Evaluating the Electroencephalographic Signal Quality of an In-Ear Wearable Device
Abstract
1. Introduction
2. Materials and Methods
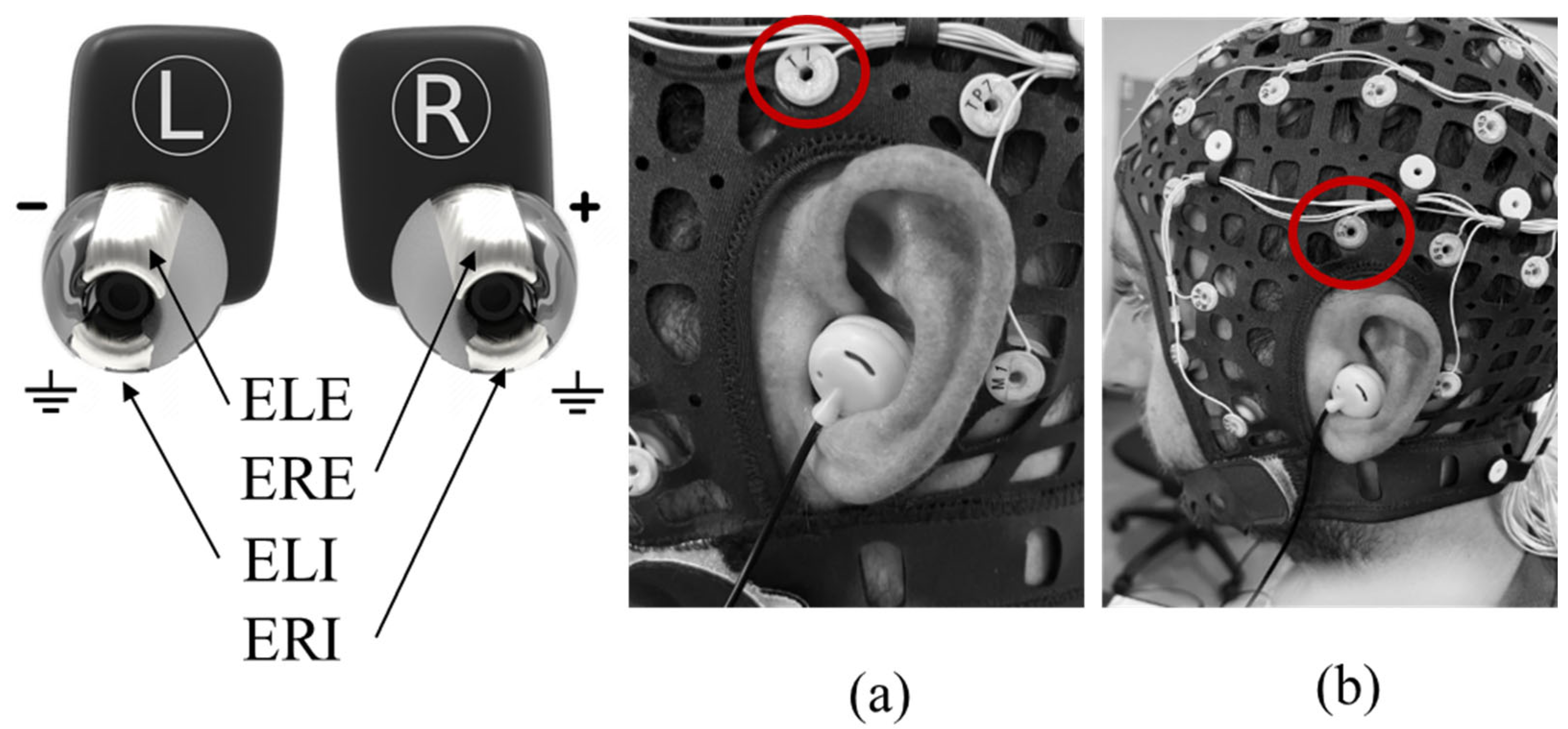
2.1. In-Ear Device
2.2. Hardware in the Loop Test
2.3. Scalp EEG Acquisition System
2.4. Synchronization System
2.5. Resting State Protocols
2.5.1. EEG Recordings
2.5.2. Data Analysis
2.6. Sleep Protocol
2.6.1. EEG Recordings
2.6.2. Data Analysis
2.7. Statistics
3. Results
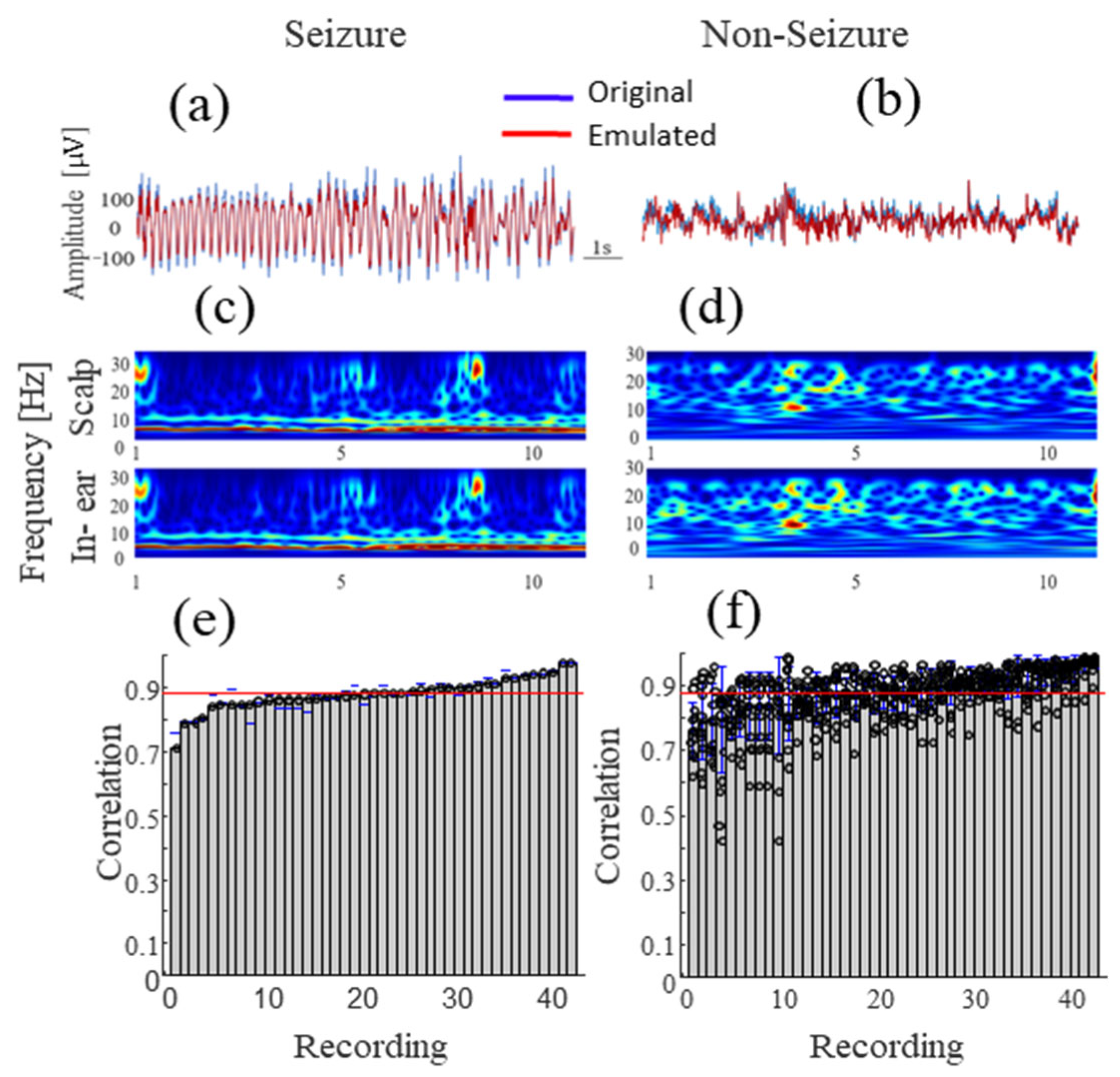
3.1. Hardware in the Loop Test
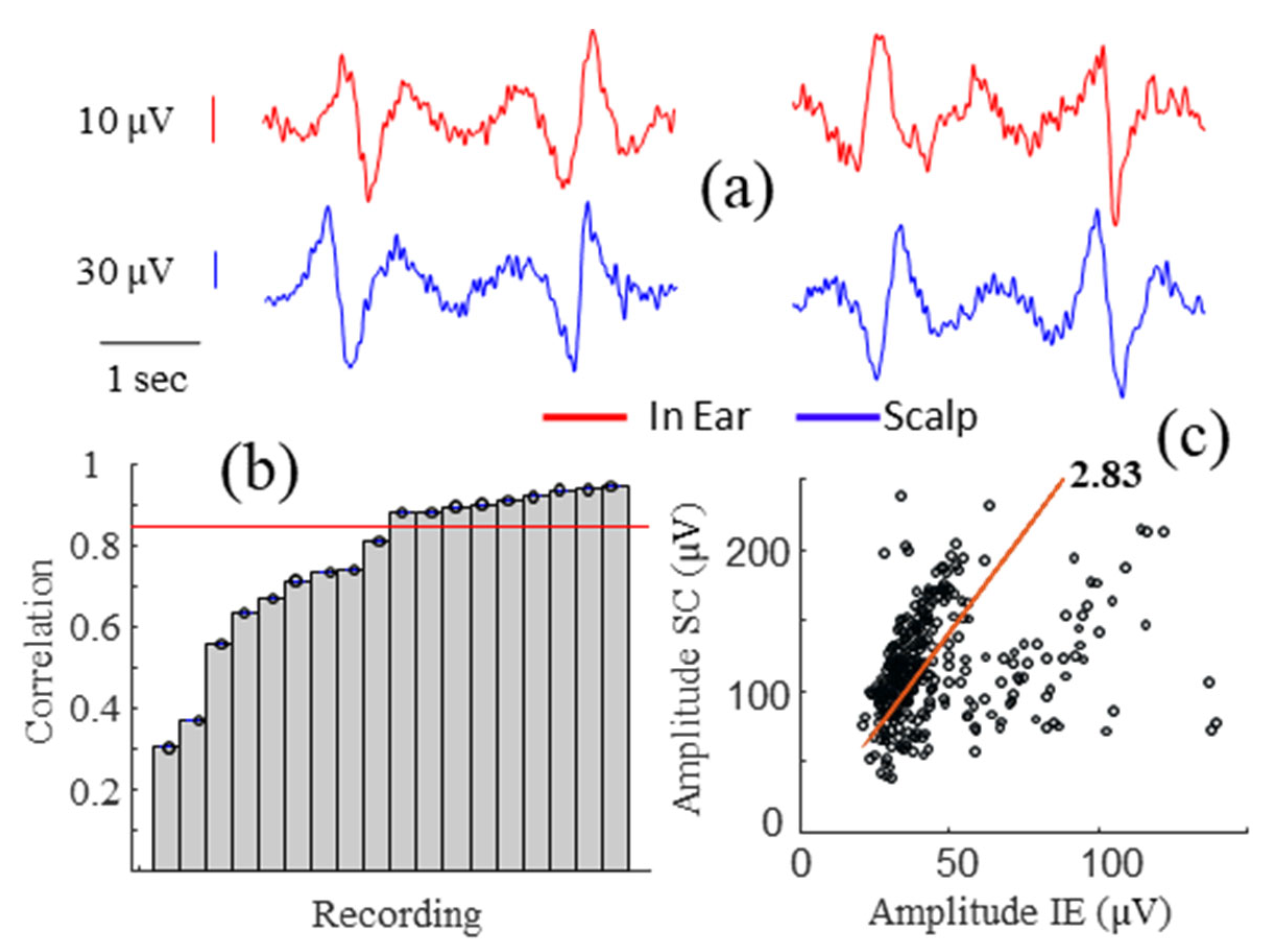
3.2. Alpha Wave Correlations
3.3. Correlations during Different Levels of Head Movements and Facial Contractions
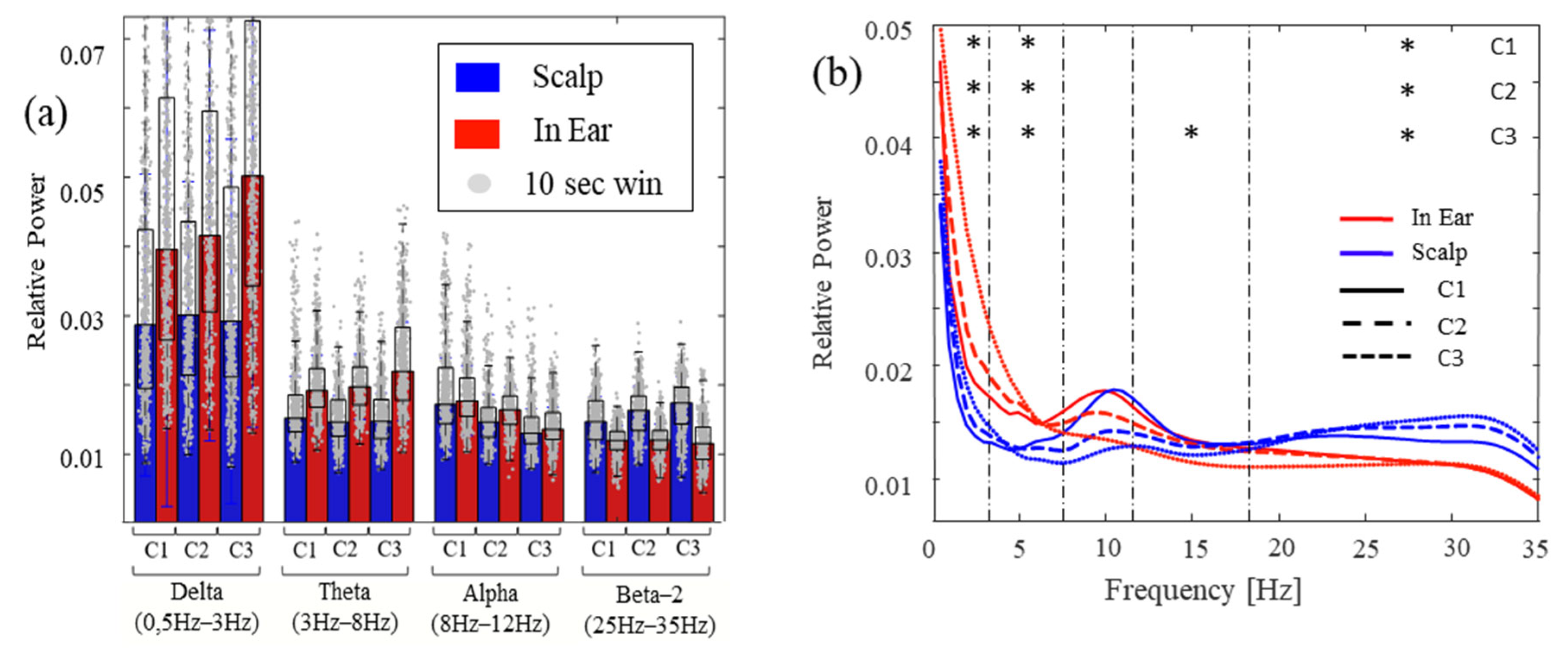
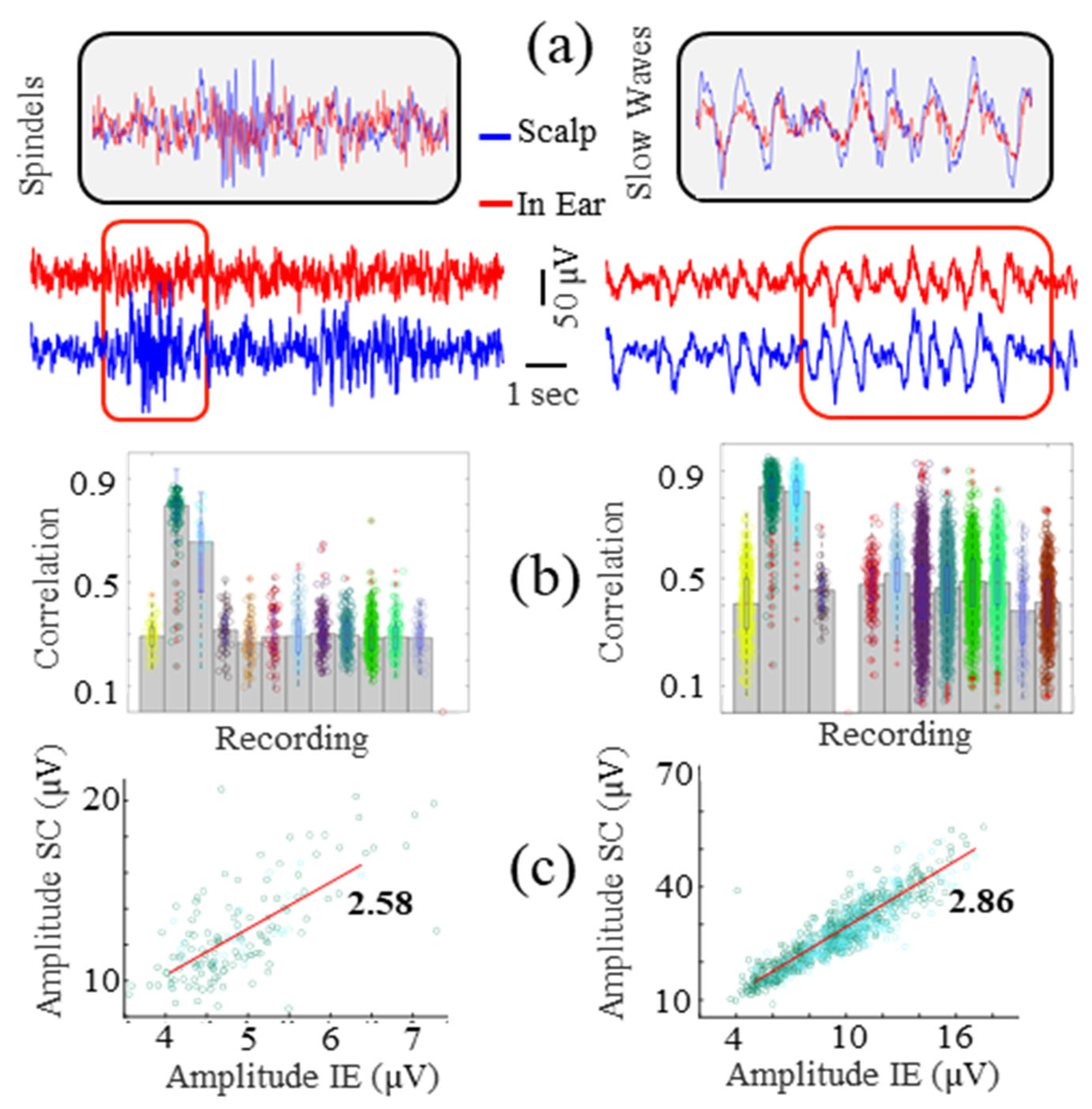
3.4. Sleep Slow Waves and Spindle Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaongoen, N.; Choi, J.; Choi, J.W.; Kwon, H.; Hwang, C.; Hwang, G.; Kim, B.H.; Jo, S. The future of wearable EEG: A review of ear-EEG technology and its applications. J. Neural Eng. 2023, 20, 051002. [Google Scholar] [CrossRef]
- Röddiger, T.; Clarke, C.; Breitling, P.; Schneegans, T.; Zhao, H.; Gellersen, H.; Beigl, M. Sensing with Earables: A Systematic Literature Review and Taxonomy of Phenomena. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2022, 6, 1–57. [Google Scholar] [CrossRef]
- Looney, D.; Park, C.; Kidmose, P.; Rank, M.L.; Ungstrup, M.; Rosenkranz, K.; Mandic, D.P. An in-the-ear platform for recording electroencephalogram. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: New York, NY, USA, 2011; pp. 6882–6885. [Google Scholar] [CrossRef]
