Advancements in Cerebrospinal Fluid Biosensors: Bridging the Gap from Early Diagnosis to the Detection of Rare Diseases
Abstract
1. Introduction
1.1. CSF’s Formation, Mechanism, and Function in the Body
1.2. CSF Collection and Challenges
1.3. Changes in CSF Associated with Various Diseases
2. An Overview of Biosensors
2.1. Electrochemical Biosensors
2.2. Optical Biosensors
2.3. Piezoelectric Biosensors
2.4. Amperometric Biosensors
2.5. Voltametric Biosensors
2.6. Development of Biosensors
2.7. Applications of Biosensors
3. Types of Biosensors
3.1. Immunosensors
3.2. Enzymatic Biosensors
3.3. Peptide-Based Biosensors
3.4. Gene-Based Biosensors
4. Multiplex Biosensors
4.1. Advancements in Multiplex Biosensors
4.2. Multiplex Biosensors in Cerebrospinal Fluid (CSF) Biosensing
5. Biosensing in Body Fluids
5.1. Saliva-Based Biosensors
5.2. Blood-Based Biosensors
5.3. Tear-Based Biosensors
6. CSF Sensing and Detection: Current State and Progress
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vernau, W.; Vernau, K.A.; Sue Bailey, C. Cerebrospinal Fluid. Clin. Biochem. Domest. Anim. 2008, 769–819. [Google Scholar] [CrossRef]
- Meldolesi, J. News about the Role of Fluid and Imaging Biomarkers in Neurodegenerative Diseases. Biomedicines 2021, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Wulf, M.; Sommer, P.; Barkovits, K.; Vorgerd, M.; Marcus, K.; Eggers, B. CSF Diagnostics: A Potentially Valuable Tool in Neurodegenerative and Inflammatory Disorders Involving Motor Neurons: A Review. Diagnostics 2021, 11, 1522. [Google Scholar] [CrossRef] [PubMed]
- Arribas, M. The history of cerebrospinal fluid: From classical antiquity to the late modern period. Neurosci. Hist 2017, 5, 105–113. [Google Scholar]
- Gastaldi, M.; Zardini, E.; Leante, R.; Ruggieri, M.; Costa, G.; Cocco, E.; De Luca, G.; Cataldo, I.; Biagioli, T.; Ballerini, C. Cerebrospinal fluid analysis and the determination of oligoclonal bands. Neurol. Sci. 2017, 38, 217–224. [Google Scholar] [CrossRef]
- Vecchio, D. The history of cerebrospinal fluid analysis in multiple sclerosis: A great development over the last centuries. J. Brain Disord. 2017, 1, 35–37. [Google Scholar]
- Ousman, S.S.; Kubes, P. Immune surveillance in the central nervous system. Nat. Neurosci. 2012, 15, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Paterson, R.W.; Warren, J.D.; Zetterberg, H.; Brien, J.T.; Fox, N.C.; Halliday, G.M.; Schott, J.M. Biomarkers in dementia: Clinical utility and new directions. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Haque, R.U.; Dammer, E.B.; Duong, D.M.; Ping, L.; Johnson, E.C.B.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Targeted mass spectrometry to quantify brain-derived cerebrospinal fluid biomarkers in Alzheimer’s disease. Clin. Proteom. 2020, 17, 19. [Google Scholar] [CrossRef]
- Mikuła, E. Recent Advancements in Electrochemical Biosensors for Alzheimer’s Disease Biomarkers Detection. Curr. Med. Chem. 2021, 28, 4049–4073. [Google Scholar] [CrossRef]
- Collins, M.A.; An, J.; Peller, D.; Bowser, R. Total protein is an effective loading control for cerebrospinal fluid western blots. J. Neurosci. Methods 2015, 251, 72–82. [Google Scholar] [CrossRef]
- Batllori, M.; Molero-Luis, M.; Ormazabal, A.; Casado, M.; Sierra, C.; García-Cazorla, A.; Kurian, M.; Pope, S.; Heales, S.J.; Artuch, R. Analysis of human cerebrospinal fluid monoamines and their cofactors by HPLC. Nat. Protoc. 2017, 12, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, H.; Panhwar, S.; Boyaci, I.H.; Tamer, U. Optical Based Transducers for Biosensors. In Biosensors; CRC Press: Boca Raton, FL, USA, 2022; pp. 57–87. [Google Scholar]
- Wethekam, L.C.; Moore, J.K. α-tubulin regulation by 5′ introns in Saccharomyces cerevisiae. Genetics 2023, 225, iyad163. [Google Scholar] [CrossRef]
- Ali, A.H. High-performance liquid chromatography (HPLC): A review. Ann. Adv. Chem. 2022, 6, 010–020. [Google Scholar]
- Brown, P.D.; Davies, S.L.; Speake, T.; Millar, I.D. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 2004, 129, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Jurado, R.; Walker, H.K. Cerebrospinal fluid. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 2016, 1862, 442–451. [Google Scholar] [CrossRef]
- Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010, 7, 1–20. [Google Scholar] [CrossRef]
- Chang, H.; Nakagawa, H. Hypothesis on the pathophysiology of syringomyelia based on simulation of cerebrospinal fluid dynamics. J. Neurol. Neurosurg. Psychiatry 2003, 74, 344–347. [Google Scholar] [CrossRef]
