Pb2+ Ion Sensors Employing Gold Etching Process: Comparative Investigation on Au Nanorods and Au Nanotriangles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Gold Seeds
2.3. Growth of Au Nanorods from the Seed Solution
2.4. Growth of Au Nanotriangles from the Seed Solution
2.5. Separation of Au Nanorods and Au Nanotriangles from Au Nanospheres
2.6. Typical Etching Process in the Presence of Pb2+/S2O32− Ions
2.7. Transmission Electron Microscopy (TEM)
2.8. Instrumentation
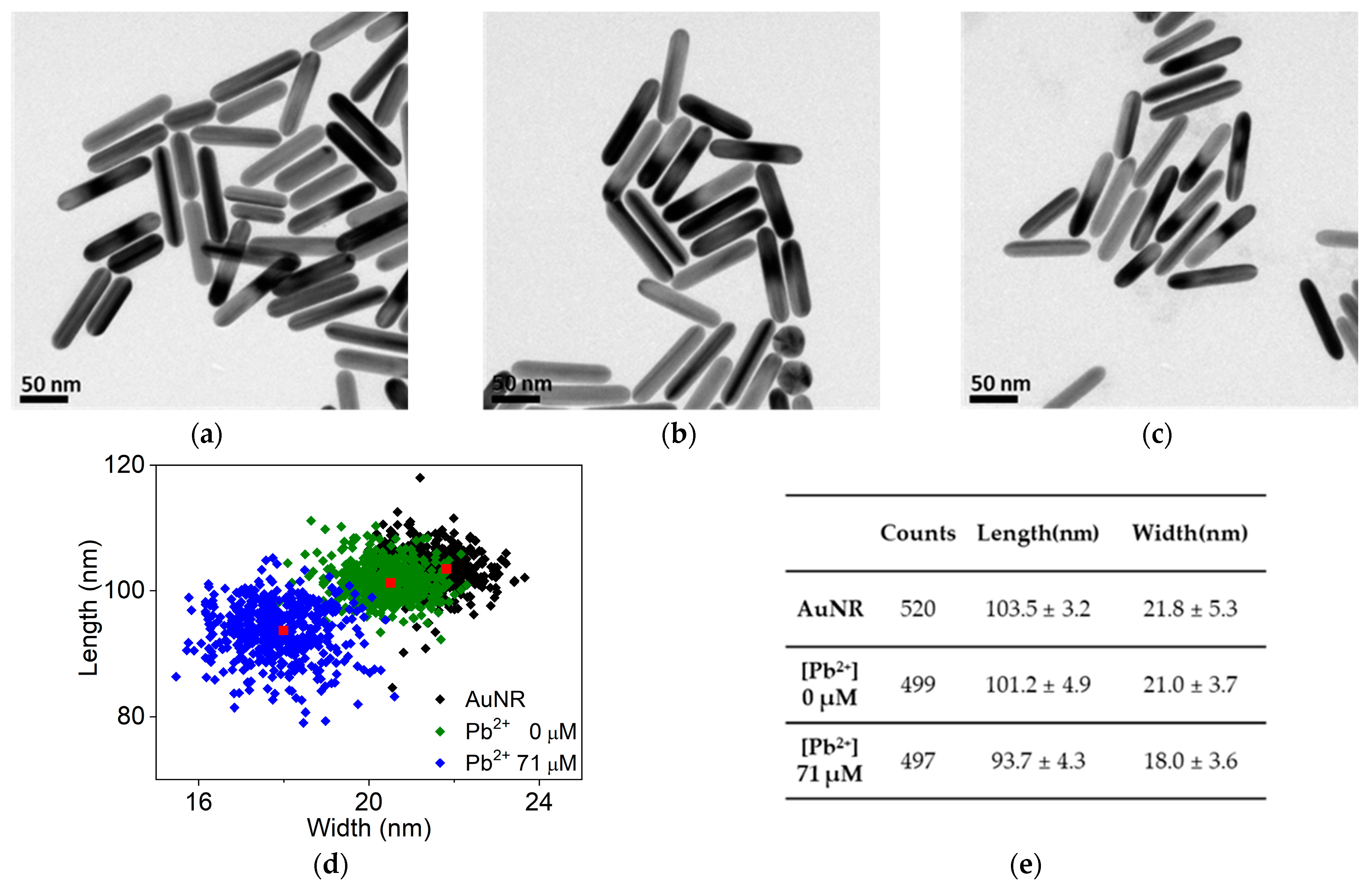
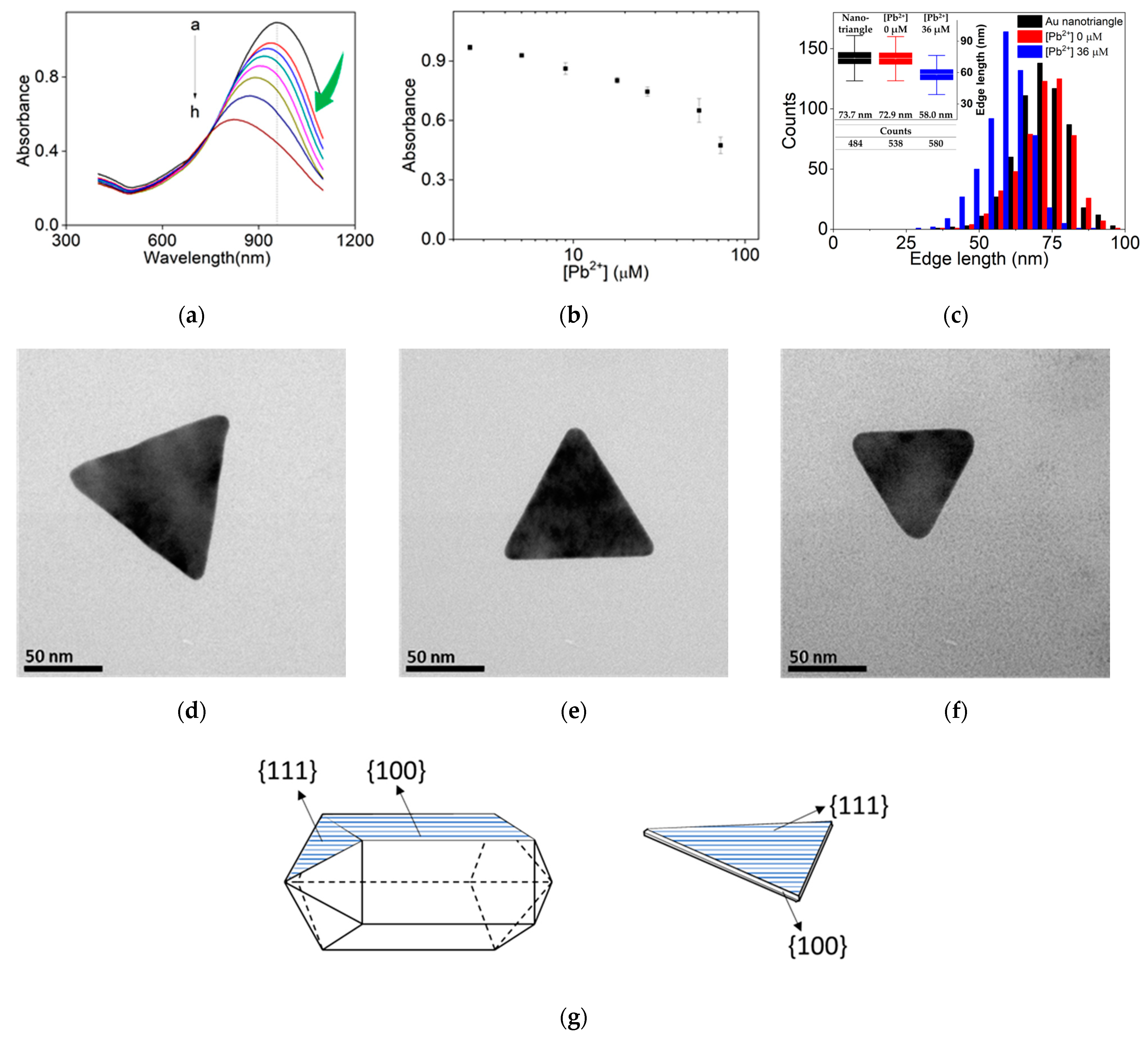
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- U.S. EPA National Primary Drinking Water Regulations (EPA 816-F-09-004); United States Environmental Protection Agency: Washington, DC, USA, 2009.