- Goverdovsky, V.; von Rosenberg, W.; Nakamura, T.; Looney, D.; Sharp, D.J.; Papavassiliou, C.; Morrell, M.J.; Mandic, D.P. Hearables: Multimodal physiological in-ear sensing. Sci. Rep. 2017, 7, 6948. [Google Scholar] [CrossRef]
- Mikkelsen, K.B.; Kappel, S.L.; Mandic, D.P.; Kidmose, P. EEG Recorded from the Ear: Characterizing the Ear-EEG Method. Front. Neurosci. 2015, 9, 438. [Google Scholar] [CrossRef]
- Correia, G.; Crosse, M.J.; Valdes, A.L. Brain Wearables: Validation Toolkit for Ear-Level EEG Sensors. Sensors 2024, 24, 1226. [Google Scholar] [CrossRef]
- Kaveh, R.; Doong, J.; Zhou, A.; Schwendeman, C.; Gopalan, K.; Burghardt, F.L.; Arias, A.C.; Maharbiz, M.M.; Muller, R. Wireless User-Generic Ear EEG. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 727–737. [Google Scholar] [CrossRef]
- Goverdovsky, V.; Looney, D.; Kidmose, P.; Mandic, D.P. In-Ear EEG from Viscoelastic Generic Earpieces: Robust and Un-obtrusive 24/7 Monitoring. IEEE Sens. J. 2016, 16, 271–277. [Google Scholar] [CrossRef]
- Tabar, Y.R.; Mikkelsen, K.B.; Shenton, N.; Kappel, S.L.; Bertelsen, A.R.; Nikbakht, R.; Toft, H.O.; Henriksen, C.H.; Hemmsen, M.C.; Rank, M.L.; et al. At-home sleep monitoring using generic ear-EEG. Front. Neurosci. 2023, 17, 987578. [Google Scholar] [CrossRef]
- Athavipach, C.; Pan-Ngum, S.; Israsena, P. A Wearable In-Ear EEG Device for Emotion Monitoring. Sensors 2019, 19, 4014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Gamper, H.; Tashev, I.; Dong, S.; Ma, S.; Remaley, J. Stress Monitoring using Multimodal Bio-sensing Headset. In Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, Honolulu, HI, USA, 25–30 April 2020; ACM: New York, NY, USA, 2020; pp. 1–7. [Google Scholar]
