Piezoelectric Behaviour in Biodegradable Carrageenan and Iron (III) Oxide Based Sensor
Abstract
1. Introduction
2. State of the Art
2.1. Carrageenan as Multifunctional Biopolymer
2.2. Iron (III) Oxide Influence on Electric Conductivity
2.3. Effect of Friction and Other Tribological Issues
2.4. Proton Type Conducting Biopolymer Coupling with N-Type Semiconductor Oxide
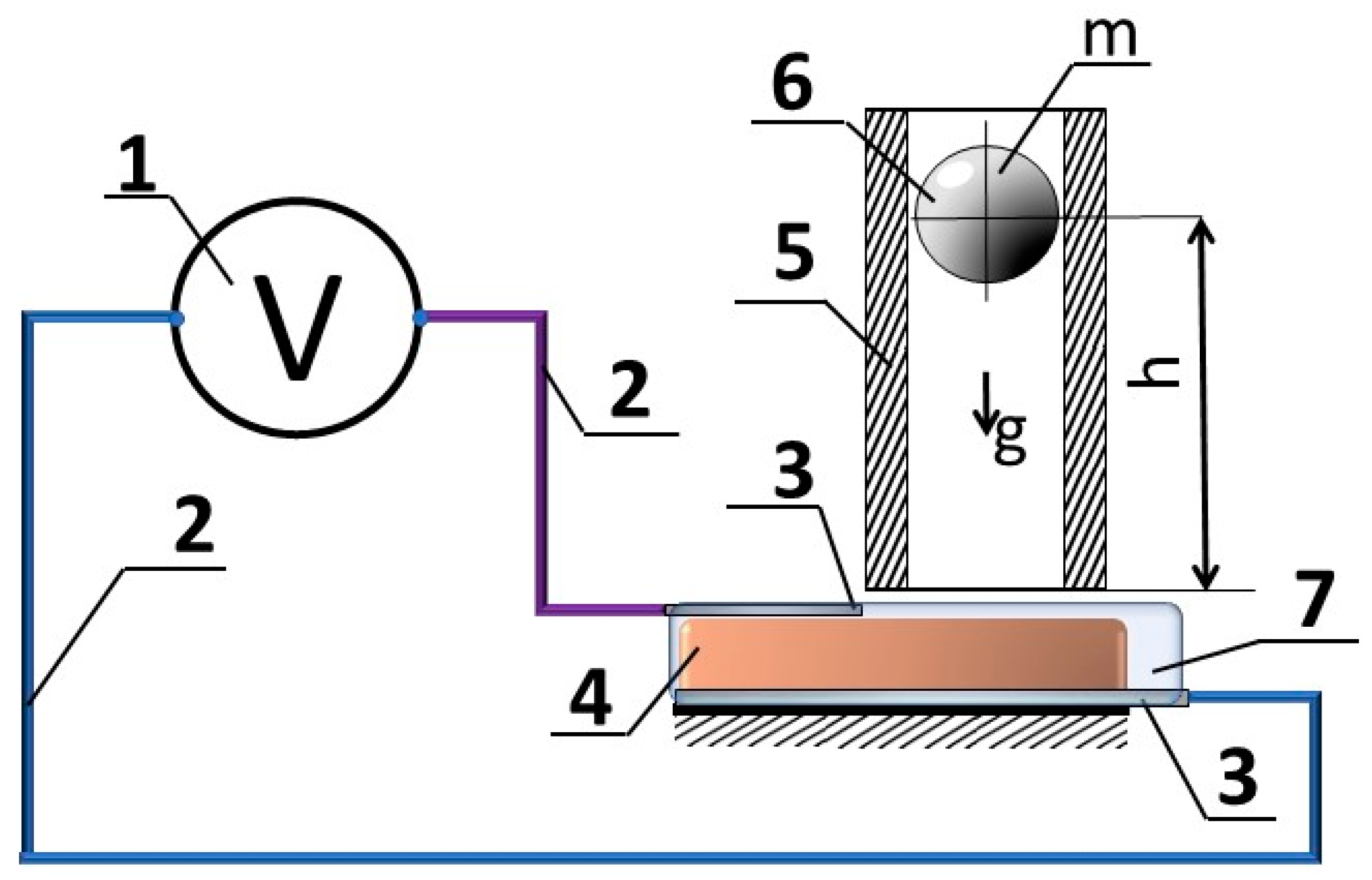
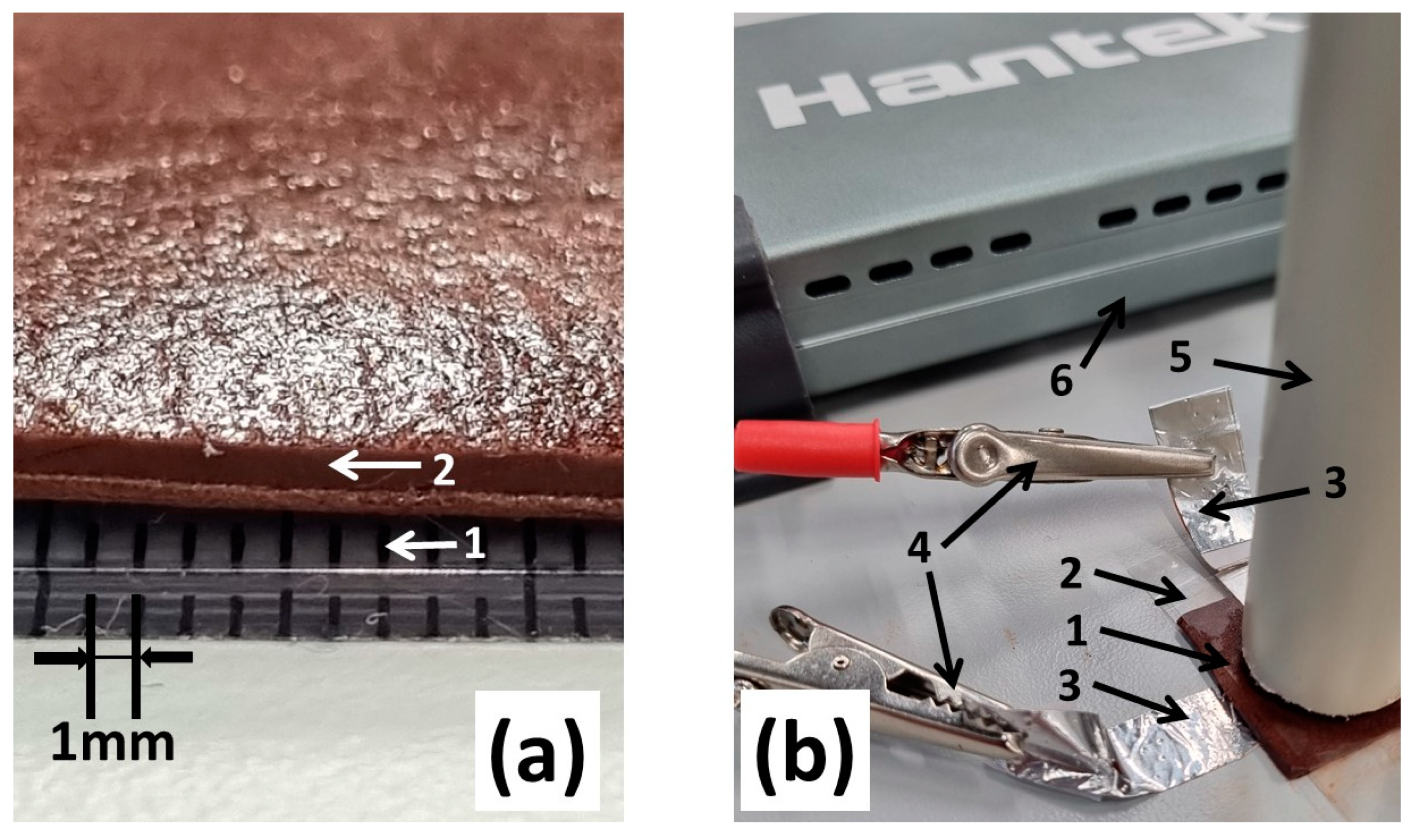
3. Materials and Methods
4. Results
4.1. Evaluation of Iron (III) Oxide Particle Size
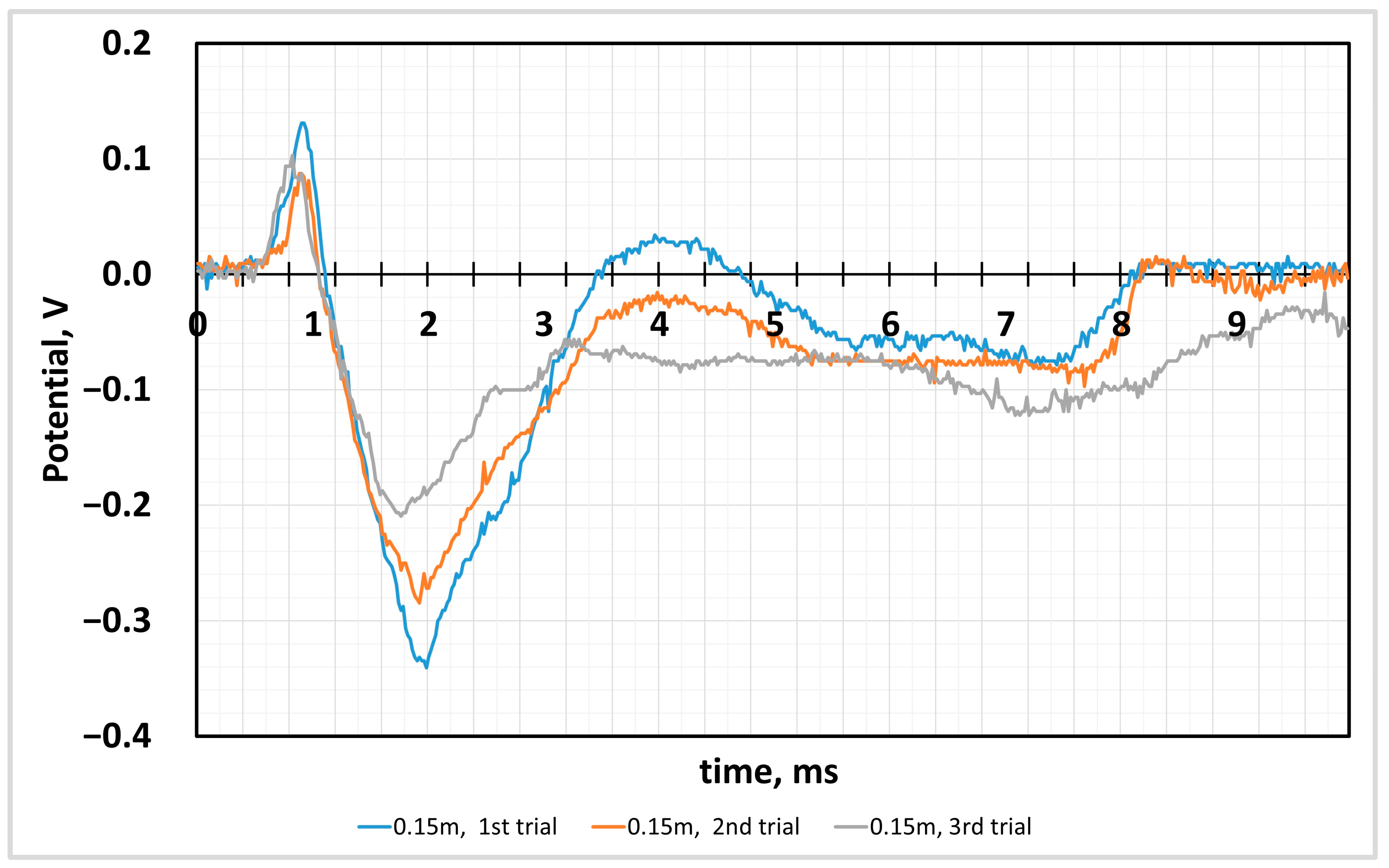
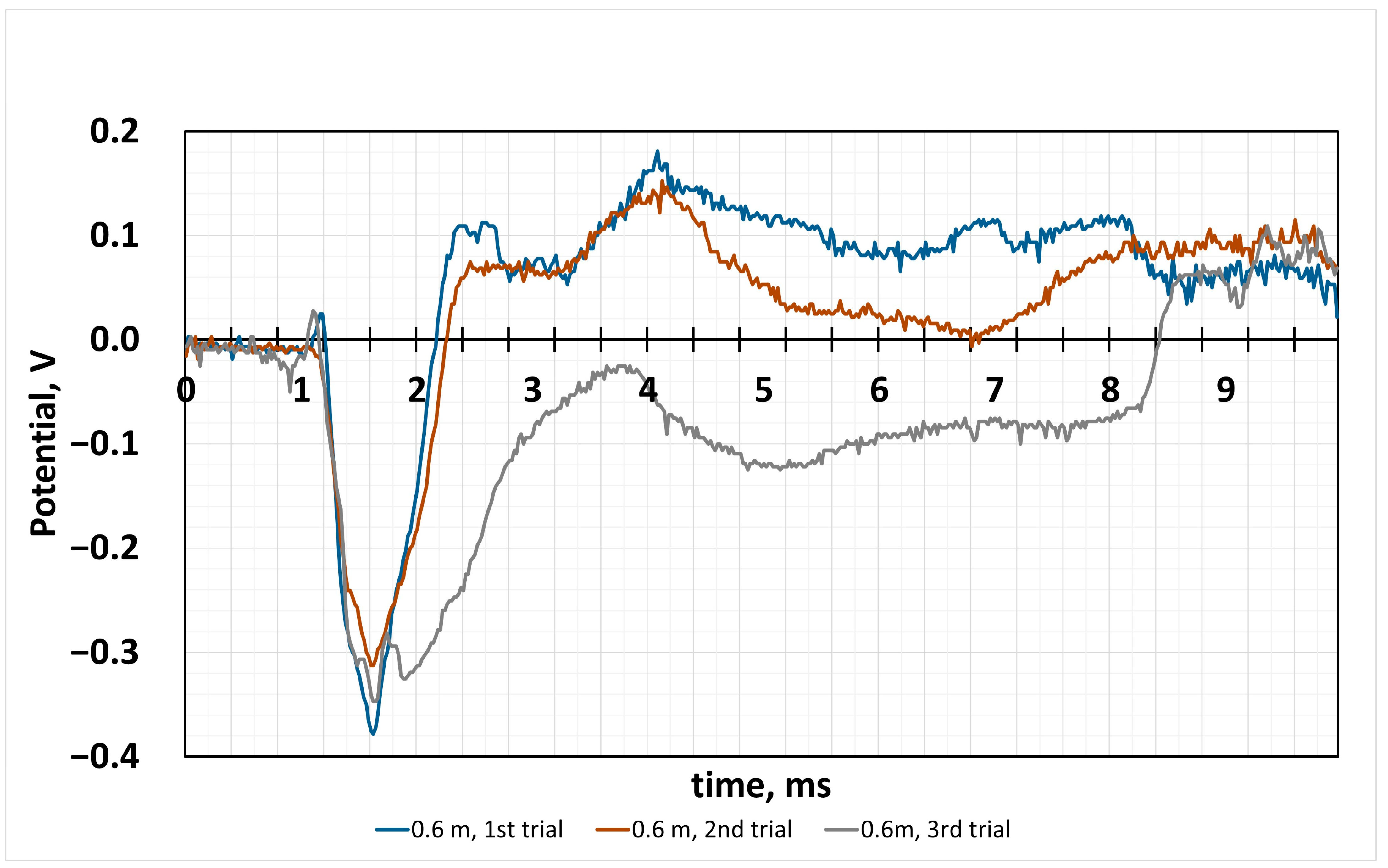
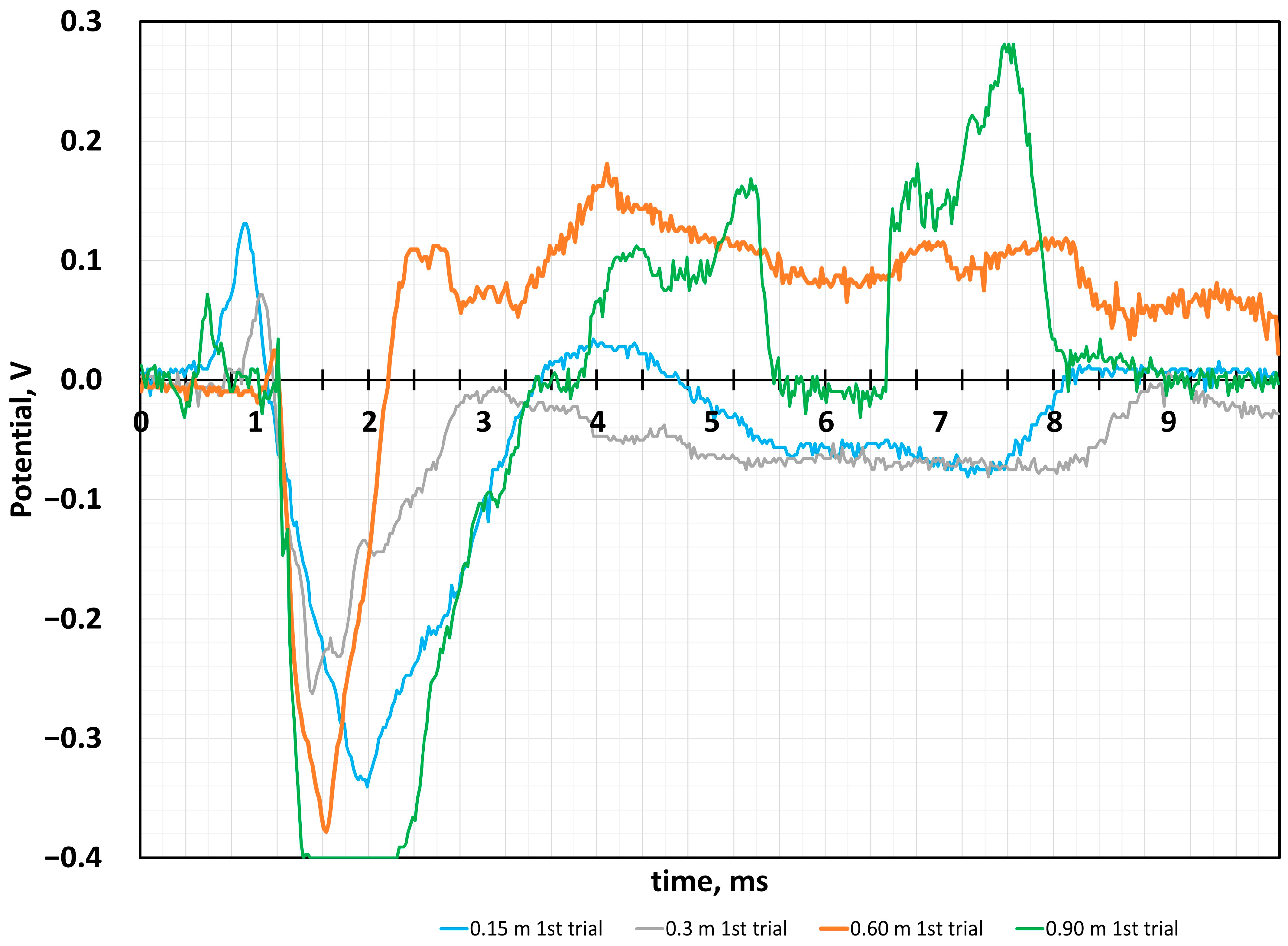
4.2. Sensor Response to the Impact
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Zhao, D.; He, J.; Zou, L. Armadillo-inspired ultra-sensitive flexible sensor for wearable electronics. Chem. Eng. J. 2023, 475, 146171. [Google Scholar] [CrossRef]
- Cheng, W.; Luo, Z.; Wang, C.; Zhao, T.; Xiang, N. Soft crawling robot integrated with liquid metal-based flexible strain sensor and closed-loop feedback control. Sens. Actuators A Phys. 2024, 371, 115316. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Li, R.; Huang, X.; Chen, T.; Xiao, X. Capacitive pressure sensor inlaid a porous dielectric layer of superelastic polydimethylsiloxane in conductive fabrics for detection of human motions. Sens. Actuators A Phys. 2020, 312, 112106. [Google Scholar] [CrossRef]
- Xiao, S.; Lao, Y.; Liu, H.; Li, D.; Wei, Q.; Li, Z.; Lu, S. Highly stretchable anti-freeze hydrogel based on aloe polysaccharides with high ionic conductivity for multifunctional wearable sensors. Int. J. Biol. Macromol. 2024, 254, 127931. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Ji, Y.; Zhao, C.; Mei, D.; Zhao, X.; Ru, J.; Li, L.; Wang, Y. The effects of ethanol content on the electrical response of IPMC for drinking perception. Sens. Actuators A Phys. 2024, 366, 114894. [Google Scholar] [CrossRef]
- AziziHariri, P.; Ebrahimi, A.H.; Zamyad, H.; Sahebian, S. Development of a phase-change material-based soft actuator for soft robotic gripper functionality: In-depth analysis of material composition, ethanol microbubble distribution and lifting capabilities. Mater. Chem. Phys. 2024, 311, 128435. [Google Scholar] [CrossRef]
- Walilko, T.J.; Viano, D.C.; Bir, C.A. Biomechanics of the head for Olympic boxer punches to the face. Br. J. Sports Med. 2005, 39, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fujimoto, Y.; Tanaka, Y. Flexible Impact Force Sensor. J. Sens. Technol. 2014, 2014, 66–80. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L. Quantification, localization, and reconstruction of impact force on interval composite structures. Int. J. Mech. Sci. 2023, 239, 107873. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Huang, T.; Sandoval Bojórquez, D.I.; Tonmoy, H.; Pané, S.; Makarov, D.; Baraban, L. Design and Development of Transient Sensing Devices for Healthcare Applications. Adv. Sci. 2024, 11, 2307232. [Google Scholar] [CrossRef]