- Hosoya, K.-i.; Tachikawa, M. Roles of organic anion/cation transporters at the blood–brain and blood–cerebrospinal fluid barriers involving uremic toxins. Clin. Exp. Nephrol. 2011, 15, 478–485. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.-F.; Strazielle, N.; Catala, M.; Silva-Vargas, V.; Doetsch, F.; Engelhardt, B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018, 135, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, S.W.; Janigro, D.; Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 2019, 16, 9. [Google Scholar] [CrossRef]
- Doherty, C.M.; Forbes, R.B. Diagnostic Lumbar Puncture. Ulst. Med J 2014, 83, 93–102. [Google Scholar]
- Tumani, H.; Huss, A.; Bachhuber, F. The cerebrospinal fluid and barriers–anatomic and physiologic considerations. Handb. Clin. Neurol. 2018, 146, 21–32. [Google Scholar]
- Rock, R.B.; Olin, M.; Baker, C.A.; Molitor, T.W.; Peterson, P.K. Central nervous system tuberculosis: Pathogenesis and clinical aspects. Clin. Microbiol. Rev. 2008, 21, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.S.; Mendes, N.T.; Martins, Á.R.; Sanvito, W.L. Cerebrospinal fluid: History, collection techniques, indications, contraindications and complications. J. Bras. Patol. Med. Lab. 2020, 56, e2822020. [Google Scholar] [CrossRef]
- Mollan, S.P.; Ali, F.; Hassan-Smith, G.; Botfield, H.; Friedman, D.I.; Sinclair, A.J. Evolving evidence in adult idiopathic intracranial hypertension: Pathophysiology and management. J. Neurol. Neurosurg. Psychiatry 2016, 87, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.V.; Jayawarna, C.; Jude, E. Post lumbar puncture headache: Diagnosis and management. Postgrad. Med. J. 2006, 82, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Lavi, R.; Rowe, J.M.; Avivi, I. Lumbar Puncture: It Is Time to Change the Needle. Eur. Neurol. 2010, 64, 108–113. [Google Scholar] [CrossRef]
- Wright, B.L.C.; Lai, J.T.F.; Sinclair, A.J. Cerebrospinal fluid and lumbar puncture: A practical review. J. Neurol. 2012, 259, 1530–1545. [Google Scholar] [CrossRef]
- Tomi, N.S.; Kränke, B.; Aberer, E. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects. J. Am. Acad. Dermatol. 2005, 53, 67–72. [Google Scholar] [CrossRef]
- Schulga, P.; Grattan, R.; Napier, C.; Babiker, M.O. How to use… lumbar puncture in children. Arch. Dis. Child Educ. Pr. Ed. 2015, 100, 264–271. [Google Scholar] [CrossRef]
- Brown, K.; Berkowitz, D. Chapter 171-Lumbar Puncture. In Pediatric Emergency Medicine; Baren, J.M., Rothrock, S.G., Brennan, J.A., Brown, L., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2008; pp. 1208–1211. [Google Scholar] [CrossRef]
- Seehusen, D.A.; Reeves, M.M.; Fomin, D.A. Cerebrospinal fluid analysis. Am. Fam. Physician 2003, 68, 1103–1108. [Google Scholar]
- Fitzgerald, J.; Fenniri, H. Cutting Edge Methods for Non-Invasive Disease Diagnosis Using E-Tongue and E-Nose Devices. Biosensors 2017, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003, 21, 79–96. [Google Scholar] [PubMed]
- Welles, E.G.; Pugh, D.G.; Wenzel, J.G.; Sorjonen, D.C. Composition of cerebrospinal fluid in healthy adult llamas. Am. J. Vet. Res. 1994, 55, 1075–1079. [Google Scholar] [CrossRef]
- Hrishi, A.P.; Sethuraman, M. Cerebrospinal Fluid (CSF) Analysis and Interpretation in Neurocritical Care for Acute Neurological Conditions. Indian J. Crit. Care Med. 2019, 23, S115–S119. [Google Scholar] [CrossRef]
- Lumb, A.B. Chapter 4-Control of Breathing. In Nunn’s Applied Respiratory Physiology, 8th ed.; Lumb, A.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 51–72.e52. [Google Scholar] [CrossRef]
- Lee, S.C.; Lueck, C.J. Cerebrospinal fluid pressure in adults. J. Neuroophthalmol. 2014, 34, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Erdem, H.; Ozturk-Engin, D.; Cag, Y.; Senbayrak, S.; Inan, A.; Kazak, E.; Savasci, U.; Elaldi, N.; Vahaboglu, H.; Hasbun, R. Central nervous system infections in the absence of cerebrospinal fluid pleocytosis. Int. J. Infect. Dis. 2017, 65, 107–109. [Google Scholar] [CrossRef]
- Martín-Ancel, A.; García-Alix, A.; Salas, S.; Del Castillo, F.; Cabañas, F.; Quero, J. Cerebrospinal fluid leucocyte counts in healthy neonates. Arch. Dis. Child Fetal Neonatal. Ed. 2006, 91, F357–F358. [Google Scholar] [CrossRef]
- Koenig, M.; Aldrich, E.M. Chapter 25-Cerebrovascular Disorders. In Cerebrospinal Fluid in Clinical Practice; Irani, D.N., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 225–231. [Google Scholar] [CrossRef]
- Leen, W.G.; Willemsen, M.A.; Wevers, R.A.; Verbeek, M.M. Cerebrospinal fluid glucose and lactate: Age-specific reference values and implications for clinical practice. PLoS ONE 2012, 7, e42745. [Google Scholar] [CrossRef] [PubMed]
- Jovel, J.; O’keefe, S.; Patterson, J.; Bording-Jorgensen, M.; Wang, W.; Mason, A.L.; Warren, K.G.; Wong, G.K.-S. Cerebrospinal Fluid in a Small Cohort of Patients with Multiple Sclerosis Was Generally Free of Microbial DNA. Front. Cell. Infect. Microbiol. 2017, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Robert Snodgrass, S.; Johanson, C.E. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp. Neurol. 2015, 273, 57–68. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef]
