Effectiveness of Telerehabilitation in Dizziness: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search and Study Selection
2.3. Data Extraction
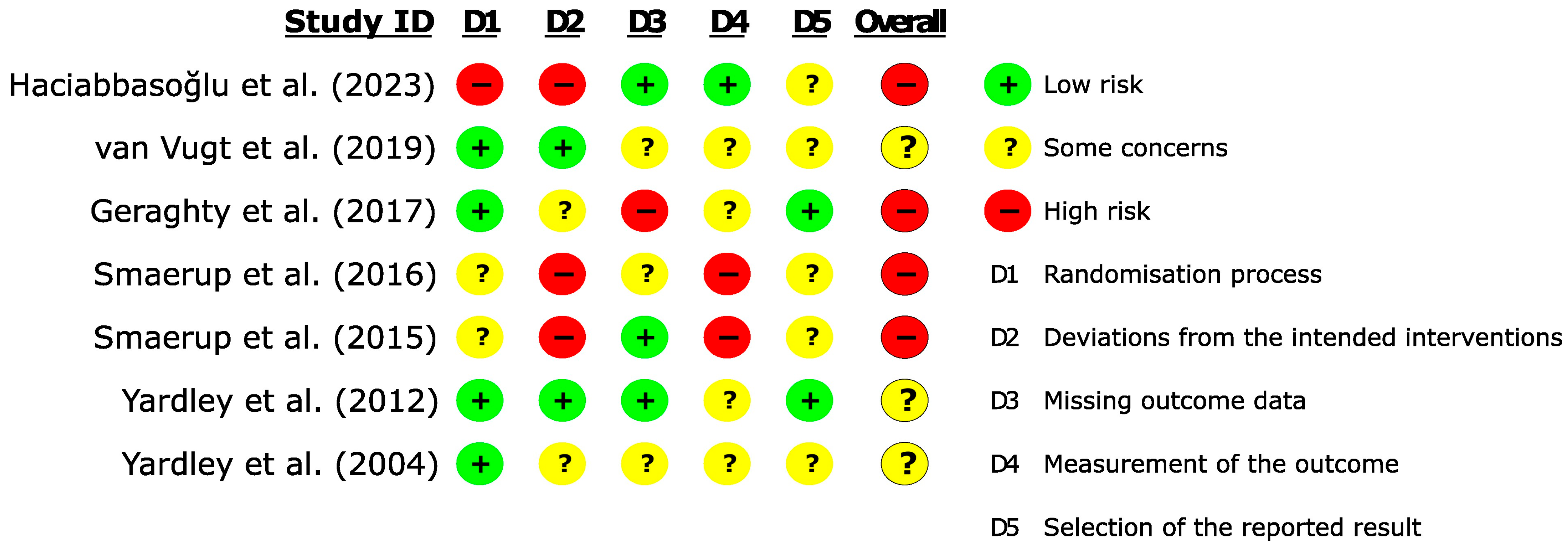
2.4. Quality Assessment
2.5. Data Analysis
3. Results
3.1. Article Selection Process
3.2. Results of Selected Articles
3.3. Methodological Evaluation of Studies
3.4. Effects of Intervention
3.4.1. Frequency and Severity of Dizziness
3.4.2. Disability
3.4.3. Anxiety
3.4.4. Depression
4. Discussion
4.1. Study Limitations
4.2. Clinical Implication and Future Study Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruthberg, J.S.; Rasendran, C.; Kocharyan, A.; Mowry, S.E.; Otteson, T.D. The Economic Burden of Vertigo and Dizziness in the United States. J. Vestib. Res. 2021, 31, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Penger, M.; Strobl, R.; Grill, E. Country-Specific and Individual Determinants of Dizziness in Europe: Results from the Survey of Health Ageing and Retirement in Europe (SHARE). Public Health 2017, 149, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Murdin, L.; Schilder, A.G.M. Epidemiology of Balance Symptoms and Disorders in the Community: A Systematic Review. Otol. Neurotol. 2015, 36, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Akdal, G.; Becker-Bense, S.; Hübinger, S.; Huppert, D.; Kentala, E.; Strobl, R.; Zwergal, A.; Celebisoy, N. Multicenter Data Banking in Management of Dizzy Patients: First Results from the DizzyNet Registry Project. J. Neurol. 2018, 265, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Müller, T.; Becker-Bense, S.; Gürkov, R.; Heinen, F.; Huppert, D.; Zwergal, A.; Strobl, R. DizzyReg: The Prospective Patient Registry of the German Center for Vertigo and Balance Disorders. J. Neurol. 2017, 264, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Bisdorff, A.R.; Staab, J.P.; Newman-Toker, D.E. Overview of the International Classification of Vestibular Disorders. Neurol. Clin. 2015, 33, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Dlugaiczyk, J.; Ertl-Wagner, B.B.; Rujescu, D.; Westhofen, M.; Dieterich, M. Vestibular Disorders. Dtsch. Ärztebl. Int. 2020, 117, 300–310. [Google Scholar] [CrossRef]
- Pfieffer, M.L.; Anthamatten, A.; Glassford, M. Assessment and Treatment of Dizziness and Vertigo. Nurse Pract. 2019, 44, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.A.; Hart, C.W. An Approach to the Dizzy Patient. Neurology 1972, 22, 323. [Google Scholar] [CrossRef]
- Kerber, K.A.; Newman-Toker, D.E. Misdiagnosing Dizzy Patients. Neurol. Clin. 2015, 33, 565–575. [Google Scholar] [CrossRef]
- Karatas, M. Central Vertigo and Dizziness: Epidemiology, Differential Diagnosis, and Common Causes. Neurologist 2008, 14, 355–364. [Google Scholar] [CrossRef]
- Seemungal, B.M. The Bárány Society Position on ‘Cervical Dizziness’. J. Vestib. Res. 2022, 32, 487–499. [Google Scholar] [CrossRef]
- Neuhauser, H.K. Neuro-Otology: Diagnosis and Management of Neuro-Otological Disorders. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 275–279. [Google Scholar]