- Christensen, C.B.; Harte, J.M.; Lunner, T.; Kidmose, P. Ear-EEG-Based Objective Hearing Threshold Estimation Evaluated on Normal Hearing Subjects. IEEE Trans. Biomed. Eng. 2018, 65, 1026–1034. [Google Scholar] [CrossRef]
- Merrill, N.; Curran, M.T.; Gandhi, S.; Chuang, J. One-Step, Three-Factor Passthought Authentication with Custom-Fit, In-Ear EEG. Front. Neurosci. 2019, 13, 354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, S.; Kwon, H.; Choi, S.H.; Park, K.S. Intelligent system for drowsiness recognition based on ear canal electroencephalography with photoplethysmography and electrocardiography. Inf. Sci. 2018, 453, 302–322. [Google Scholar] [CrossRef]
- Mikkelsen, K.B.; Villadsen, D.B.; Otto, M.; Kidmose, P. Automatic sleep staging using ear-EEG. Biomed. Eng. Online 2017, 16, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zibrandtsen, I.; Kidmose, P.; Christensen, C.; Kjaer, T. Ear-EEG detects ictal and interictal abnormalities in focal and generalized epilepsy—A comparison with scalp EEG monitoring. Clin. Neurophysiol. 2017, 128, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.; Hsu, S.-H.; Martin, S.; Dwyer, J.; Chen, D.F.; Sameni, R.; Waters, S.H.; Borodin, K.; Clifford, G.D.; Levey, A.I.; et al. Using a standalone ear-EEG device for focal-onset seizure detection. Bioelectron. Med. 2024, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Frederiksen, K.S.; Andersen, B.B.; Høgh, P.; Kidmose, P.; Fabricius, M.; Hribljan, M.C.; Hemmsen, M.C.; Rank, M.L.; Waldemar, G.; et al. Detection of subclinical epileptiform discharges in Alzheimer’s disease using long-term outpatient EEG monitoring. Neurobiol. Dis. 2023, 183, 106149. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Kjær, T.W.; Hribljan, M.C.; Andersen, B.B.; Høgh, P.; Kidmose, P.; Fabricius, M.; Hemmsen, M.C.; Rank, M.L.; Waldemar, G.; et al. Subclinical Epileptiform Activity in Dementia with Lewy Bodies. Mov. Disord. 2023, 38, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Kidmose, P.; Looney, D.; Mandic, D.P. Auditory evoked responses from Ear-EEG recordings. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 586–589. [Google Scholar] [CrossRef]
- Zibrandtsen, I.; Kidmose, P.; Otto, M.; Ibsen, J.; Kjaer, T. Case comparison of sleep features from ear-EEG and scalp-EEG. Sleep Sci. 2016, 9, 69–72. [Google Scholar] [CrossRef]
- Mikkelsen, K.B.; Tabar, Y.R.; Kappel, S.L.; Christensen, C.B.; Toft, H.O.; Hemmsen, M.C.; Rank, M.L.; Otto, M.; Kidmose, P. Accurate whole-night sleep monitoring with dry-contact ear-EEG. Sci. Rep. 2019, 9, 16824. [Google Scholar] [CrossRef]
- Kappel, S.L.; Looney, D.; Mandic, D.P.; Kidmose, P. Physiological artifacts in scalp EEG and ear-EEG. Biomed. Eng. Online 2017, 16, 103. [Google Scholar] [CrossRef]
- Merrill, N.; Curran, M.T.; Yang, J.-K.; Chuang, J. Classifying mental gestures with in-ear EEG. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; pp. 130–135. [Google Scholar] [CrossRef]
- Kappel, S.L.; Rank, M.L.; Toft, H.O.; Andersen, M.; Kidmose, P. Dry-Contact Electrode Ear-EEG. IEEE Trans. Biomed. Eng. 2018, 66, 150–158. [Google Scholar] [CrossRef]