- Swanson, P.A., 2nd; McGavern, D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015, 11, 44–54. [Google Scholar] [CrossRef]
- Davis, R.; Jeffery, K.; Atkins, B.L. Hypoglycorrhachia in herpes simplex encephalitis. Clin. Infect. Dis. 2004, 38, 1506–1507. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, N.; Legoff, J.; Mengelle, C.; Mercier-Delarue, S.; N’Guyen, Y.; Renois, F.; Tissier, F.; Simon, F.; Izopet, J.; Andréoletti, L. Virological diagnosis of central nervous system infections by use of PCR coupled with mass spectrometry analysis of cerebrospinal fluid samples. J. Clin. Microbiol. 2014, 52, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Suthar, R.; Sankhyan, N. Bacterial Infections of the Central Nervous System. Indian J. Pediatr. 2019, 86, 60–69. [Google Scholar] [CrossRef]
- Benninger, F.; Steiner, I. Chapter 12-CSF in acute and chronic infectious diseases. In Handbook of Clinical Neurology; Deisenhammer, F., Teunissen, C.E., Tumani, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 146, pp. 187–206. [Google Scholar]
- Prasad, K.; Karlupia, N. Prevention of bacterial meningitis: An overview of Cochrane systematic reviews. Respir. Med. 2007, 101, 2037–2043. [Google Scholar] [CrossRef]
- Issa, M.; Mölling, P.; Bäckman, A.; Unemo, M.; Sulaiman, N.; Olcén, P. PCR of cerebrospinal fluid for diagnosis of bacterial meningitis during meningococcal epidemics; an example from Sudan. Scand. J Infect Dis. 2003, 35, 719–723. [Google Scholar] [CrossRef]
- Borha, A.; Parienti, J.-J.; Emery, E.; Coskun, O.; Khouri, S.; Derlon, J.-M. Granulome cérébral à Candida albicans chez un patient immunocompétent. Cas clinique. Neurochirurgie 2009, 55, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Góralska, K.; Blaszkowska, J.; Dzikowiec, M. Neuroinfections caused by fungi. Infection 2018, 46, 443–459. [Google Scholar] [CrossRef]
- Nash, T.E. Parasitic Diseases that Cause Seizures. Epilepsy Curr. 2014, 14, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.; Tucci, V.; Galwankar, S. Infections of the nervous system. Int. J. Crit. Illn. Inj. Sci. 2012, 2, 82–97. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The Cerebrospinal Fluid in Multiple Sclerosis. Front. Immunol. 2019, 10, 438156. [Google Scholar] [CrossRef]
- Le, W.-D.; Rowe, D.B.; Jankovic, J.; Xie, W.; Appel, S.H. Effects of Cerebrospinal Fluid From Patients With Parkinson Disease on Dopaminergic Cells. Arch. Neurol. 1999, 56, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Toedebusch, C.M.; Bachrach, M.D.; Garcia, V.B.; Johnson, G.C.; Katz, M.L.; Shaw, G.; Coates, J.R.; Garcia, M.L. Cerebrospinal Fluid Levels of Phosphorylated Neurofilament Heavy as a Diagnostic Marker of Canine Degenerative Myelopathy. J. Vet. Intern. Med. 2017, 31, 513–520. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J.; Nair, P.P. Diagnostic approach to peripheral neuropathy. Ann. Indian Acad. Neurol. 2008, 11, 89–97. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Wunderlich, G.; Fink, G.R.; Sommer, C. Diagnosis of peripheral neuropathy. Neurol. Res. Pract. 2020, 2, 20. [Google Scholar] [CrossRef]
- Corado, C.R.; Pinkstaff, J.; Jiang, X.; Galban, E.M.; Fisher, S.J.; Scholler, O.; Russell, C.; Bagel, J.H.; PA, O.D.; Ory, D.S.; et al. Cerebrospinal fluid and serum glycosphingolipid biomarkers in canine globoid cell leukodystrophy (Krabbe Disease). Mol. Cell. Neurosci. 2020, 102, 103451. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Zanier, E.R.; Refai, D.; Zipfel, G.J.; Zoerle, T.; Longhi, L.; Esparza, T.J.; Spinner, M.L.; Bateman, R.J.; Brody, D.L.; Stocchetti, N. Neurofilament light chain levels in ventricular cerebrospinal fluid after acute aneurysmal subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2011, 82, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.; Alyahya, B.; Sivilotti, M.L.A.; Bullard, M.J.; Émond, M.; Sutherland, J.; Worster, A.; Hohl, C.; Lee, J.S.; Eisenhauer, M.A.; et al. Differentiation between traumatic tap and aneurysmal subarachnoid hemorrhage: Prospective cohort study. BMJ Br. Med. J. 2015, 350, h568. [Google Scholar] [CrossRef] [PubMed]
- Khoury, N.T.; Hossain, M.M.; Wootton, S.H.; Salazar, L.; Hasbun, R. Meningitis with a negative cerebrospinal fluid Gram stain in adults: Risk classification for an adverse clinical outcome. Mayo Clin. Proc. 2012, 87, 1181–1188. [Google Scholar] [CrossRef]