- Hall, C.D.; Herdman, S.J.P.; Whitney, S.L.D.; Anson, E.R.; Carender, W.J.P.; Hoppes, C.W.P.; Cass, S.P.; Christy, J.B.; Cohen, H.S.O.; Fife, T.D.M.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Updated Clinical Practice Guideline from the Academy of Neurologic Physical Therapy of the American Physical Therapy Association. J. Neurol. Phys. Ther. 2022, 46, 118–177. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, D.; Tian, E.; Wang, J.; Guo, Z.; Kong, W. Central Vestibular Dysfunction: Don’t Forget Vestibular Rehabilitation. Expert Rev. Neurother. 2022, 22, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Vestel, C. Systematic Review and Meta-Analysis of the Therapeutic Management of Patients with Cervicogenic Dizziness. J. Man. Manip. Ther. 2022, 30, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Russo, V.; Spitoni, G.F.; Ciancarelli, I.; Paolucci, S.; Manzari, L.; Morone, G. Efficacy of Vestibular Rehabilitation in Patients with Neurologic Disorders: A Systematic Review. Arch. Phys. Med. Rehabil. 2021, 102, 1379–1389. [Google Scholar] [CrossRef]
- Regauer, V.; Seckler, E.; Müller, M.; Bauer, P. Physical Therapy Interventions for Older People with Vertigo, Dizziness and Balance Disorders Addressing Mobility and Participation: A Systematic Review. BMC Geriatr. 2020, 20, 494. [Google Scholar] [CrossRef]
- Heydari, M.; Ahadi, M.; Jalaei, B.; Maarefvand, M.; Talebi, H. The Additional Effect of Vestibular Rehabilitation Therapy on Residual Dizziness after Successful Modified Epley Procedure for Posterior Canal Benign Paroxysmal Positional Vertigo. Am. J. Audiol. 2021, 30, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, F.S. Rehabilitation in Vestibular Injuries. Proc. R. Soc. Med. 1946, 39, 273–278. [Google Scholar] [CrossRef]
- Han, B.I.; Song, H.S.; Kim, J.S. Vestibular Rehabilitation Therapy: Review of Indications, Mechanisms, and Key Exercises. J. Clin. Neurol. 2011, 7, 184. [Google Scholar] [CrossRef]
- Klatt, B.N. A Conceptual Framework for the Progression of Balance Exercises in Persons with Balance and Vestibular Disorders. Phys. Med. Rehabil. Int. 2015, 2, 1044. [Google Scholar] [PubMed]
- Yu, Y.-C.; Xue, H.; Zhang, Y.; Zhou, J. Cognitive Behavior Therapy as Augmentation for Sertraline in Treating Patients with Persistent Postural-Perceptual Dizziness. BioMed Res. Int. 2018, 2018, 8518631. [Google Scholar] [CrossRef] [PubMed]
- Herdman, D.; Norton, S.; Murdin, L.; Frost, K.; Pavlou, M.; Moss-Morris, R. The INVEST Trial: A Randomised Feasibility Trial of Psychologically Informed Vestibular Rehabilitation versus Current Gold Standard Physiotherapy for People with Persistent Postural Perceptual Dizziness. J. Neurol. 2022, 269, 4753–4763. [Google Scholar] [CrossRef] [PubMed]
- Shaver, J. The State of Telehealth Before and After the COVID-19 Pandemic. Prim. Care Clin. Off. Pract. 2022, 49, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hou, M.; Liu, L.; Wang, X. Knowledge Structure and Emerging Trends of Telerehabilitation in Recent 20 Years: A Bibliometric Analysis via CiteSpace. Front. Public Health 2022, 10, 904855. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Pak, S.S.; Keller, D.R.; Gustavson, A.M.; Barnes, D.E. Physical Therapist Telehealth Delivery at 1 Year Into COVID-19. Phys. Ther. 2022, 102, pzac121. [Google Scholar] [CrossRef]