- Tautan, A.-M.; Mihajlovic, V.; Chen, Y.-H.; Grundlehner, B.; Penders, J.; Serdijn, W. Signal Quality in Dry Electrode EEG and the Relation to Skin-electrode Contact Impedance Magnitude. In Proceedings of the International Conference on Biomedical Electronics and Devices, ESEO, Angers, France, 3–6 March 2014; SCITEPRESS—Science and and Technology Publications: Setúbal, Portugal, 2014; pp. 12–22. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef]
- Detti, P. Siena Scalp EEG Database; PhysioNet, MIT Laboratory for Computational Physiology: Cambridge, MA, USA. [CrossRef]
- Detti, P.; Vatti, G.; de Lara, G.Z.M. EEG Synchronization Analysis for Seizure Prediction: A Study on Data of Noninvasive Recordings. Processes 2020, 8, 846. [Google Scholar] [CrossRef]
- Sinha, S.R.; Sullivan, L.; Sabau, D.; San-Juan, D.; Dombrowski, K.E.; Halford, J.J.; Hani, A.J.; Drislane, F.W.; Stecker, M.M. American Clinical Neurophysiology Society Guideline 1: Minimum Technical Requirements for Performing Clinical Electroencephalography. J. Clin. Neurophysiol. 2016, 33, 303–307. [Google Scholar] [CrossRef]
- BSCN|Practice of Electroencephalography. Consulté le: 12 Février 2024. [En ligne]. Disponible sur. Available online: https://www.bscn.org.uk/content_wide.aspx?group=guidelines&page=guidelines_eeg (accessed on 12 February 2024).
- Morlet, J.; Arens, G.; Fourgeau, E.; Giard, D. Wave propagation and sampling theory—Part II: Sampling theory and complex waves. Geophysics 1982, 47, 222–236. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Perslev, M.; Darkner, S.; Kempfner, L.; Nikolic, M.; Jennum, P.J.; Igel, C. U-Sleep: Resilient high-frequency sleep staging. npj Digit. Med. 2021, 4, 72. [Google Scholar] [CrossRef]