- Blinder, T.; Lewerenz, J. Cerebrospinal Fluid Findings in Patients With Autoimmune Encephalitis—A Systematic Analysis. Front. Neurol. 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.L.; Glantz, M.J.; Connor, J.R. Detection of cancer cells in the cerebrospinal fluid: Current methods and future directions. Fluids Barriers CNS 2011, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Lu, G.; Zorofchian, S.; Vantaku, V.; Putluri, V.; Yan, Y.; Arevalo, O.; Zhu, P.; Riascos, R.F.; Sreekumar, A.; et al. Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol. Commun. 2018, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C.H.; Brown, S.J.; Fuller, H.R.; Riddell, J.; Osman, A.; Chowdhury, J.; Kumar, N.; Johnson, W.E.; Wright, K.T. The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord 2017, 55, 114–125. [Google Scholar] [CrossRef]
- Wright, K.T.; Masri, W.E.; Osman, A.; Chowdhury, J.; Johnson, W.E.B. Concise Review: Bone Marrow for the Treatment of Spinal Cord Injury: Mechanisms and Clinical Applications. Stem Cells 2011, 29, 169–178. [Google Scholar] [CrossRef]
- Alcolea, D.; Pegueroles, J.; Munoz, L.; Camacho, V.; López-Mora, D.; Fernández-León, A.; Le Bastard, N.; Huyck, E.; Nadal, A.; Olmedo, V. Agreement of amyloid PET and CSF biomarkers for Alzheimer’s disease on Lumipulse. Ann. Clin. Transl. Neurol. 2019, 6, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Contador, J.; Pérez-Millán, A.; Tort-Merino, A.; Balasa, M.; Falgàs, N.; Olives, J.; Castellví, M.; Borrego-Écija, S.; Bosch, B.; Fernández-Villullas, G.; et al. Longitudinal brain atrophy and CSF biomarkers in early-onset Alzheimer’s disease. NeuroImage Clin. 2021, 32, 102804. [Google Scholar] [CrossRef]
- Magliozzi, R.; Cross, A.H. Can CSF biomarkers predict future MS disease activity and severity? Mult. Scler. J. 2020, 26, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Patti, F. CSF biomarkers in multiple sclerosis: Beyond neuroinflammation. Neuroimmunol. Neuroinflammation 2021, 8, 14–41. [Google Scholar] [CrossRef]
- Pouw, M.H.; Kwon, B.K.; Verbeek, M.M.; Vos, P.E.; van Kampen, A.; Fisher, C.G.; Street, J.; Paquette, S.J.; Dvorak, M.F.; Boyd, M.C.; et al. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: A descriptive analysis of 16 subjects. Spinal Cord 2014, 52, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Rath, J.; Zulehner, G.; Schober, B.; Grisold, A.; Krenn, M.; Cetin, H.; Zimprich, F. Cerebrospinal fluid analysis in Guillain–Barré syndrome: Value of albumin quotients. J. Neurol. 2021, 268, 3294–3300. [Google Scholar] [CrossRef] [PubMed]
- Agah, E.; Saleh, F.; Sanjari Moghaddam, H.; Saghazadeh, A.; Tafakhori, A.; Rezaei, N. CSF and blood biomarkers in amyotrophic lateral sclerosis: Protocol for a systematic review and meta-analysis. Syst. Rev. 2018, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Drannik, A.; Martin, J.; Peterson, R.; Ma, X.; Jiang, F.; Turnbull, J. Cerebrospinal fluid from patients with amyotrophic lateral sclerosis inhibits sonic hedgehog function. PLoS ONE 2017, 12, e0171668. [Google Scholar] [CrossRef]
- Ahmed, S.; Rakib, A.; Nasrin, F.; Chowdhury, R.H.; Azad, A.K.; Hasan, C.M.M. Assessment of Cerebrospinal Fluid (CSF) to Differentiate between Bacterial Meningitis and Viral Meningitis. J. Adv. Med. Pharm. Sci. 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Stephane, G.H. Estimation of CSF Lactate as a Diagnostic Marker to Differentiate Pyogenic Meningitis from Nonpyogenic Meningitis. Ph.D Thesis, Rajiv Gandhi University of Health Sciences, Bengaluru, India, 2019. [Google Scholar]
- Marchegiani, F.; Matacchione, G.; Ramini, D.; Marcheselli, F.; Recchioni, R.; Casoli, T.; Mercuri, E.; Lazzarini, M.; Giorgetti, B.; Cameriere, V.; et al. Diagnostic performance of new and classic CSF biomarkers in age-related dementias. Aging 2019, 11, 2420–2429. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Steinacker, P.; Polischi, B.; Mammana, A.; Bartoletti-Stella, A.; Oeckl, P.; Baiardi, S.; Zenesini, C.; Huss, A.; Cortelli, P.; et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimer’s Res. Ther. 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Tetyana, P.; Shumbula, P.M.; Njengele-Tetyana, Z. Biosensors: Design, development and applications. In Nanopores; IntechOpen: London, UK, 2021. [Google Scholar]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent advances in optical biosensors for sensing applications: A review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Qiu, Y.; Wu, H.; Qin, W.; Liao, Y.; Yu, Q.; Cheng, H. Stretchable piezoelectric energy harvesters and self-powered sensors for wearable and implantable devices. Biosens. Bioelectron. 2020, 168, 112569. [Google Scholar] [CrossRef]
- Deng, W.; Zhou, Y.; Libanori, A.; Chen, G.; Yang, W.; Chen, J. Piezoelectric nanogenerators for personalized healthcare. Chem. Soc. Rev. 2022, 51, 3380–3435. [Google Scholar] [CrossRef]
- Bollella, P. Enzyme-based amperometric biosensors: 60 years later… Quo Vadis? Anal. Chim. Acta 2022, 1234, 340517. [Google Scholar] [CrossRef]
- Gulaboski, R.; Mirceski, V. Application of voltammetry in biomedicine-Recent achievements in enzymatic voltammetry. Maced. J. Chem. Chem. Eng. 2020, 39, 153–166. [Google Scholar] [CrossRef]