- Needleman, H. Lead Poisoning. Annu. Rev. Med. 2004, 55, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Shokrollahi, A.; Niknam, K.; Niknam, E.; Najibi, A.; Soylak, M. Cloud point extraction and flame atomic absorption spectrometric determination of cadmium(II), lead(II), palladium(II) and silver(I) in environmental samples. J. Hazard. Mater. 2009, 168, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Afonso, D.D.; Baytak, S.; Arslan, Z. Simultaneous generation of hydrides of bismuth, lead and tin in the presence of ferricyanide and application to determination in biominerals by ICP-AES. J. Anal. At. Spectrom. 2010, 25, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Wojciechowski, M.; Wagner, B.; Bulska, E.; Maksymiuk, K. Laser Ablation Inductively Coupled Plasma Mass Spectrometry Assisted Insight into Ion-Selective Membranes. Anal. Chem. 2006, 78, 5584–5589. [Google Scholar] [CrossRef] [PubMed]
- Deibler, K.; Basu, P. Continuing Issues with Lead: Recent Advances in Detection. Eur. J. Inorg. Chem. 2013, 2013, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Vantelon, D.; Lanzirotti, A.; Scheinost, A.C.; Kretzschmar, R. Spatial Distribution and Speciation of Lead around Corroding Bullets in a Shooting Range Soil Studied by Micro-X-ray Fluorescence and Absorption Spectroscopy. Environ. Sci. Technol. 2005, 39, 4808–4815. [Google Scholar] [CrossRef]
- Zhang, R.; Li, L.; Sultanbawa, Y.; Xu, Z.P. X-ray fluorescence imaging of metals and metalloids in biological systems. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 169–188. [Google Scholar]
- Wei, Y.; Yang, R.; Yu, X.-Y.; Wang, L.; Liu, J.-H.; Huang, X.-J. Stripping voltammetry study of ultra-trace toxic metal ions on highly selectively adsorptive porous magnesium oxide nanoflowers. Analyst 2012, 137, 2183–2191. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Guo, L.; Zhou, X.; Chen, P.; Hong, S.; Kim, D.-H. Solid-Phase Colorimetric Sensor Based on Gold Nanoparticle-Loaded Polymer Brushes: Lead Detection as a Case Study. Anal. Chem. 2013, 85, 4094–4099. [Google Scholar] [CrossRef]
- Shrivas, K.; Sahu, B.; Deb, M.K.; Thakur, S.S.; Sahu, S.; Kurrey, R.; Kant, T.; Patle, T.K.; Jangde, R. Colorimetric and paper-based detection of lead using PVA capped silver nanoparticles: Experimental and theoretical approach. Microchem. J. 2019, 150, 104156. [Google Scholar] [CrossRef]
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Brown, R.J.C.; Bhardwaj, N.; Kim, K.-H. Recent advances in the application of noble metal nanoparticles in colorimetric sensors for lead ions. Environ. Sci. Nano 2021, 8, 863–889. [Google Scholar] [CrossRef]
- Nguyen, H.; Sung, Y.; O’Shaughnessy, K.; Shan, X.; Shih, W.C. Smartphone Nanocolorimetry for On-Demand Lead Detection and Quantitation in Drinking Water. Anal. Chem. 2018, 90, 11517–11522. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Hosseini, M.; Tavakoli, O. Aptamer-based colorimetric determination of Pb2+ using a paper-based microfluidic platform. Anal. Methods 2018, 10, 4438–4444. [Google Scholar] [CrossRef]
- Sahu, B.; Kurrey, R.; Deb, M.K.; Shrivas, K.; Karbhal, I.; Khalkho, B.R. A simple and cost-effective paper-based and colorimetric dual-mode detection of arsenic(iii) and lead(ii) based on glucose-functionalized gold nanoparticles. RSC Adv. 2021, 11, 20769–20780. [Google Scholar] [CrossRef]