- Khan, S.A.; Ahmad, H.; Zhu, G.; Pang, H.; Zhang, Y. Three-Dimensional Printing of Hydrogels for Flexible Sensors: A Review. Gels 2024, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Dudek, G.; Turczyn, R.; Strzelewicz, A.; Rybak, A.; Krasowska, M.; Grzywna, Z.J. Preparation and Characterization of Iron Oxides—Polymer Composite Membranes. Sep. Sci. Technol. 2012, 47, 1390–1394. [Google Scholar] [CrossRef]
- Liew, J.W.Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Wan Daud, W.R. Synthesis and characterization of modified κ-carrageenan for enhanced proton conductivity as polymer electrolyte membrane. PLoS ONE 2017, 12, e0185313. [Google Scholar] [CrossRef]
- Perumal, P.; Selvin, P.C. Red algae-derived k-carrageenan-based proton-conducting electrolytes for the wearable electrical devices. J. Solid State Electrochem. 2020, 24, 2249–2260. [Google Scholar] [CrossRef]
- Zainuddin, N.K.; Rasali, N.M.J.; Mazuki, N.F.; Saadiah, M.A.; Samsudin, A.S. Investigation on favourable ionic conduction based on CMC-K carrageenan proton conducting hybrid solid bio-polymer electrolytes for applications in EDLC. Int. J. Hydrogen Energy 2020, 45, 8727–8741. [Google Scholar] [CrossRef]
- Mirzaei, A.; Esmkhani, M.; Zallaghi, M.; Nezafat, Z.; Javanshir, S. Biomedical and Environmental Applications of Carrageenan-Based Hydrogels: A Review. J. Polym. Environ. 2022, 31, 1679–1705. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Nie, M.; Wen, H.; Xu, W.; Fan, L.; Cao, Q. Preparation and characterization of carboxymethyl chitosan sulfate/oxidized konjac glucomannan hydrogels. Int. J. Biol. Macromol. 2018, 113, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Wang, L.F. Mechanical and water barrier properties of agar/κ-carrageenan/konjac glucomannan ternary blend biohydrogel films. Carbohydr. Polym. 2013, 96, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Vermonden, T.; Klumperman, B. The past, present and future of hydrogels. Eur. Polym. J. 2015, 72, 341–343. [Google Scholar] [CrossRef]
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomater. 2014, 10, 1671–1682. [Google Scholar] [CrossRef]
- Monjazeb Marvdashti, L.; Koocheki, A.; Yavarmanesh, M. Alyssum homolocarpum seed gum-polyvinyl alcohol biodegradable composite film: Physicochemical, mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 155, 280–293. [Google Scholar] [CrossRef]