- Song, L.; Zhuge, Y.; Zuo, X.; Li, M.; Wang, F. DNA walkers for biosensing development. Adv. Sci. 2022, 9, 2200327. [Google Scholar] [CrossRef]
- Khattab, A.M. Basics of Biological Sensors. In Handbook of Nanosensors: Materials and Technological Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–43. [Google Scholar]
- Tripathi, A.; Bonilla-Cruz, J. Review on healthcare biosensing nanomaterials. ACS Appl. Nano Mater. 2023, 6, 5042–5074. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Masih, J.; Kumar, R. Biosensor: Application in Environmental Management. In Harnessing Microbial Potential for Multifarious Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 455–488. [Google Scholar]
- Simon, E. Biological and Medical Sensors 12. In Sensors in Science and Technology; Springer: Wiesbaden, Germany, 2022; p. 651. [Google Scholar] [CrossRef]
- Chaudhary, V.; Khanna, V.; Ahmed Awan, H.T.; Singh, K.; Khalid, M.; Mishra, Y.K.; Bhansali, S.; Li, C.Z.; Kaushik, A. Towards hospital-on-chip supported by 2D MXenes-based 5(th) generation intelligent biosensors. Biosens. Bioelectron. 2023, 220, 114847. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-printed electrodes: Fabrication, modification, and biosensing applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Aydin, M.; Aydin, E.B.; Sezgintürk, M.K. Advances in immunosensor technology. Adv. Clin. Chem. 2021, 102, 1–62. [Google Scholar]
- Li, Z.; Zhang, J.; Huang, Y.; Zhai, J.; Liao, G.; Wang, Z.; Ning, C. Development of electroactive materials-based immunosensor towards early-stage cancer detection. Coord. Chem. Rev. 2022, 471, 214723. [Google Scholar] [CrossRef]
- Cavalcante, F.T.; de A. Falcão, I.R.; da S. Souza, J.E.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.; de Oliveira, A.L.; de Sousa, M.C.; dos Santos, J.C. Designing of nanomaterials-based enzymatic biosensors: Synthesis, properties, and applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, T.; Chu, Z.; Jin, W. Recent advances in electrochemical enzymatic biosensors based on regular nanostructured materials. J. Electroanal. Chem. 2021, 893, 115328. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Zhang, L.; Zhao, J.; Jiang, Z.; Wang, W. Peptide-derived biosensors and their applications in tumor immunology-related detection. Anal. Chem. 2021, 94, 431–441. [Google Scholar] [CrossRef]
- Vanova, V.; Mitrevska, K.; Milosavljevic, V.; Hynek, D.; Richtera, L.; Adam, V. Peptide-based electrochemical biosensors utilized for protein detection. Biosens. Bioelectron. 2021, 180, 113087. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lu, Y. Advances in cell-free biosensors: Principle, mechanism, and applications. Biotechnol. J. 2020, 15, 2000187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, Z.; Ding, Y.; Xie, M. A pH-engineering regenerative DNA tetrahedron ECL biosensor for the assay of SARS-CoV-2 RdRp gene based on CRISPR/Cas12a trans-activity. Chem. Eng. J. 2022, 429, 132472. [Google Scholar] [CrossRef] [PubMed]
- Glatz, R.T.; Ates, H.C.; Mohsenin, H.; Weber, W.; Dincer, C. Designing electrochemical microfluidic multiplexed biosensors for on-site applications. Anal. Bioanal. Chem. 2022, 414, 6531–6540. [Google Scholar] [CrossRef] [PubMed]
- Beduk, T.; Beduk, D.; Hasan, M.R.; Guler Celik, E.; Kosel, J.; Narang, J.; Salama, K.N.; Timur, S. Smartphone-based multiplexed biosensing tools for health monitoring. Biosensors 2022, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-P.; Yang, T.-H.; Wang, J.-C.; Chuang, H.-S. Recent Trends and Innovations in Bead-Based Biosensors for Cancer Detection. Sensors 2024, 24, 2904. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Hunter, H.; Zhou, Z.; Sun, Y.; Cheng, X.; Ma, J.; Su, L.; Zhang, X.; Serpe, M.J. Portable point-of-care diagnostic devices: An updated review. Anal. Methods 2021, 13, 5418–5435. [Google Scholar] [CrossRef] [PubMed]
- Kharati, M.; Foroutanparsa, S.; Rabiee, M.; Salarian, R.; Rabiee, N.; Rabiee, G. Early diagnosis of multiple sclerosis based on optical and electrochemical biosensors: Comprehensive perspective. Curr. Anal. Chem. 2020, 16, 557–569. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Chen, R.; Wang, X.; Wang, Y.; Wei, J.; Zhang, K.; Zhang, C. Electrochemical biosensing of circulating microRNA-21 in cerebrospinal fluid of medulloblastoma patients through target-induced redox signal amplification. Microchim. Acta 2022, 189, 105. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, J.U.; Jeon, M.J.; Kim, S.; Sim, S.J. Detection of multiplex exosomal miRNAs for clinically accurate diagnosis of Alzheimer’s disease using label-free plasmonic biosensor based on DNA-Assembled advanced plasmonic architecture. Biosens. Bioelectron. 2022, 199, 113864. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Huang, Y.; Du, Y.; Zhang, Y.; Cui, Y.; Kong, D.-m. Development of the DNA-based biosensors for high performance in detection of molecular biomarkers: More rapid, sensitive, and universal. Biosens. Bioelectron. 2022, 197, 113739. [Google Scholar] [CrossRef]