- Werneke, M.W.; Deutscher, D.; Grigsby, D.; Tucker, C.A.; Mioduski, J.E.; Hayes, D. Telerehabilitation During the COVID-19 Pandemic in Outpatient Rehabilitation Settings: A Descriptive Study. Phys. Ther. 2021, 101, pzab110. [Google Scholar] [CrossRef] [PubMed]
- Seron, P.; Oliveros, M.-J.; Gutierrez-Arias, R.; Fuentes-Aspe, R.; Torres-Castro, R.C.; Merino-Osorio, C.; Nahuelhual, P.; Inostroza, J.; Jalil, Y.; Solano, R.; et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys. Ther. 2021, 101, pzab053. [Google Scholar] [CrossRef]
- Kocyigit, B.F.; Assylbek, M.I.; Yessirkepov, M. Telerehabilitation: Lessons from the COVID-19 Pandemic and Future Perspectives. Rheumatol. Int. 2024, 44, 577–582. [Google Scholar] [CrossRef]
- Baroni, M.P.; Jacob, M.F.A.; Rios, W.R.; Fandim, J.V.; Fernandes, L.G.; Chaves, P.I.; Fioratti, I.; Saragiotto, B.T. The State of the Art in Telerehabilitation for Musculoskeletal Conditions. Arch. Physiother. 2023, 13, 1. [Google Scholar] [CrossRef]
- León-Salas, B.; González-Hernández, Y.; Infante-Ventura, D.; de Armas-Castellano, A.; García-García, J.; García-Hernández, M.; Carmona-Rodríguez, M.; Olazarán, J.; Dobato, J.L.; Rodríguez-Rodríguez, L.; et al. Telemedicine for Neurological Diseases: A Systematic Review and Meta-Analysis. Eur. J. Neurol. 2023, 30, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Bonanno, M.; Torregrossa, W.; Cacciante, L.; Celesti, A.; Rifici, C.; Tonin, P.; De Luca, R.; Quartarone, A. Benefits of Telerehabilitation for Patients with Severe Acquired Brain Injury: Promising Results from a Multicenter Randomized Controlled Trial Using Nonimmersive Virtual Reality. J. Med. Internet Res. 2023, 25, e45458. [Google Scholar] [CrossRef] [PubMed]
- Shem, K.; Irgens, I.; Alexander, M.G.S. Mechanisms of Telerehabilitation. In Telerehabilitation 5–20; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Cottrell, M.A.; Russell, T.G. Telehealth for Musculoskeletal Physiotherapy. Musculoskelet. Sci. Pract. 2020, 48, 102193. [Google Scholar] [CrossRef] [PubMed]
- Green, K.E.; Pogson, J.M.; Otero-Millan, J.; Gold, D.R.; Tevzadze, N.; Tehrani, A.S.S.; Zee, D.S.; Newman-Toker, D.E.; Kheradmand, A. Opinion and Special Articles: Remote Evaluation of Acute Vertigo: Strategies and Technological Considerations. Neurology 2021, 96, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.G.; Bronstein, A.; Carmona, S.; Cha, Y.-H.; Cho, C.; Ghasia, F.F.; Gold, D.; Green, K.E.; Helmchen, C.; Ibitoye, R.T.; et al. Consensus on Virtual Management of Vestibular Disorders: Urgent Versus Expedited Care. Cerebellum 2021, 20, 4–8. [Google Scholar] [CrossRef]