- Hilliou, L. Structure–Elastic Properties Relationships in Gelling Carrageenans. Polymers 2021, 13, 4120. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Falqué, E.; Domínguez, H.; Torres, M.D. Green Extraction of Carrageenans from Mastocarpus stellatus. Polymers 2022, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.T.; Cuong, D.X.; Thuy, L.H.; Thuan, P.T.; Tuyen, D.T.T.; Mo, V.T.; Dong, D.H. Carrageenan of Red Algae Eucheuma gelatinae: Extraction, Antioxidant Activity, Rheology Characteristics, and Physicochemistry Characterization. Molecules 2022, 27, 1268. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wallace, G.G. Flexible Electrodes and Electrolytes for Energy Storage. Electrochim. Acta 2015, 175, 87–95. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Nouri, A.; Tavakkoli Yaraki, M.; Ghorbanpour, M.; Wang, S. Biodegradable κ-carrageenan/nanoclay nanocomposite films containing Rosmarinus officinalis L. extract for improved strength and antibacterial performance. Int. J. Biol. Macromol. 2018, 115, 227–235. [Google Scholar] [CrossRef]
- Ozolina, S.; Zaimis, U. Some Aspects of Extraction and Application of Seaweed Furcellaria lumbricalis Carrageenan in Production of Recycled Paper. In Proceedings of the 21st International Scientific Conference on Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2022. [Google Scholar] [CrossRef]
- Shafie, M.H.; Kamal, M.L.; Zulkiflee, F.F.; Hasan, S.; Uyup, N.H.; Abdullah, S.; Mohamed Hussin, N.A.; Tan, Y.C.; Zafarina, Z. Application of Carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. J. Indian Chem. Soc. 2022, 99, 100613. [Google Scholar] [CrossRef]
- Thevanayagam, H.; Mohamed, S.M.; Chu, W.L. Assessment of UVB-photoprotective and antioxidative activities of carrageenan in keratinocytes. J. Appl. Phycol. 2014, 26, 1813–1821. [Google Scholar] [CrossRef]
- Ijaz, U.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kazi, M.; Shah, S.A.; Mahmood, A.; Maniruzzaman, M. Biofunctional Hyaluronic Acid/κ-Carrageenan Injectable Hydrogels for Improved Drug Delivery and Wound Healing. Polymers 2022, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef]
- Cielecka, I.; Szustak, M.; Gendaszewska-Darmach, E.; Kalinowska, H.; Ryngajllo, M.; Maniukiewicz, W.; Bielecki, S. Novel Bionanocellulose/κ-Carrageenan Composites for Tissue Engineering. Appl. Sci. 2018, 8, 1352. [Google Scholar] [CrossRef]
- Lu, S.; Ma, Z.; Qin, L.; Ding, M.; Wang, Z.; Liu, J.; Zhang, R.; Zhang, Y.; Dong, G. Effect of molecular helical structure on self-growing and damping of single network κ-carrageenan hydrogel. Int. J. Biol. Macromol. 2023, 249, 126082. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A. The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review. Clin. Epidemiol. Glob. Health 2021, 12, 100826. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Davydova, V.N.; Krylova, N.V.; Iunikhina, O.V.; Volod’ko, A.V.; Pimenova, E.A.; Shchelkanov, M.Y.; Yermak, I.M. Physicochemical Properties and Antiherpetic Activity of κ-Carrageenan Complex with Chitosan. Mar. Drugs 2023, 21, 238. [Google Scholar] [CrossRef]