- Malon, R.S.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. Biomed. Res. Int. 2014, 2014, 962903. [Google Scholar] [CrossRef] [PubMed]
- John, R.V.; Devasiya, T.; VR, N.; Adigal, S.; Lukose, J.; Kartha, V.; Chidangil, S. Cardiovascular biomarkers in body fluids: Progress and prospects in optical sensors. Biophys. Rev. 2022, 14, 1023–1050. [Google Scholar] [CrossRef] [PubMed]
- Guest, F.L.; Guest, P.C.; Martins-de-Souza, D. The emergence of point-of-care blood-based biomarker testing for psychiatric disorders: Enabling personalized medicine. Biomark. Med. 2016, 10, 431–443. [Google Scholar] [CrossRef]
- Verber, N. Biochemical Biomarkers of Disease in a Deeply-Phenotyped Cohort of Patients with Motor Neuron Disease. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2021. [Google Scholar]
- Kaštelan, S.; Braš, M.; Pjevač, N.; Bakija, I.; Tomić, Z.; Pjevač Keleminić, N.; Gverović Antunica, A. Tear biomarkers and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 13429. [Google Scholar] [CrossRef]
- Espinosa, P.S.; Rizvi, Z.; Sharma, P.; Hindi, F.; Filatov, A. Neurological complications of coronavirus disease (COVID-19): Encephalopathy, MRI brain and cerebrospinal fluid findings: Case 2. Cureus 2020, 12, e7930. [Google Scholar] [CrossRef]
- Dobrocky, T.; Mosimann, P.J.; Zibold, F.; Mordasini, P.; Raabe, A.; Ulrich, C.T.; Gralla, J.; Beck, J.; Piechowiak, E.I. Cryptogenic cerebrospinal fluid leaks in spontaneous intracranial hypotension: Role of dynamic CT myelography. Radiology 2018, 289, 766–772. [Google Scholar] [CrossRef]
- Ruggeri, M.; Rojas, A.; Chai, O.; Purzyc, H.; Hanael, E.; Rapoport, K.; Barnoon, I.; Konstantin, L.; Baneth, G.; Shamir, M. Detection of intraspinal Spirocerca lupi in canine cerebrospinal fluid by polymerase chain reaction. J. Comp. Pathol. 2019, 170, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tani, N.; Ikeda, T.; Aoki, Y.; Shida, A.; Oritani, S.; Ishikawa, T. Evaluation of screening for drug use using postmortem prolactin levels in serum and cerebrospinal fluid. Hum. Exp. Toxicol. 2019, 38, 1244–1253. [Google Scholar] [CrossRef]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S. Cryo-electron microscopy of extracellular vesicles from cerebrospinal fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, L.; Gao, A.; Li, Y.; Jiang, Z.; Hu, F.; Shao, B.; Liu, Y.; Zhang, X. Risk Factors for Infections Related to Lumbar Drainage in Spontaneous Subarachnoid Hemorrhage. Neurocrit Care 2016, 25, 243–249. [Google Scholar] [CrossRef]
- Hassan, Q.; Li, S.; Ferrag, C.; Kerman, K. Electrochemical biosensors for the detection and study of α-synuclein related to Parkinson’s disease—A review. Anal. Chim. Acta 2019, 1089, 32–39. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Hu, F.; Ding, J.; Wang, X. Pathogenesis and pathophysiology of idiopathic normal pressure hydrocephalus. CNS Neurosci. Ther. 2020, 26, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.; Hader, W.; Bullivant, K.; Brindle, M.; Riva-Cambrin, J. Calgary Shunt Protocol, an adaptation of the Hydrocephalus Clinical Research Network shunt protocol, reduces shunt infections in children. J. Neurosurg. Pediatr. 2019, 23, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Richard, K.E.; Block, F.R.; Weiser, R.R. First clinical results with a telemetric shunt-integrated ICP-sensor. Neurol. Res. 1999, 21, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.R.; Arafa, H.M.; Kwon, K.; Deng, Y.; Su, C.-J.; Reeder, J.T.; Freudman, J.; Stankiewicz, I.; Chen, H.-M.; Loza, R.; et al. Continuous, noninvasive wireless monitoring of flow of cerebrospinal fluid through shunts in patients with hydrocephalus. NPJ Digit. Med. 2020, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Stamos, B.; Dasgupta, P.K. Inline Shunt Flow Monitor for Hydrocephalus. Anal. Chem. 2017, 89, 8170–8176. [Google Scholar] [CrossRef]
- Qin, C.; Olivencia-Yurvati, A.H.; Williams Jr, A.G.; Eskildsen, D.; Mallet, R.T.; Dasgupta, P.K. Inline flow sensor for ventriculoperitoneal shunts: Experimental evaluation in swine. Med. Eng. Phys. 2019, 67, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Gamero, M.; Kim, W.S.; Hong, S.; Vorobiev, D.; Morgan, C.D.; Park, S.I. Multimodal Sensing Capabilities for the Detection of Shunt Failure. Sensors 2021, 21, 1747. [Google Scholar] [CrossRef] [PubMed]
- Pennell, T.; Yi, J.L.; Kaufman, B.A.; Krishnamurthy, S. Noninvasive measurement of cerebrospinal fluid flow using an ultrasonic transit time flow sensor: A preliminary study. J. Neurosurg. Pediatr. 2016, 17, 270–277. [Google Scholar] [CrossRef]