- Schoo, D.P.; Ward, B.K. New Frontiers in Managing the Dizzy Patient. Otolaryngol. Clin. N. Am. 2021, 54, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Bertholon, P.; Thai-Van, H.; Bouccara, D.; Esteve-Fraysse, M.-J.; Wiener-Vacher, S.; Ionescu, E. Guidelines of the French Society of Otorhinolaryngology (SFORL) for Teleconsultation in Patients with Vertigo during the COVID-19 Pandemic. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Harrell, R.G.; Schubert, M.C.; Oxborough, S.; Whitney, S.L. Vestibular Rehabilitation Telehealth During the SAEA-CoV-2 (COVID-19) Pandemic. Front. Neurol. 2022, 12, 781482. [Google Scholar] [CrossRef]
- Meldrum, D.; Murray, D.; Vance, R.; Coleman, S.; McConnell, S.; Hardiman, O.; Walsh, R.M. Toward a Digital Health Intervention for Vestibular Rehabilitation: Usability and Subjective Outcomes of a Novel Platform. Front. Neurol. 2022, 13, 836796. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schiavenato, M.; Chu, F. PICO: What It Is and What It Is Not. Nurse Educ. Pract. 2021, 56, 103194. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Beech, S.; Zander, L.; Evans, T.; Weinman, J. A Randomized Controlled Trial of Exercise Therapy for Dizziness and Vertigo in Primary Care. Br. J. Gen. Pract. 1998, 48, 1136–1140. [Google Scholar]
- Söderman, A.-C.H.; Bergenius, J.; Bagger-Sjöbäck, D.; Tjell, C.; Langius, A. Patients’ Subjective Evaluations of Quality of Life Related to Disease-Specific Symptoms, Sense of Coherence, and Treatment in MéNièRe’s Disease. Otol. Neurotol. 2001, 22, 526–533. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Newman, C.W. The Development of the Dizziness Handicap Inventory. Arch. Otolaryngol.-Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The Validity of the Hospital Anxiety and Depression Scale. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Piker, E.G.; Kaylie, D.M.; Garrison, D.; Tucci, D.L. Hospital Anxiety and Depression Scale: Factor Structure, Internal Consistency and Convergent Validity in Patients with Dizziness. Audiol. Neurotol. 2015, 20, 394–399. [Google Scholar] [CrossRef]
- Löwe, B.; Decker, O.; Müller, S.; Brähler, E.; Schellberg, D.; Herzog, W.; Herzberg, P.Y. Validation and Standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the General Population. Med. Care 2008, 46, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kjærgaard, M.; Arfwedson Wang, C.E.; Waterloo, K.; Jorde, R. A Study of the Psychometric Properties of the Beck Depression Inventory-II, the Montgomery and Åsberg Depression Rating Scale, and the Hospital Anxiety and Depression Scale in a Sample from a Healthy Population. Scand. J. Psychol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Gorenstein, C. Psychometric Properties of the Beck Depression Inventory-II: A Comprehensive Review. Rev. Bras. Psiquiatr. 2013, 35, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.; Li, C.; Aslakson, R.; Agrawal, Y. Systematic Review of Patient-Reported Outcome Measures in Clinical Vestibular Research. Arch. Phys. Med. Rehabil. 2015, 96, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zang, J. The Effect of Accompanying Anxiety and Depression on Patients with Different Vestibular Syndromes. Front. Aging Neurosci. 2023, 15, 1208392. [Google Scholar] [CrossRef] [PubMed]