- Tian, W.; Wang, D.; Fan, H.; Yang, L.; Ma, G. A Plasma Biochemical Analysis of Acute Lead Poisoning in a Rat Model by Chemometrics-Based Fourier Transform Infrared Spectroscopy: An Exploratory Study. Front. Chem. 2018, 6, 261. [Google Scholar] [CrossRef]
- Lehmann, S.; Fischer, M.; Rosin, A.; Gerdes, T.; Krenkel, W. The feasibility of CO2-laser-induced breakdown spectroscopy for fast lead determination in glass cullet. Int. J. Appl. Glass Sci. 2020, 11, 369–379. [Google Scholar] [CrossRef]
- Song, R.; Zhang, Q.; Chu, Y.; Zhang, L.; Dai, H.; Wu, W. Fluorescent cellulose nanocrystals for the detection of lead ions in complete aqueous solution. Cellulose 2019, 26, 9553–9565. [Google Scholar] [CrossRef]
- Chen, H.; Shao, S.; Yu, Y.; Huang, Y.; Zhu, X.; Zhang, S.; Fan, J.; Yin, G.Y.; Chi, B.; Wan, M.; et al. A dual-responsive biosensor for blood lead detection. Anal. Chim. Acta 2020, 1093, 131–141. [Google Scholar] [CrossRef]
- Silva, I.B.; de Araújo, D.M.; Vocciante, M.; Ferro, S.; Martínez-Huitle, C.A.; Dos Santos, E.V. Electrochemical Determination of Lead Using A Composite Sensor Obtained from Low-Cost Green Materials: Graphite/Cork. Appl. Sci. 2021, 11, 2355. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.-Y.; Yoon, J.; Czarnik, A.W. Chelation-enhanced fluorescence chemosensing of Pb(II), an inherently quenching metal ion. J. Mol. Recognit. 1996, 9, 297–303. [Google Scholar] [CrossRef]
- Meng, X.; Cao, D.; Hu, Z.; Han, X.; Li, Z.; Ma, W. A highly sensitive and selective chemosensor for Pb2+ based on quinoline–coumarin. RSC Adv. 2018, 8, 33947–33951. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Das, G. “Turn-on” Pb2+ sensing and rapid detection of biothiols in aqueous medium and real samples. Analyst 2019, 144, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Zhang, J.; Zhang, D.; Zhu, M.; Gong, L.; Su, C.; Lu, W.; Bian, Y.; Jiang, J. A phthalocyanine-porphyrin triad for ratiometric fluorescent detection of Lead(II) ions. Dye. Pigment. 2020, 173, 107941. [Google Scholar] [CrossRef]
- Métivier, R.; Leray, I.; Valeur, B. A highly sensitive and selective fluorescent molecular sensor for Pb(ii) based on a calix[4]arene bearing four dansyl groups. Chem. Commun. 2003, 8, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-M.; Bu, J.-H.; Zheng, Q.-Y.; Chen, C.-F.; Huang, Z.-T. Highly selective fluorescent sensing of Pb2+ by a new calix[4]arene derivative. Tetrahedron Lett. 2006, 47, 1905–1908. [Google Scholar] [CrossRef]
- Buie, N.M.; Talanov, V.S.; Butcher, R.J.; Talanova, G.G. New Fluorogenic Dansyl-Containing Calix[4]arene in the Partial Cone Conformation for Highly Sensitive and Selective Recognition of Lead(II). Inorg. Chem. 2008, 47, 3549–3558. [Google Scholar] [CrossRef]
- Kim, I.-B.; Dunkhorst, A.; Gilbert, J.; Bunz, U.H.F. Sensing of Lead Ions by a Carboxylate-Substituted PPE: Multivalency Effects. Macromolecules 2005, 38, 4560–4562. [Google Scholar] [CrossRef]
- Narkwiboonwong, P.; Tumcharern, G.; Potisatityuenyong, A.; Wacharasindhu, S.; Sukwattanasinitt, M. Aqueous sols of oligo(ethylene glycol) surface decorated polydiacetylene vesicles for colorimetric detection of Pb2+. Talanta 2011, 83, 872–878. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y. A Highly Sensitive and Selective Catalytic DNA Biosensor for Lead Ions. J. Am. Chem. Soc. 2000, 122, 10466–10467. [Google Scholar] [CrossRef]