- Butar, B.F.; Park, J.W. Permutation tests for comparing two populations. J. Math. Sci. Math. Educ. 2008, 3, 19–30. [Google Scholar]
- Holt, C.A.; Sullivan, S.P. Permutation tests for experimental data. Exp. Econ. 2023, 26, 775–812. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Daniel, W.W.; Cross, C.L. Biostatistics—A Foundation for Analysis in the Health Sciences, 10th ed.; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-118-30279-8. [Google Scholar]
- Theiler, J.; Eubank, S.; Longtin, A.; Galdrikian, B.; Farmer, J.D. Testing for nonlinearity in time series: The method of surrogate data. Phys. D Nonlinear Phenom. 1992, 58, 77–94. [Google Scholar] [CrossRef]
- Haaga, K.A.; Datseris, G. TimeseriesSurrogates.jl: A Julia package for generating surrogate data. J. Open Source Softw. 2022, 7, 4414. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- McLaren, J.R.; Jing, J.; Westover, M.B.; Nascimento, F.A. Journal Club: Criteria for Defining Interictal Epileptiform Discharges in EEG. Neurology 2022, 99, 430–432. [Google Scholar] [CrossRef]
- Kappel, S.L.; Makeig, S.; Kidmose, P. Ear-EEG Forward Models: Improved Head-Models for Ear-EEG. Front. Neurosci. 2019, 13, 943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henao, D.; Navarrete, M.; Juez, J.Y.; Dinh, H.; Gómez, R.; Valderrama, M.; Le Van Quyen, M. Auditory closed-loop stimulation on sleep slow oscillations using in-ear EEG sensors. J. Sleep Res. 2022, 31, e13555. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, N.; Zhang, Y.; Zheng, N.; Li, H.; Jiao, Y.; Cheng, J.; Wang, Y.; Zhang, X.; Chen, Y.; et al. Conformal in-ear bioelectronics for visual and auditory brain-computer interfaces. Nat. Commun. 2023, 14, 4213. [Google Scholar] [CrossRef]