- Senel, M.; Dervisevic, E.; Alhassen, S.; Dervisevic, M.; Alachkar, A.; Cadarso, V.J.; Voelcker, N.H. Microfluidic Electrochemical Sensor for Cerebrospinal Fluid and Blood Dopamine Detection in a Mouse Model of Parkinson’s Disease. Anal. Chem. 2020, 92, 12347–12355. [Google Scholar] [CrossRef]
- Jalalvand, E.; Robertson, B.; Tostivint, H.; Löw, P.; Wallén, P.; Grillner, S. Cerebrospinal Fluid-Contacting Neurons Sense pH Changes and Motion in the Hypothalamus. J. Neurosci. 2018, 38, 7713–7724. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.K.; Miyakoda, K.; Saito, A.; Yasoda, Y.; Kajiwara, K.; Kimura, M.; Tsuchiya, K. Microneedle pH Sensor: Direct, Label-Free, Real-Time Detection of Cerebrospinal Fluid and Bladder pH. ACS Appl. Mater. Interfaces 2017, 9, 21651–21659. [Google Scholar] [CrossRef]
- Booth, M.A.; Gowers, S.A.N.; Hersey, M.; Samper, I.C.; Park, S.; Anikeeva, P.; Hashemi, P.; Stevens, M.M.; Boutelle, M.G. Fiber-Based Electrochemical Biosensors for Monitoring pH and Transient Neurometabolic Lactate. Anal. Chem. 2021, 93, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Cordella, A.; Mercuri, N.B.; D’Amico, A.; Palombi, A.; Zompanti, A.; Grasso, S.; Pennazza, G.; Santonico, M. Design of an Innovative Methodology for Cerebrospinal Fluid Analysis: Preliminary Results. Sensors 2021, 21, 3767. [Google Scholar] [CrossRef]
- Goh, J.H.; Mason, A.; Al-Shamma’a, A.I.; Field, M.; Shackcloth, M.; Browning, P. Non Invasive Microwave Sensor for the Detection of Lactic Acid in Cerebrospinal Fluid (CSF). J. Phys. Conf. Ser. 2011, 307, 012017. [Google Scholar] [CrossRef]
- Stephani, C.; Choi, A.H.K.; Moerer, O. Point-of-care detection of lactate in cerebrospinal fluid. Intensive Care Med. Exp. 2021, 9, 18. [Google Scholar] [CrossRef]
- Fisher, O.; Benson, R.A.; Imray, C.H. The clinical application of purine nucleosides as biomarkers of tissue Ischemia and hypoxia in humans in vivo. Biomark. Med. 2019, 13, 953–964. [Google Scholar] [CrossRef]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable Sensor System for Detection of Lactate in Sweat. Sci. Rep. 2018, 8, 15890. [Google Scholar] [CrossRef]
- Yang, X.; Fu, T.; Kota, P.K.; Tjia, M.; Nguyen, C.M.; Chiao, J.-C. Lactate Sensors on Flexible Substrates. Biosensors 2016, 6, 48. [Google Scholar] [CrossRef]
- Abbasi, H.Y.; Tehrani, Z.; Devadoss, A.; Ali, M.M.; Moradi-Bachiller, S.; Albani, D.; Guy, O.J. Graphene based electrochemical immunosensor for the ultra-sensitive label free detection of Alzheimer’s beta amyloid peptides Aβ (1–42). Nanoscale Adv. 2021, 3, 2295–2304. [Google Scholar] [CrossRef]
- Nabers, A.; Ollesch, J.; Schartner, J.; Kötting, C.; Genius, J.; Hafermann, H.; Klafki, H.; Gerwert, K.; Wiltfang, J. Amyloid-β-Secondary Structure Distribution in Cerebrospinal Fluid and Blood Measured by an Immuno-Infrared-Sensor: A Biomarker Candidate for Alzheimer’s Disease. Anal. Chem. 2016, 88, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Valkova, P.; Pohanka, M. Novel Trends in Electrochemical Biosensors for Early Diagnosis of Alzheimer’s Disease. Int. J. Anal. Chem. 2021, 2021, 9984876. [Google Scholar] [CrossRef] [PubMed]
- Karki, H.P.; Jang, Y.; Jung, J.; Oh, J. Advances in the development paradigm of biosample-based biosensors for early ultrasensitive detection of alzheimer’s disease. J. Nanobiotechnology 2021, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Scarano, S.; Lisi, S.; Ravelet, C.; Peyrin, E.; Minunni, M. Detecting Alzheimer’s disease biomarkers: From antibodies to new bio-mimetic receptors and their application to established and emerging bioanalytical platforms—A critical review. Anal. Chim. Acta 2016, 940, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Shabaninejad, Z.; Movahedpour, A.; Sahebkar, A.; Mohammadi, S.; Hosseindoost, S.; Ebrahimi, M.S.; Savardashtaki, A.; Karimipour, M.; Mirzaei, H. Biosensors for detection of Tau protein as an Alzheimer’s disease marker. Int. J. Biol. Macromol. 2020, 162, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wark, A.W.; Lee, H.J. Femtomolar detection of tau proteins in undiluted plasma using surface plasmon resonance. Anal. Chem. 2016, 88, 7793–7799. [Google Scholar] [CrossRef] [PubMed]
- Carlin, N.; Martic-Milne, S. Anti-tau antibodies based electrochemical sensor for detection of tau protein biomarkers. J. Electrochem. Soc. 2018, 165, G3018. [Google Scholar] [CrossRef]
- Li, D.; Scarano, S.; Lisi, S.; Palladino, P.; Minunni, M. Real-time tau protein detection by sandwich-based piezoelectric biosensing: Exploring tubulin as a mass enhancer. Sensors 2018, 18, 946. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, D.; Yun, G.; Kim, H.; Kim, H.-G.; Lee, K.M.; Hong, I.K.; Park, K.-C.; San Lee, J.; Hwang, K.S. Screening for cerebral amyloid angiopathy based on serological biomarkers analysis using a dielectrophoretic force-driven biosensor platform. Lab A Chip 2021, 21, 4557–4565. [Google Scholar] [CrossRef]