- Swearingen, C.B.; Wernette, D.P.; Cropek, D.M.; Lu, Y.; Sweedler, J.V.; Bohn, P.W. Immobilization of a Catalytic DNA Molecular Beacon on Au for Pb(II) Detection. Anal. Chem. 2005, 77, 442–448. [Google Scholar] [CrossRef]
- Wernette, D.P.; Swearingen, C.B.; Cropek, D.M.; Lu, Y.; Sweedler, J.V.; Bohn, P.W. Incorporation of a DNAzyme into Au-coated nanocapillary array membranes with an internal standard for Pb(ii) sensing. Analyst 2006, 131, 41–47. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Zhang, X.; Tang, Y. A sensitive biosensor with a DNAzyme for lead(ii) detection based on fluorescence turn-on. Analyst 2015, 140, 4642–4647. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Ma, L.-H.; Fang, B.-Y.; Zhao, Y.-D.; Hu, X.-B. Graphene oxide-assisted Au nanoparticle strip biosensor based on GR-5 DNAzyme for rapid lead ion detection. Colloids Surf. B Biointerfaces 2018, 169, 305–312. [Google Scholar] [CrossRef]
- Kim, Y.; Johnson, R.C.; Hupp, J.T. Gold Nanoparticle-Based Sensing of “Spectroscopically Silent” Heavy Metal Ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Berlina, A.N.; Zherdev, A.V.; Pridvorova, S.M.; Gaur, M.S.; Dzantiev, B.B. Rapid Visual Detection of Lead and Mercury via Enhanced Crosslinking Aggregation of Aptamer-Labeled Gold Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5489–5495. [Google Scholar] [CrossRef]
- Dehghani, Z.; Hosseini, M.; Mohammadnejad, J.; Ganjali, M.R. Novel colorimetric sensor based on peroxidase-like activity of chitosan-stabilized Au/Pt nanoclusters for trace lead. Anal. Methods 2019, 11, 684–690. [Google Scholar] [CrossRef]
- Solra, M.; Bala, R.; Wangoo, N.; Soni, G.K.; Kumar, M.; Sharma, R.K. Optical pico-biosensing of lead using plasmonic gold nanoparticles and a cationic peptide-based aptasensor. Chem. Commun. 2020, 56, 289–292. [Google Scholar] [CrossRef]
- Sahu, S.; Sharma, S.; Ghosh, K.K. Novel formation of Au/Ag bimetallic nanoparticles from a mixture of monometallic nanoparticles and their application for the rapid detection of lead in onion samples. New J. Chem. 2020, 44, 15010–15017. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C. Gold nanoparticles as colorimetric probes in food analysis: Progress and challenges. Food Chem. 2023, 429, 136887. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Song, Z.; Peng, J.; Yang, M.; Zhi, H.; He, H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020, 127, 115880. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, X.; Han, Y.; Luan, X.; Wei, G. Biomimetic gold nanomaterials for biosensing, bioimaging and biotherapy: A mini-review. Sens. Diagn. 2023, 2, 320–336. [Google Scholar] [CrossRef]
- Sanchis-Gual, R.; Coronado-Puchau, M.; Mallah, T.; Coronado, E. Hybrid nanostructures based on gold nanoparticles and functional coordination polymers: Chemistry, physics and applications in biomedicine, catalysis and magnetism. Coord. Chem. Rev. 2023, 480, 215025. [Google Scholar] [CrossRef]