- Kidmose, P.; Looney, D.; Jochumsen, L.; Mandic, D.P. Ear-EEG from generic earpieces: A feasibility study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 543–546. [Google Scholar] [CrossRef]
- Occhipinti, E.; Davies, H.J.; Hammour, G.; Mandic, D.P. Hearables: Artefact removal in Ear-EEG for continuous 24/7 monitoring. In Proceedings of the 2022 International Joint Conference on Neural Networks (IJCNN), Padua, Italy, 18–23 July 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Seok, D.; Lee, S.; Kim, M.; Cho, J.; Kim, C. Motion Artifact Removal Techniques for Wearable EEG and PPG Sensor Systems. Front. Electron. 2021, 2, 685513. [Google Scholar] [CrossRef]



| Features | NAOX Earbuds |
|---|---|
| Skin Contact Location | Ear canal |
| Electrodes | 2 actives and dry silver electrodes by ear tip |
| Channel | 1 single bipolar EEG channel left ear–right ear |
| Sampling Frequency | 250 Hz |
| Transfer of Data | Bluetooth 2.4 GHz |
| Data Format | The European Data Format (EDF) |
| Autonomy | ~10 h |
| Weight | 20 g |
| Input noise | <6 μV peak to valley |
| CMRR | >80 dB at 50 Hz |
| Tasks | Duration 1 | Condition |
|---|---|---|
| Alpha paradigm | 5 | C1 |
| Artifact paradigm | 2 | C3 |
| Hyperventilation | 2-3-2 | C3 |
| PVT | 10 | C2 |
| Reading text | 10 | C3 |
| Emotion recognition | 5 | C2 |
| Relaxation period | 10 | C1 |
| IE vs. Scalp p-Value | Delta | Theta | Alpha | Beta-1 (12–18 Hz) | Beta-2 (18–35 Hz) |
|---|---|---|---|---|---|
| C1 | 0.00625 | 0.029375 | 0.648125 | 0.548125 | 0.001875 |
| C2 | 0.006875 | 0.00062461 | 0.20375 | 0.775625 | 0.00062461 |