- Radziej, K.; Probst, T.; Limburg, K.; Dinkel, A.; Dieterich, M.; Lahmann, C. The Longitudinal Effect of Vertigo and Dizziness Symptoms on Psychological Distress: Symptom-Related Fears and Beliefs as Mediators. J. Nerv. Ment. Dis. 2018, 206, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Prell, T.; Axer, H. Avoidance Behavior in Patients with Chronic Dizziness: A Prospective Observational Study. J. Clin. Med. 2022, 11, 7473. [Google Scholar] [CrossRef] [PubMed]
- Omara, A.; Basiouny, E.M.; Shabrawy, M.E.; Shafei, R.R.E. The Correlation between Anxiety, Depression, and Vertigo: A Cross-Sectional Study. Egypt. J. Otolaryngol. 2022, 38, 143. [Google Scholar] [CrossRef]
- Rizk, H.G.; Liu, Y.F. Interviewing and Counseling the Dizzy Patient with Focus on Quality of Life. Otolaryngol. Clin. N. Am. 2021, 54, 853–861. [Google Scholar] [CrossRef]
- Ciorba, A.; Bianchini, C.; Scanelli, G.; Pala, M.; Zurlo, A.; Aimoni, C. The Impact of Dizziness on Quality-of-Life in the Elderly. Eur. Arch. Otorhinolaryngol. 2017, 274, 1245–1250. [Google Scholar] [CrossRef]
- Weidt, S.; Bruehl, A.B.; Straumann, D.; Hegemann, S.C.; Krautstrunk, G.; Rufer, M. Health-Related Quality of Life and Emotional Distress in Patients with Dizziness: A Cross-Sectional Approach to Disentangle Their Relationship. BMC Health Serv. Res. 2014, 14, 317. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Collaboration, T.C. Review Manager (RevMan), 5.4; The Cochrane Collaboration: London, UK, 2020. [Google Scholar]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Front Matter. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-53662-8. [Google Scholar]
- Szturm, T.; Hochman, J.; Wu, C.; Lisa, L.; Reimer, K.; Wonneck, B.; Giacobbo, A. Games and Telerehabilitation for Balance Impairments and Gaze Dysfunction: Protocol of a Randomized Controlled Trial. JMIR Res. Protoc. 2015, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, A.W.A.; Essery, R.; Kirby, S.; Stuart, B.; Turner, D.; Little, P.; Bronstein, A.; Andersson, G.; Carlbring, P.; Yardley, L. Internet-Based Vestibular Rehabilitation for Older Adults with Chronic Dizziness: A Randomized Controlled Trial in Primary Care. Ann. Fam. Med. 2017, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vugt, V.A.; Wouden, J.C.; Essery, R.; Yardley, L.; Twisk, J.W.R.; Horst, H.E.; Maarsingh, O. Internet Based Vestibular Rehabilitation with and without Physiotherapy Support for Adults Aged 50 and Older with a Chronic Vestibular Syndrome in General Practice: Three Armed Randomised Controlled Trial. BMJ 2019, 367, l5922. [Google Scholar] [CrossRef]
- Yardley, L.; Barker, F.; Muller, I.; Turner, D.; Kirby, S.; Mullee, M.; Morris, A.; Little, P. Clinical and cost effectiveness of booklet based vestibular rehabilitation for chronic dizziness in primary care: Single blind, parallel group, pragmatic, randomised controlled trial. BMJ 2012, 344, e2237. [Google Scholar] [CrossRef] [PubMed]