- Pangestuti, R.; Shin, K.H.; Kim, S.K. Anti-Photoaging and Potential Skin Health Benefits of Seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef]
- Fauzi, M.A.R.D.; Pudjiastuti, P.; Wibowo, A.C.; Hendradi, E. Preparation, Properties and Potential of Carrageenan-Based Hard Capsules for Replacing Gelatine: A Review. Polymers 2021, 13, 2666. [Google Scholar] [CrossRef]
- Tarman, K.; Sadi, U.; Santoso, J.; Hardjito, L. Carrageenan and its Enzymatic Extraction. In Encyclopedia of Marine Biotechnology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 147–159. [Google Scholar] [CrossRef]
- Pogozhykh, D.; Posokhov, Y.; Myasoedov, V.; Gubina-Vakulyck, G.; Chumachenko, T.; Knigavko, O.; Polikarpova, H.; Kalashnyk-Vakulenko, Y.; Sharashydze, K.; Nakonechna, O.; et al. Experimental Evaluation of Food-Grade Semi-Refined Carrageenan Toxicity. Int. J. Mol. Sci. 2021, 22, 11178. [Google Scholar] [CrossRef]
- Avila, L.B.; Barreto, E.R.C.; Moraes, C.C.; Morais, M.M.; da Rosa, G.S. Promising New Material for Food Packaging: An Active and Intelligent Carrageenan Film with Natural Jaboticaba Additive. Foods 2022, 11, 792. [Google Scholar] [CrossRef]
- Kokkuvayil Ramadas, B.; Rhim, J.W.; Roy, S. Recent Progress of Carrageenan-Based Composite Films in Active and Intelligent Food Packaging Applications. Polymers 2024, 16, 1001. [Google Scholar] [CrossRef]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef]
- Lee, C. Carrageenans as Broad-Spectrum Microbicides: Current Status and Challenges. Mar. Drugs 2020, 18, 435. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Kadyirov, A.I.; Ziganshina, S.A.; Kazantseva, M.A.; Zueva, O.S.; Gubaidullin, A.T.; Zuev, Y.F. Supramolecular Structure and Mechanical Performance of κ-Carrageenan–Gelatin Gel. Polymers 2022, 14, 4347. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kim, S.S.; Lee, J. Novel synergistic transparent k-Carrageenan/Xanthan gum/Gellan gum hydrogel film: Mechanical, thermal and water barrier properties. Int. J. Biol. Macromol. 2018, 118, 561–568. [Google Scholar] [CrossRef]
- Humayun, S.; Premarathna, A.D.; Rjabovs, V.; Howlader, M.M.; Darko, C.N.S.; Mok, I.K.; Tuvikene, R. Biochemical Characteristics and Potential Biomedical Applications of Hydrolyzed Carrageenans. Mar. Drugs 2023, 21, 269. [Google Scholar] [CrossRef]