| C3 | 0.0025 | 0.00062461 | 0.935 | 0.039375 | 0.00062461 |
| Pattern | Number of Recording | Correlation Mean ± Std | Statistically Significant Cases (p < 0.05) |
|---|---|---|---|
| Seizures | 45 | 0.87 ± 0.08 | 45/45 |
| Non-Seizures | 45 | 0.86 ± 0.09 | 45/45 |
| Alpha waves | 18 | 0.76 ± 0.19 | 15/18 |
| Artifacts | 18 | 0.87 ± 0.19 | 18/18 |
| Spindles | 12 | 0.38 ± 0.15 | 11/12 |
| Slow waves | 12 | 0.52 ± 0.21 | 11/12 |
| Sleep phases | 8 | 0.39 ± 0.11 | 8/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazuelo, J.; Juez, J.Y.; Moumane, H.; Pyrzowski, J.; Mayor, L.; Segura-Quijano, F.E.; Valderrama, M.; Le Van Quyen, M. Evaluating the Electroencephalographic Signal Quality of an In-Ear Wearable Device. Sensors 2024, 24, 3973. https://doi.org/10.3390/s24123973
Pazuelo J, Juez JY, Moumane H, Pyrzowski J, Mayor L, Segura-Quijano FE, Valderrama M, Le Van Quyen M. Evaluating the Electroencephalographic Signal Quality of an In-Ear Wearable Device. Sensors. 2024; 24(12):3973. https://doi.org/10.3390/s24123973
Chicago/Turabian StylePazuelo, Jeremy, Jose Yesith Juez, Hanane Moumane, Jan Pyrzowski, Liliana Mayor, Fredy Enrique Segura-Quijano, Mario Valderrama, and Michel Le Van Quyen. 2024. "Evaluating the Electroencephalographic Signal Quality of an In-Ear Wearable Device" Sensors 24, no. 12: 3973. https://doi.org/10.3390/s24123973
APA StylePazuelo, J., Juez, J. Y., Moumane, H., Pyrzowski, J., Mayor, L., Segura-Quijano, F. E., Valderrama, M., & Le Van Quyen, M. (2024). Evaluating the Electroencephalographic Signal Quality of an In-Ear Wearable Device. Sensors, 24(12), 3973. https://doi.org/10.3390/s24123973