- Liu, S.; Min, X.; Xiang, M.; Wang, J.; Tang, L.; Liu, L. Nanoanalysis of the leaching process simulation of Pb in agricultural soil. Environ. Pollut. 2022, 306, 119402. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Gad, G.M.A.; Hegazy, M.A. Optoelectronic properties of gold nanoparticles synthesized by using wet chemical method. Mater. Res. Express 2019, 6, 085024. [Google Scholar] [CrossRef]
- Chen, J.; Gan, F. Polarization-switchable plasmonic emitters based on laser-induced bubbles. Opto-Electron. Adv. 2022, 5, 200100-1. [Google Scholar] [CrossRef]
- Jing, J.; Liu, K.; Jiang, J.; Xu, T.; Wang, S.; Liu, T. Highly sensitive and stable probe refractometer based on configurable plasmonic resonance with nano-modified fiber core. Opto-Electron. Adv. 2023, 6, 220072. [Google Scholar] [CrossRef]
- Yuan, Z.; Hu, C.-C.; Chang, H.-T.; Lu, C. Gold nanoparticles as sensitive optical probes. Analyst 2016, 141, 1611–1626. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Zhou, W.; Sun, L.; Samanta, D.; Mirkin, C.A. Corner-, edge-, and facet-controlled growth of nanocrystals. Sci. Adv. 2021, 7, eabf1410. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Xue, X.; Wang, H.; Xue, Z. Gold nanorod etching-based multicolorimetric sensors: Strategies and applications. J. Mater. Chem. C 2019, 7, 4610–4621. [Google Scholar] [CrossRef]
- Zhang, Y.; Leng, Y.; Miao, L.; Xin, J.; Wu, A. The colorimetric detection of Pb2+ by using sodium thiosulfate and hexadecyl trimethyl ammonium bromide modified gold nanoparticles. Dalton Trans. 2013, 42, 5485–5490. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, Y.-Q.; Li, J.-J.; Zhao, J.-W. Colorimetric detection of lead(ii) ions based on accelerating surface etching of gold nanorods to nanospheres: The effect of sodium thiosulfate. RSC Adv. 2016, 6, 25611–25619. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Ke, C.-Y.; Chen, T.-H.; Lu, L.-C.; Tseng, W.-L. Understanding thiol-induced etching of luminescent gold nanoclusters. RSC Adv. 2014, 4, 26050–26056. [Google Scholar] [CrossRef]
- Ha, T.H.; Koo, H.-J.; Chung, B.H. Shape-Controlled Syntheses of Gold Nanoprisms and Nanorods Influenced by Specific Adsorption of Halide Ions. J. Phys. Chem. C 2007, 111, 1123–1130. [Google Scholar] [CrossRef]
- Smith, D.K.; Korgel, B.A. The Importance of the CTAB Surfactant on the Colloidal Seed-Mediated Synthesis of Gold Nanorods. Langmuir 2008, 24, 644–649. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chang, H.-T.; Shiang, Y.-C.; Hung, Y.-L.; Chiang, C.-K.; Huang, C.-C. Colorimetric Assay for Lead Ions Based on the Leaching of Gold Nanoparticles. Anal. Chem. 2009, 81, 9433–9439. [Google Scholar] [CrossRef]
- Link, S.; Mohamed, M.B.; El-Sayed, M.A. Simulation of the Optical Absorption Spectra of Gold Nanorods as a Function of Their Aspect Ratio and the Effect of the Medium Dielectric Constant. J. Phys. Chem. B 1999, 103, 3073–3077. [Google Scholar] [CrossRef]
- Lan, Y.-J.; Lin, Y.-W. A non-aggregation colorimetric method for trace lead(ii) ions based on the leaching of gold nanorods. Anal. Methods 2014, 6, 7234–7242. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Vegliò, F.; Ubaldini, S. Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Dreier, T.A.; Ackerson, C.J. Radicals Are Required for Thiol Etching of Gold Particles. Angew. Chem. Int. Ed. 2015, 54, 9249–9252. [Google Scholar] [CrossRef] [PubMed]