- Lim, W.; Kim, G.J.; Kim, H.W.; Lee, J.; Zhang, X.; Kang, M.G.; Seo, J.W.; Cha, J.M.; Park, H.J.; Lee, M.Y.; et al. Kappa-Carrageenan-Based Dual Crosslinkable Bioink for Extrusion Type Bioprinting. Polymers 2020, 12, 2377. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, A.; Li, Y.; Chang, Y.; Xue, C.; Tang, Q. The risk of carrageenan-induced colitis is exacerbated under high-sucrose/high-salt diet. Int. J. Biol. Macromol. 2022, 210, 475–482. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.S. Biological activity of algal derived carrageenan: A comprehensive review in light of human health and disease. Int. J. Biol. Macromol. 2023, 238, 124085. [Google Scholar] [CrossRef]
- Qamar, S.A.; Junaid, M.; Riasat, A.; Jahangeer, M.; Bilal, M.; Mu, B.-Z.; Qamar, S.A.; Mu, B.-Z.; Junaid, M.; Riasat, A.; et al. Carrageenan-Based Hybrids with Biopolymers and Nano-Structured Materials for Biomimetic Applications. Starch 2024, 76, 2200018. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Azeman, N.H.; Mobarak, N.N.; Mokhtar, M.H.H.; Bakar, A.A.A. Effect of Active Site Modification towards Performance Enhancement in Biopolymer κ-Carrageenan Derivatives. Polymers 2020, 12, 2040. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Mohamad Ibrahim, M.N.; Sekeri, S.H.; Ansari, M.T.; Nanda, A.; Ahmad, G. Bacteria Mediated Synthesis of Iron Oxide Nanoparticles and Their Antibacterial, Antioxidant, Cytocompatibility Properties. J. Clust. Sci. 2021, 32, 1083–1094. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Townsend, T.K.; Sabio, E.M.; Browning, N.D.; Osterloh, F.E. Photocatalytic water oxidation with suspended alpha-Fe2O3 particles-effects of nanoscaling. Energy Environ. Sci. 2011, 4, 4270–4275. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, H.; Kuang, C.; Wang, W.; Hu, Y.; Yang, X. Boosting charge transfer for α-Fe2O3 semiconductor with the coupling of chiral monolayer. Mater. Lett. 2022, 308, 131130. [Google Scholar] [CrossRef]
- Yang, Y.; Ratner, M.A.; Schatz, G.C. Computational modeling of octahedral iron oxide clusters: Hexaaquairon(III) and its dimers. J. Phys. Chem. C 2013, 117, 21706–21717. [Google Scholar] [CrossRef]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Kralj, S.; Jagodic, M.; Hanzel, D.; Makovec, D. Magnetic properties of novel superparamagnetic iron oxide nanoclusters and their peculiarity under annealing treatment. Appl. Surf. Sci. 2014, 322, 255–264. [Google Scholar] [CrossRef]
- Kopanja, L.; Milosevic, I.; Panjan, M.; Damnjanovic, V.; Tadic, M. Sol–gel combustion synthesis, particle shape analysis and magnetic properties of hematite (α-Fe2O3) nanoparticles embedded in an amorphous silica matrix. Appl. Surf. Sci. 2016, 362, 380–386. [Google Scholar] [CrossRef]
- Soysal, F.; Çıplak, Z.; Getiren, B.; Gökalp, C.; Yıldız, N. Synthesis and characterization of reduced graphene oxide-iron oxide-polyaniline ternary nanocomposite and determination of its photothermal properties. Mater. Res. Bull. 2020, 124, 110763. [Google Scholar] [CrossRef]
