergibay, M.; Kappassov, Z.; Tosi, D.; Molardi, C. Investigation of High-Resolution Distributed Fiber Sensing System Embedded in Flexible Silicone Carpet for 2D Pressure Mapping. Sensors 2022, 22, 8800. [Google Scholar] [CrossRef] [PubMed]
- Gent, A.N. On the Relation between Indentation Hardness and Young’s Modulus. Rubber Chem. Technol. 1958, 31, 896–906. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, K.; Badar, M.; Yi, X.; Lu, P.; Buric, M.; Mao, Z.-H.; Chen, K.P. Improving OFDR Distributed Fiber Sensing by Fibers With Enhanced Rayleigh Backscattering and Image Processing. IEEE Sens. J. 2022, 22, 18471–18478. [Google Scholar] [CrossRef]
- Yan, A.; Huang, S.; Li, S.; Chen, R.; Ohodnicki, P.; Buric, M.; Lee, S.; Li, M.-J.; Chen, K.P. Distributed Optical Fiber Sensors with Ultrafast Laser Enhanced Rayleigh Backscattering Profiles for Real-Time Monitoring of Solid Oxide Fuel Cell Operations. Sci. Rep. 2017, 7, 9360. [Google Scholar] [CrossRef]
- Loranger, S.; Gagné, M.; Lambin-Iezzi, V.; Kashyap, R. Rayleigh Scatter Based Order of Magnitude Increase in Distributed Temperature and Strain Sensing by Simple UV Exposure of Optical Fibre. Sci. Rep. 2015, 5, 11177. [Google Scholar] [CrossRef]




| Criteria | The Dental Bite Based on SMF-28 | The Dental Bite Based on NP-Doped Fiber |
|---|---|---|
| The thickness | 6 mm | 6 mm |
| The material | Simple and cheap SMF-28 | Nanoparticle-doped fibers |
| The resolution | 2 mm × 2 mm | 2 mm × 2 mm |
| The sensing points | ~232 | ~200 |
| The pressure sensitivity | 1.672 pm/kPa | 2.376 pm/kPa |
| The arrangement of fiber lines | Spiral topology | SLMux |
| The covering area | Posterior and anterior regions of dental arc | Only posterior region of dental arc |
| The limitations | Unsuitable for in vivo measurements, low sensitivity | The anterior part of the dental arch cannot be measured. |
| Topology | Material | R2 | Sensitivity (nm/kg) | Sensitivity (pm/kPa) |
|---|---|---|---|---|
| Spiral-based silicone bite | SC18 | 0.98 | 1.672 | 0.535 |
| SC40 | 0.99 | 0.875 | 0.28 | |
| SLMux-based silicone bite | SC18 | 0.99 | 2.376 | 0.762 |
| SC40 | 0.98 | 2.037 | 0.653 |
| Topology | Material | A | B | C | D | Z | Variance | |
|---|---|---|---|---|---|---|---|---|
| Mean value of wavelength shift | Spiral-based silicone bite | SC18 | 1.477 | 1.485667 | 1.2555 | 0.7845 | 1.16875 | 0.082 |
| SC40 | 1.033667 | 1.0175 | 0.998667 | 0.799167 | 0.87475 | 0.01 | ||
| SLMux-based silicone bite | SC18 | 1.789333 | 1.927667 | 1.945 | 1.438167 | 0.055 | ||
| SC40 | 1.612833 | 1.6995 | 1.539667 | 1.297667 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katrenova, Z.; Alisherov, S.; Yergibay, M.; Kappasov, Z.; Blanc, W.; Tosi, D.; Molardi, C. Bite Force Mapping Based on Distributed Fiber Sensing Network Approach. Sensors 2024, 24, 537. https://doi.org/10.3390/s24020537
Katrenova Z, Alisherov S, Yergibay M, Kappasov Z, Blanc W, Tosi D, Molardi C. Bite Force Mapping Based on Distributed Fiber Sensing Network Approach. Sensors. 2024; 24(2):537. https://doi.org/10.3390/s24020537
Chicago/Turabian StyleKatrenova, Zhanerke, Shakhrizat Alisherov, Madina Yergibay, Zhanat Kappasov, Wilfried Blanc, Daniele Tosi, and Carlo Molardi. 2024. "Bite Force Mapping Based on Distributed Fiber Sensing Network Approach" Sensors 24, no. 2: 537. https://doi.org/10.3390/s24020537
APA StyleKatrenova, Z., Alisherov, S., Yergibay, M., Kappasov, Z., Blanc, W., Tosi, D., & Molardi, C. (2024). Bite Force Mapping Based on Distributed Fiber Sensing Network Approach. Sensors, 24(2), 537. https://doi.org/10.3390/s24020537