- Haciabbasoğlu, R.; Araci, A.; Günizi, H. Are Telerehabilitation Exercise Practices Effective in Patients Diagnosed with Benign Paroxysmal Positional Vertigo? Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 557–567. [Google Scholar] [CrossRef]
- Smaerup, M.; Grönvall, E.; Larsen, S.B.; Laessoe, U.; Henriksen, J.-J.; Damsgaard, E.M. Computer-Assisted Training as a Complement in Rehabilitation of Patients with Chronic Vestibular Dizziness—A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Smaerup, M.; Laessoe, U.; Grönvall, E.; Henriksen, J.-J.; Damsgaard, E.M. The Use of Computer-Assisted Home Exercises to Preserve Physical Function after a Vestibular Rehabilitation Program: A Randomized Controlled Study. Rehabil. Res. Pract. 2016, 2016, 7026317. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Donovan-Hall, M.; Smith, H.E.; Walsh, B.M.; Mullee, M.; Bronstein, A.M. Effectiveness of Primary Care–Based Vestibular Rehabilitation for Chronic Dizziness. Ann. Intern. Med. 2004, 141, 598. [Google Scholar] [CrossRef]
- Essery, R.; Kirby, S.; Geraghty, A.W.A.; Andersson, G.; Carlbring, P.; Bronstein, A.; Little, P.; Yardley, L. The Development of Balance Retraining: An Online Intervention for Dizziness in Adults Aged 50 Years and Older. Am. J. Audiol. 2015, 24, 276–279. [Google Scholar] [CrossRef]
- Boyd, R.N. Move It to Improve It (Mitii): Study Protocol of a Randomised Controlled Trial of a Novel Web-Based Multimodal Training Program for Children and Adolescents with Cerebral Palsy. BMJ Open 2013, 3, 002853. [Google Scholar] [CrossRef] [PubMed]
- Beukes, E.W.; Manchaiah, V.; Allen, P.M.; Baguley, D.M.; Andersson, G. Internet-Based Interventions for Adults with Hearing Loss, Tinnitus, and Vestibular Disorders: A Systematic Review and Meta-Analysis. Trends Hear. 2019, 23, 233121651985174. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Kamo, T.; Smith, L.; Harrington-Benton, N.A.; Judd, O.; Kaski, D.; Maarsingh, O.R.; MacKeith, S.; Ray, J.; Vugt, V.A.; et al. Non-pharmacological Interventions for Persistent Postural-perceptual Dizziness (PPPD). Cochrane Database Syst. Rev. 2023, CD015333. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-Y.; Song, N.; Zhou, Z.-R.; Li, Z.-F.; Yang, X. Can Virtual Reality-Assisted Therapy Offer Additional Benefits to Patients with Vestibular Disorders Compared with Conventional Vestibular Physical Therapy? A Meta-Analysis. Arch. Phys. Med. Rehabil. 2023, 104, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Suso-Martí, L.; La Touche, R.; Herranz-Gómez, A.; Angulo-Díaz-Parreño, S.; Paris-Alemany, A.; Cuenca-Martínez, F. Effectiveness of Telerehabilitation in Physical Therapist Practice: An Umbrella and Mapping Review with Meta–Meta-Analysis. Phys. Ther. 2021, 101, pzab075. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.F.; Oliveira, V.C.; Borges, P.R.T.; Dutra, F.C.M.S.; Mancini, M.C.; Kirkwood, R.N.; Resende, R.A.; Sampaio, R.F. Effectiveness of Exercises by Telerehabilitation on Pain, Physical Function and Quality of Life in People with Physical Disabilities: A Systematic Review of Randomised Controlled Trials with GRADE Recommendations. Br. J. Sports Med. 2021, 55, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.B.; Mukherjee, T.; Shah, P.V.; Ambode, O.I.; Johnson, E.G.; Daher, N.S. Home Exercise Program Adherence Strategies in Vestibular Rehabilitation: A Systematic Review. Phys. Ther. Rehabil. Sci. 2016, 5, 53–62. [Google Scholar] [CrossRef]