- Vassallo, M.; Martella, D.; Barrera, G.; Celegato, F.; Coïsson, M.; Ferrero, R.; Olivetti, E.S.; Troia, A.; Sözeri, H.; Parmeggiani, C.; et al. Improvement of Hyperthermia Properties of Iron Oxide Nanoparticles by Surface Coating. ACS Omega 2022, 8, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.G.; Lopez-Barbera, J.F.; Lafuente, A.; Özel, F.; Fantechi, E.; Muro-Cruces, J.; Hémadi, M.; Sepulveda, B.; Nogues, J. Iron oxide nanoparticles (Fe3O4, γ-Fe2O3 and FeO) as photothermal heat mediators in the first, second and third biological windows. Phys. Rep. 2023, 1043, 1–35. [Google Scholar] [CrossRef]
- Serna, C.J.; Bødker, F.; Mørup, S.; Morales, M.P.; Sandiumenge, F.; Veintemillas-Verdaguer, S. Spin frustration in maghemite nanoparticles. Solid State Commun. 2001, 118, 437–440. [Google Scholar] [CrossRef]
- Dutta, P.; Manivannan, A.; Seehra, M.S.; Shah, N.; Huffman, G.P. Magnetic properties of nearly defect-free maghemite nanocrystals. Phys. Rev. B—Condens. Matter Mater. Phys. 2004, 70, 174428. [Google Scholar] [CrossRef]
- de Montferrand, C.; Lalatonne, Y.; Bonnin, D.; Lièvre, N.; Lecouvey, M.; Monod, P.; Russier, V.; Motte, L.; de Montferrand, C.; Lalatonne, Y.; et al. Size-Dependent Nonlinear Weak-Field Magnetic Behavior of Maghemite Nanoparticles. Small 2012, 8, 1945–1956. [Google Scholar] [CrossRef]
- De Montferrand, C.; Lalatonne, Y.; Bonnin, D.; Motte, L.; Monod, P. Non-linear magnetic behavior around zero field of an assembly of superparamagnetic nanoparticles. Analyst 2012, 137, 2304–2308. [Google Scholar] [CrossRef]
- De Montferrand, C.; Hu, L.; Milosevic, I.; Russier, V.; Bonnin, D.; Motte, L.; Brioude, A.; Lalatonne, Y. Iron oxide nanoparticles with sizes, shapes and compositions resulting in different magnetization signatures as potential labels for multiparametric detection. Acta Biomater. 2013, 9, 6150–6157. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Díaz-García, Á.; Law, J.Y.; Romero, A.; Franco, V.; Guerrero, A. Quantifying the Structure and Properties of Nanomagnetic Iron Oxide Particles for Enhanced Functionality through Chemical Synthesis. Nanomaterials 2023, 13, 2242. [Google Scholar] [CrossRef] [PubMed]
- Iordanova, N.; Dupuis, M.; Rosso, K.M. Charge transport in metal oxides: A theoretical study of hematite α-Fe2O3. J. Chem. Phys. 2005, 122, 144305. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, C.M. The surface structure of α-Fe2O3 (001) by scanning tunneling microscopy: Implications for interfacial electron transfer reactions. Am. Mineral. 1999, 84, 1061–1070. [Google Scholar] [CrossRef]
- He, Y.P.; Miao, Y.M.; Li, C.R.; Wang, S.Q.; Cao, L.; Xie, S.S.; Yang, G.Z.; Zou, B.S.; Burda, C. Size and structure effect on optical transitions of iron oxide nanocrystals. Phys. Rev. B—Condens. Matter Mater. Phys. 2005, 71, 125411. [Google Scholar] [CrossRef]