- Mayet, N.; Servat, K.; Kokoh, K.B.; Napporn, T.W. Probing the Surface of Noble Metals Electrochemically by Underpotential Deposition of Transition Metals. Surfaces 2019, 2, 257–276. [Google Scholar] [CrossRef]
- Lane, L.A.; Xue, R.; Nie, S. Emergence of two near-infrared windows for in vivo and intraoperative SERS. Curr. Opin. Chem. Biol. 2018, 45, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, X.; An, Q.; Zhou, R.; Xu, W.; Xu, D.; Lin, Q.; Cao, X. Gold nanostar as an ultrasensitive colorimetric probe for picomolar detection of lead ion. Anal. Chim. Acta 2021, 1160, 338380. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.L.T.; Kim, E.J.; Chang, S.-K.; Park, T.J. Sensitive detection of lead ions using sodium thiosulfate and surfactant-capped gold nanoparticles. BioChip J. 2016, 10, 65–73. [Google Scholar] [CrossRef]
- Dai, D.; Xu, D.; Cheng, X.; He, Y. Direct imaging of single gold nanoparticle etching: Sensitive detection of lead ions. Anal. Methods 2014, 6, 4507–4511. [Google Scholar] [CrossRef]
- Biberian, J.P.; Rhead, G.E. Spontaneous alloying of a gold substrate with lead monolayers. J. Phys. F Metal Phys. 1973, 3, 675. [Google Scholar] [CrossRef]
- Perdereau, J.; Biberian, J.P.; Rhead, G.E. Adsorption and surface alloying of lead monolayers on (111) and (110) faces of gold. J. Phys. F Metal. Phys. 1974, 4, 798. [Google Scholar] [CrossRef]
- Lee, Y.-F.; Huang, C.-C. Colorimetric Assay of Lead Ions in Biological Samples Using a Nanogold-Based Membrane. ACS Appl. Mater. Interfaces 2011, 3, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-F.; Nan, F.-H.; Chen, M.-J.; Wu, H.-Y.; Ho, C.-W.; Chen, Y.-Y.; Huang, C.-C. Detection and removal of mercury and lead ions by using gold nanoparticle-based gel membrane. Anal. Methods 2012, 4, 1709–1717. [Google Scholar] [CrossRef]
- Soleymani, M.; Li, L.; Sadri, F.; Ghahreman, A. The electrochemical catalytic role of Pb2+ in thiosulfate gold oxidation process. Miner. Eng. 2022, 184, 107676. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Li, Q.; Liu, X.; Yang, Y.; Jiang, T. Role of foreign ions in the thiourea leaching of gold. Miner. Eng. 2023, 202, 108265. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, C.; Shi, J.; Pridgen, E.M.; Frieder, J.; Wu, J.; Farokhzad, O.C. DNA Self-Assembly of Targeted Near-Infrared-Responsive Gold Nanoparticles for Cancer Thermo-Chemotherapy. Angew. Chem. 2012, 124, 12023–12027. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.J.; Ha, T.H. Pb2+ Ion Sensors Employing Gold Etching Process: Comparative Investigation on Au Nanorods and Au Nanotriangles. Sensors 2024, 24, 497. https://doi.org/10.3390/s24020497
Park EJ, Ha TH. Pb2+ Ion Sensors Employing Gold Etching Process: Comparative Investigation on Au Nanorods and Au Nanotriangles. Sensors. 2024; 24(2):497. https://doi.org/10.3390/s24020497
Chicago/Turabian StylePark, Eun Jin, and Tai Hwan Ha. 2024. "Pb2+ Ion Sensors Employing Gold Etching Process: Comparative Investigation on Au Nanorods and Au Nanotriangles" Sensors 24, no. 2: 497. https://doi.org/10.3390/s24020497
APA StylePark, E. J., & Ha, T. H. (2024). Pb2+ Ion Sensors Employing Gold Etching Process: Comparative Investigation on Au Nanorods and Au Nanotriangles. Sensors, 24(2), 497. https://doi.org/10.3390/s24020497