- Özcan, N.; Medetalibeyoglu, H.; Akyıldırım, O.; Atar, N.; Yola, M.L. Electrochemical detection of amyloid-β protein by delaminated titanium carbide MXene/multi-walled carbon nanotubes composite with molecularly imprinted polymer. Mater. Today Commun. 2020, 23, 101097. [Google Scholar] [CrossRef]
- You, M.; Yang, S.; An, Y.; Zhang, F.; He, P. A novel electrochemical biosensor with molecularly imprinted polymers and aptamer-based sandwich assay for determining amyloid-β oligomer. J. Electroanal. Chem. 2020, 862, 114017. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Biosens. Bioelectron. 2020, 167, 112511. [Google Scholar] [CrossRef] [PubMed]
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater. Sci. Eng. C 2021, 122, 111916. [Google Scholar] [CrossRef]
- Ganesana, M.; Trikantzopoulos, E.; Maniar, Y.; Lee, S.T.; Venton, B.J. Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectron. 2019, 130, 103–109. [Google Scholar] [CrossRef]
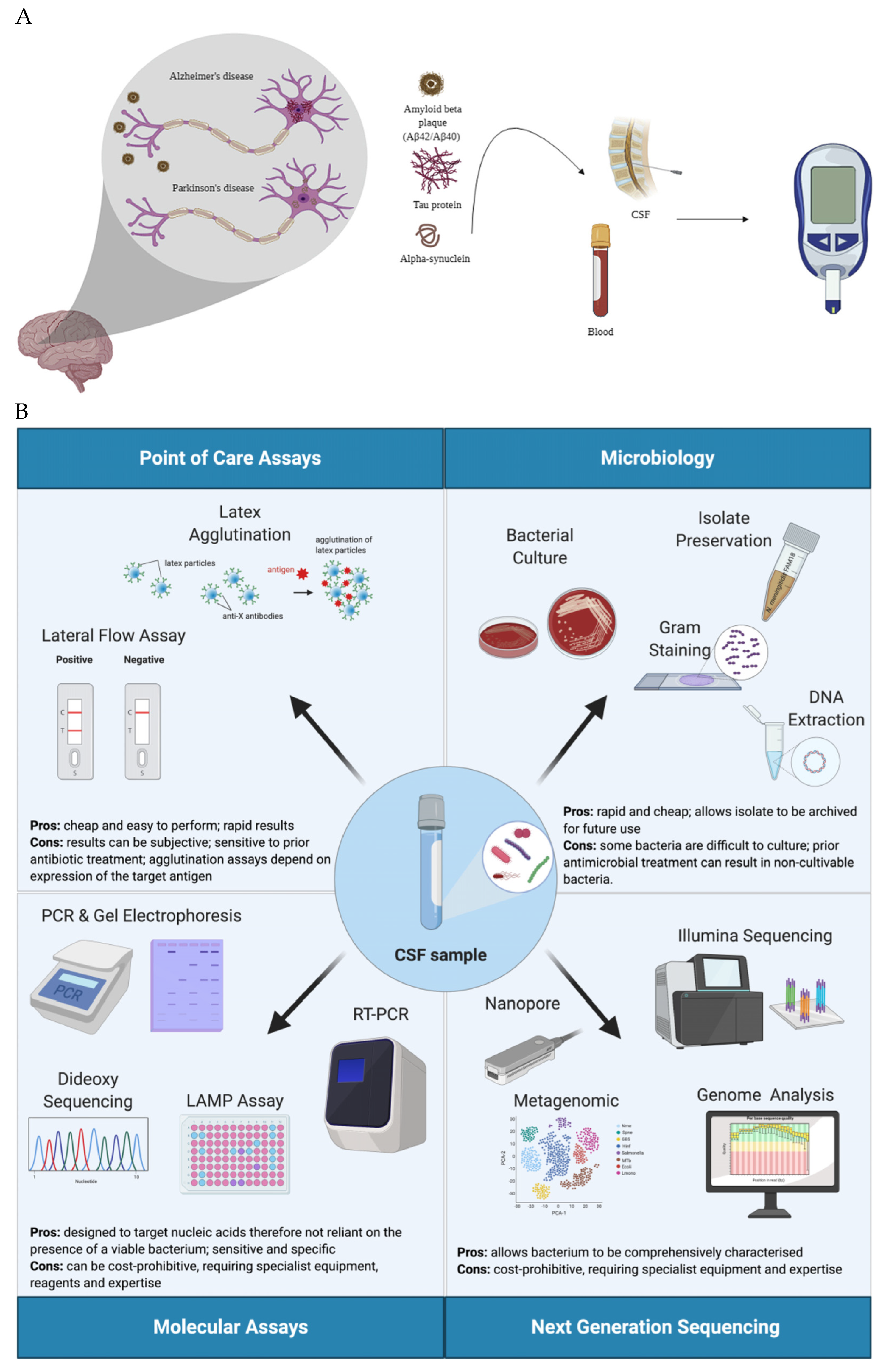
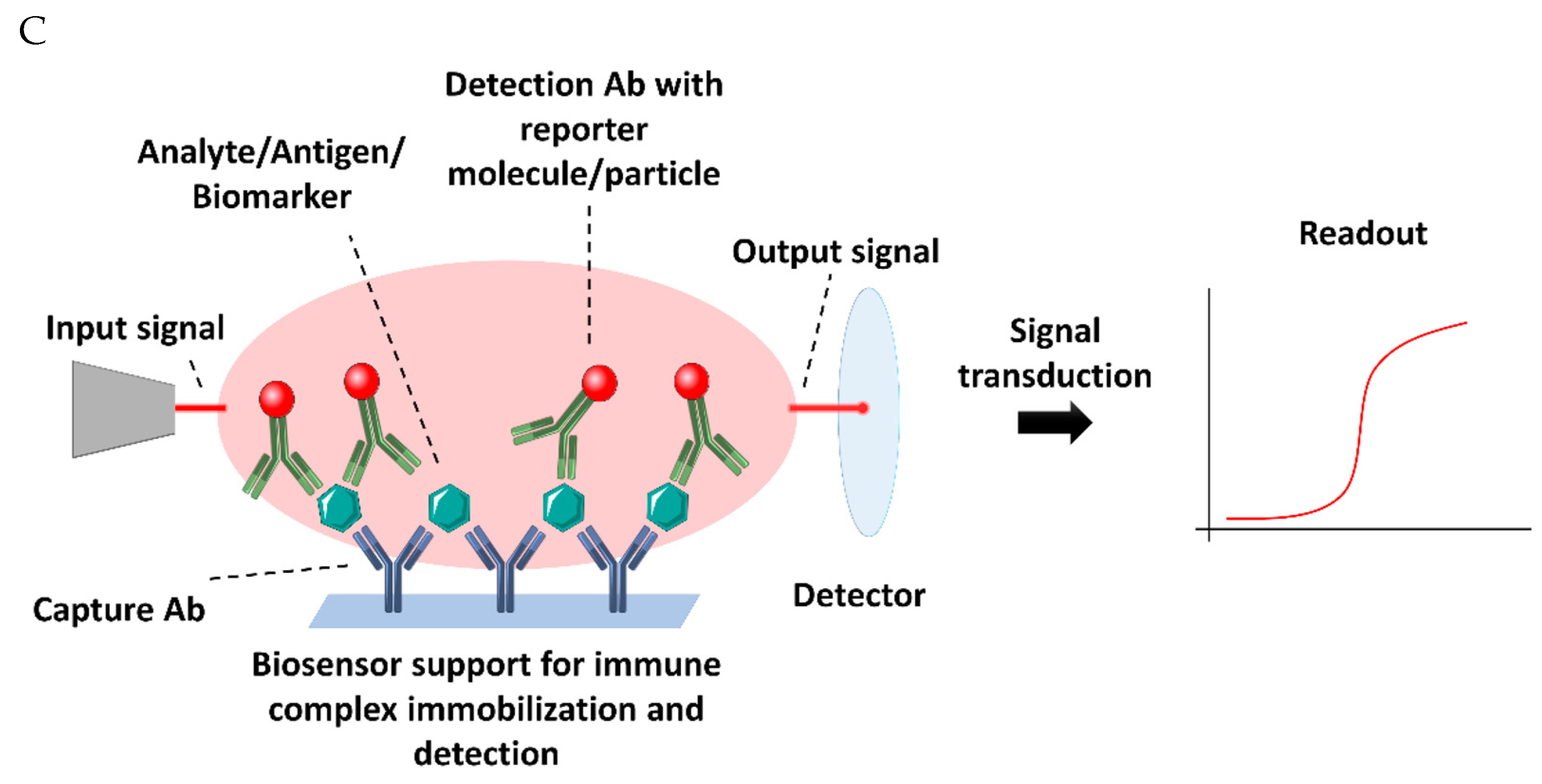
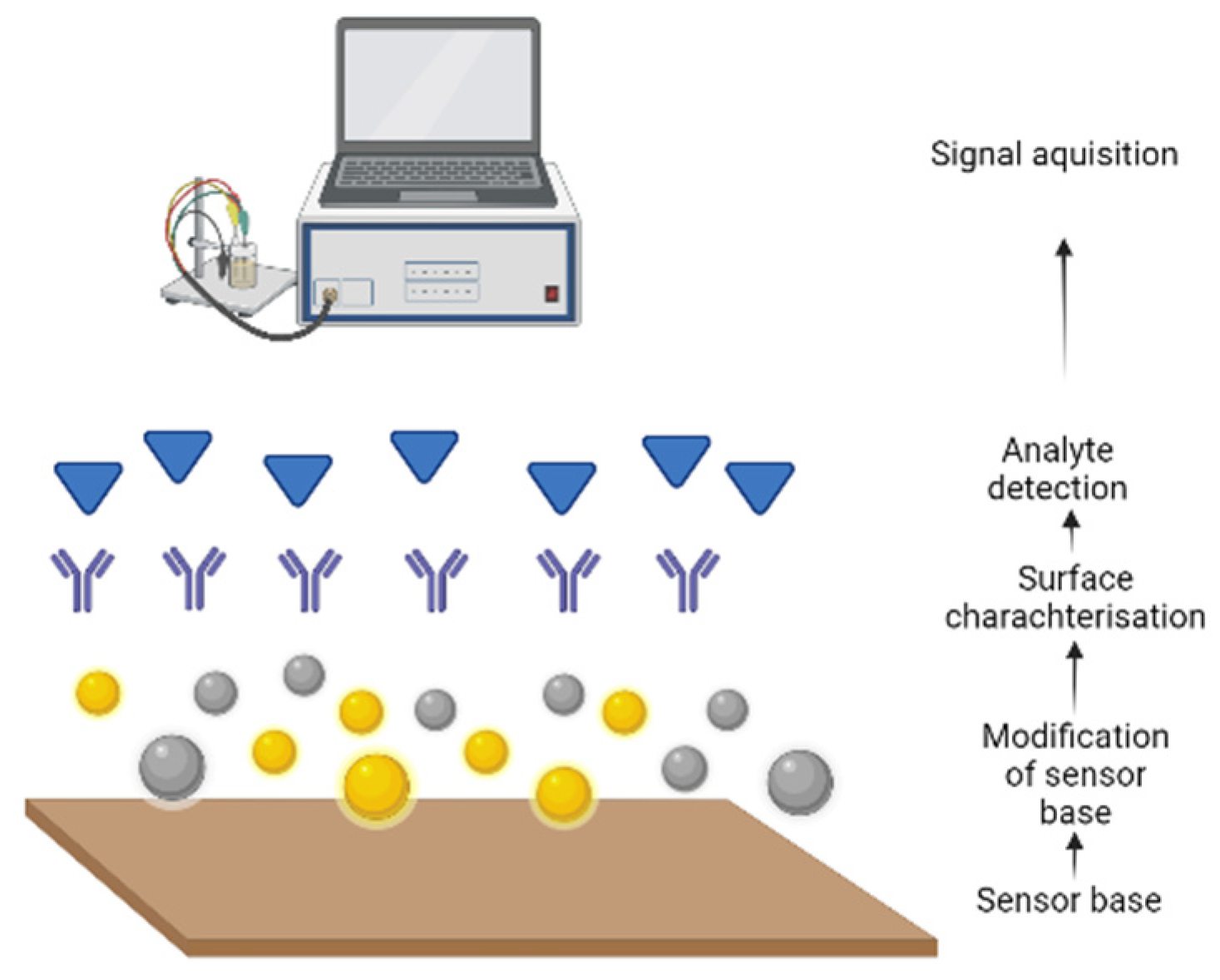
| Quantity | References | |
|---|---|---|
| Colour | No colour | [39,40] |
| pH | 7.4 | [41] |
| Pressure | 50–200 mm H2O | [42] |
| Overall cell count | <5 cells per mL | [43] |
| Red blood cells | 0 | [40] |
| White blood cells | 0–5 (lymphocytes) | [44] |
| Protein | 15–40 mg/dL | [45] |
| Glucose | 50–80 mg/dL | [46] |
| Lactate | 1–3 mmol/L | [46] |
| Microorganisms | No | [47] |
| Mg, K, Na, Ca | Trace | [48] |
| Clinical Condition | Biomarkers in CSF | References |
|---|---|---|
| Alzheimer’s | Aβ1-40, Aβ1-42, tTAU, pTAU, cortisol | [77,78] |
| Multiple sclerosis | OCBs (oligoclonal bands) Increased IgA, IgM CRTAC, Tetranectin, autotaxin-T Immunoglobulins: Ig ϒ1, Ig heavy chain V-III, and Ig-k-chain | [79,80] |
| Spinal cord injuries | Pro-inflammatory cytokines in CSF, NSEs, S100β, and NFH | [81] |
| Guillain–Barre Syndrome | Increased CSF protein level | [82] |
| Amyotrophic lateral sclerosis | Total protein concentration, IL-1β, and TNF-α | [83,84] |
| Meningitis | Increased lactate level, CSF glucose/blood glucose < 0.4 | [85,86] |
| Dementia | Tau protein, Aβ1-42, NF light | [87,88] |
| Biomarker | Electrochemical Sensor Substrate | Related Disease | LOD | References |
|---|---|---|---|---|
| Tau protein | Biosensor cell lines, Sandwich-based antibody | Alzheimer’s | 316 pg–100 ng 34 ng/L | [118,123] |
| Aβ1-42, Aβ1-40, Amyloid-β | Dielectrophoretic force-driven MXene/multi-wall carbon printed nanotube (molecularly imprinted) Molecularly imprinted polymers and aptamers Gold-based plasmon resonance biosensors | Alzheimer’s | Pg/mL 1.0 fg mL−1–100.0 fg mL−1 1.22 pg mL−1 2.4 pg/mL | [162,163,164,165] |
| Dopamine | Aptamer-based biosensor Microfluidic Au-based biosensor Silica-functionalised fluorescent carbon dots | Alzheimer’s Parkinson’s Parkinson’s | µM 0.1 nM 41.2 nM | [142,164,166] |
| Glutamate | In vivo sensing biosensor (Pt wire-based) Carbon–Pt microparticle-based biosensor | Brain Glutamate monitoring Brain Glutamate | 0.044 µM 0.03 µM | [167] |
| Serotonin | Reusable aptasensor (Au-based) Flexible WS2/Graphene/Polyimide Electrode-based biosensor | Alzheimer’s and Parkinson’s NA | [130] | |
| Acetylcholine | EDOT-based solid-state biosensor Amperometry biosensors | Alzheimer’s and Parkinson’s | [131] | |
| Glycated albumin | His6-RAGE VC1-modified electrodes used as biosensors Aptamer-conjugated magnetic nanoparticles used to precipitate glycated albumin | Alzheimer’s | [132] | |
| Cortisol | NA | Delirium, bacterial meningitis, Alzheimer’s disease | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatami-Fard, G.; Anastasova-Ivanova, S. Advancements in Cerebrospinal Fluid Biosensors: Bridging the Gap from Early Diagnosis to the Detection of Rare Diseases. Sensors 2024, 24, 3294. https://doi.org/10.3390/s24113294
Hatami-Fard G, Anastasova-Ivanova S. Advancements in Cerebrospinal Fluid Biosensors: Bridging the Gap from Early Diagnosis to the Detection of Rare Diseases. Sensors. 2024; 24(11):3294. https://doi.org/10.3390/s24113294
Chicago/Turabian StyleHatami-Fard, Ghazal, and Salzitsa Anastasova-Ivanova. 2024. "Advancements in Cerebrospinal Fluid Biosensors: Bridging the Gap from Early Diagnosis to the Detection of Rare Diseases" Sensors 24, no. 11: 3294. https://doi.org/10.3390/s24113294
APA StyleHatami-Fard, G., & Anastasova-Ivanova, S. (2024). Advancements in Cerebrospinal Fluid Biosensors: Bridging the Gap from Early Diagnosis to the Detection of Rare Diseases. Sensors, 24(11), 3294. https://doi.org/10.3390/s24113294