- Van Sang, L.; Yano, A.; Isohashi, A.; Sugimura, N.; Washizu, H. Smoothed particle hydrodynamics study of friction of the coarse-grained α-Al2O3/α-Al2O3 and α-Fe2O3/α-Fe2O3 contacts in behavior of the spring interfacial potential. Tribol. Int. 2019, 135, 296–304. [Google Scholar] [CrossRef]
- Mann, D.J.; Zhong, L.; Hase, W.L. Effect of surface stiffness on the friction of sliding model hydroxylated α-alumina surfaces. J. Phys. Chem. B 2001, 105, 12032–12045. [Google Scholar] [CrossRef]
- Gao, J.; Luedtke, W.D.; Landman, U. Friction control in thin-film lubrication. J. Phys. Chem. B 1998, 102, 5033–5037. [Google Scholar] [CrossRef]
- Robbins, M.O.; Smith, E.D. Connecting molecular-scale and macroscopic tribology. Langmuir 1996, 12, 4543–4547. [Google Scholar] [CrossRef]
- Wu, H.; Wu, G.; Wang, L. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: Facile synthesis and electromagnetic properties. Powder Technol. 2015, 269, 443–451. [Google Scholar] [CrossRef]
- Qin, Q.; Olimov, D.; Yin, L. Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene. Chemosensors 2022, 10, 267. [Google Scholar] [CrossRef]
- Ishii, M.; Yamashita, Y.; Watanabe, S.; Ariga, K.; Takeya, J. Doping of molecular semiconductors through proton-coupled electron transfer. Nature 2023, 622, 285–291. [Google Scholar] [CrossRef]
- Klug, J.N.; Lutz, J.; Meijer, J.B. N-type doping of silicon by proton implantation. In Proceedings of the 2011 14th European Conference on Power Electronics and Application, Birmingham, UK, 30 August–1 September 2011. IEEE Conference Publication; IEEE Xplore. [Google Scholar]
- Shah, M.A.K.Y.; Lu, Y.; Mushtaq, N.; Yousaf, M.; Akbar, M.; Rauf, S.; Dong, Y.; Lund, P.D.; Zhu, B.; Asghar, M.I. Enabling high ionic conductivity in semiconductor electrolyte membrane by surface engineering and band alignment for LT-CFCs. J. Memb. Sci. 2023, 668, 121264. [Google Scholar] [CrossRef]
- Rasool, S.; Akbar, N.; Shah, M.A.K.Y.; Afzal, M.; Sarfraz; Zhu, B. Insight of proton transport phenomena in semiconductor ionic materials. J. Power Sources 2024, 598, 234148. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Macromolecules in ionic liquids: Progress, challenges, and opportunities. Macromolecules 2008, 41, 3739–3749. [Google Scholar] [CrossRef]
- Colomer, M.T.; Zenzinger, K. Mesoporous α-Fe2O3 membranes as proton conductors: Synthesis by microwave-assisted sol–gel route and effect of their textural characteristics on water uptake and proton conductivity. Microporous Mesoporous Mater. 2012, 161, 123–133. [Google Scholar] [CrossRef]
- Zaimis, U.; Ozolina, S.; Kukuskins, A. Recycled Algae Paper Density Control System for Quality Screening with Adjustable Light Source Wavelength. In Proceedings of the 21st International Scientific Conference on Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2022. [Google Scholar] [CrossRef]
- Žaimis, U.; Petronienė, J.J.; Dzedzickis, A.; Bučinskas, V. Biodegradable Carrageenan-Based Force Sensor: An Experimental Approach. Sensors 2023, 23, 9423. [Google Scholar] [CrossRef] [PubMed]
- Šutinys, E.; Dzedzickis, A.; Samukaitė-Bubnienė, U.; Bučinskas, V. Novel synthetic iron (III) oxide-based force sensor. Sens. Actuators A Phys. 2021, 331, 113043. [Google Scholar] [CrossRef]
- Schwertmann, U.; Taylor, R.M. Iron Oxides. In Minerals in Soil Environments; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 379–438. [Google Scholar] [CrossRef]
- Masthoff, I.C.; Kraken, M.; Menzel, D.; Litterst, F.J.; Garnweitner, G. Study of the growth of hydrophilic iron oxide nanoparticles obtained via the non-aqueous sol–gel method. J. Sol-Gel Sci. Technol. 2016, 77, 553–564. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y.; Qian, J. Compression sensibility of magnetic-concentrated fly ash mortar under uniaxial loading. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2012, 27, 999–1003. [Google Scholar] [CrossRef]
- Morris, E.R.; Williams, Q. Electrical resistivity of Fe3O4 to 48 GPa: Compression-induced changes in electron hopping at mantle pressures. J. Geophys. Res. Solid Earth 1997, 102, 18139–18148. [Google Scholar] [CrossRef]
- Alam, K. Electrical and Electronic Properties of Materials; IntechOpen: London, UK, 2019. [Google Scholar]
- Williams, A.G.B.; Scherer, M.M. Spectroscopic evidence for Fe(II)-Fe(III) electron transfer at the iron oxide-water interface. Environ. Sci. Technol. 2004, 38, 4782–4790. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Petrović, Ž.; Metikoš-Huković, M.; Babić, R. The electrochemical transfer reactions and the structure of the iron|oxide layer|electrolyte interface. Electrochim. Acta 2012, 75, 406–413. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bučinskas, V.; Udris, D.; Dzedzickis, A.; Petronienė, J.J. Piezoelectric Behaviour in Biodegradable Carrageenan and Iron (III) Oxide Based Sensor. Sensors 2024, 24, 4622. https://doi.org/10.3390/s24144622
Bučinskas V, Udris D, Dzedzickis A, Petronienė JJ. Piezoelectric Behaviour in Biodegradable Carrageenan and Iron (III) Oxide Based Sensor. Sensors. 2024; 24(14):4622. https://doi.org/10.3390/s24144622
Chicago/Turabian StyleBučinskas, Vytautas, Dainius Udris, Andrius Dzedzickis, and Jūratė Jolanta Petronienė. 2024. "Piezoelectric Behaviour in Biodegradable Carrageenan and Iron (III) Oxide Based Sensor" Sensors 24, no. 14: 4622. https://doi.org/10.3390/s24144622
APA StyleBučinskas, V., Udris, D., Dzedzickis, A., & Petronienė, J. J. (2024). Piezoelectric Behaviour in Biodegradable Carrageenan and Iron (III) Oxide Based Sensor. Sensors, 24(14), 4622. https://doi.org/10.3390/s24144622