- Meldrum, D.; Burrows, L.; Cakrt, O.; Kerkeni, H.; Lopez, C.; Tjernstrom, F.; Vereeck, L.; Zur, O.; Jahn, K. Vestibular rehabilitation in Europe: A survey of clinical and research practice. J. Neurol. 2020, 267, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Muller, I.; Kirby, S.; Yardley, L. Understanding Patient Experiences of Self-Managing Chronic Dizziness: A Qualitative Study of Booklet-Based Vestibular Rehabilitation, with or without Remote Support. BMJ Open 2015, 5, 007680. [Google Scholar] [CrossRef]
- Eldøen, G.; Kvalheim, S.E.; Thesen, T.; Mygland, Å.; Ljøstad, U.; Bakke, S.; Holo, M.H.; Løge, I.; Jonsbu, E. Web-based Vestibular Rehabilitation in Persistent Postural-perceptual Dizziness. Brain Behav. 2021, 11, e2346. [Google Scholar] [CrossRef]
- Hovareshti, P.; Roeder, S.; Holt, L.S.; Gao, P.; Xiao, L.; Zalkin, C.; Ou, V.; Tolani, D.; Klatt, B.N.; Whitney, S.L. VestAid: A Tablet-Based Technology for Objective Exercise Monitoring in Vestibular Rehabilitation. Sensors 2021, 21, 8388. [Google Scholar] [CrossRef]
- Beh, S.C. The Neuropsychology of Dizziness and Related Disorders. Otolaryngol. Clin. N. Am. 2021, 54, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Carender, W.J.; Grzesiak, M.; Telian, S.A. Vestibular Physical Therapy and Fall Risk Assessment. Otolaryngol. Clin. N. Am. 2021, 54, 1015–1036. [Google Scholar] [CrossRef]
- Hülse, R.; Biesdorf, A.; Hörmann, K.; Stuck, B.; Erhart, M.; Hülse, M.; Wenzel, A. Peripheral Vestibular Disorders: An Epidemiologic Survey in 70 Million Individuals. Otol. Neurotol. 2019, 40, 88–95. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.N.; Hillier, S.L. Vestibular Rehabilitation for Unilateral Peripheral Vestibular Dysfunction. Cochrane Database Syst. Rev. 2015, CD005397. [Google Scholar] [CrossRef] [PubMed]





| Author and Year | Population | Interventions | Outcome Measures | Effects (Experimental vs. Control Comparison) | Conclusion |
|---|---|---|---|---|---|
| Haciabbasoğlu et al., 2023 [70] | 44 individuals with diagnosis of “positional vertigo”. Age = 18–65 years. Experimental group: 22 individuals. Control group: 22 individuals. | Experimental group: Vestibular rehabilitation adaptation exercises performed at home, and vestibular rehabilitation balance exercises in telerehabilitation. Telerehabilitation with therapist via WhatsApp video call, 25–30 min 2 times a day for 6 weeks. Exercises in frequency autonomy not specified. Control group: Vestibular rehabilitation adaptation exercises performed at home 2–3 times/day for 6 weeks. The exercises were shown in person, and sent via WhatsApp together with other material additional | Primary Outcome:
| Primary Outcome (6 weeks):
| TR applications are effective and clinically applicable in patients with BPPV. |
| Van Vugt et al., 2019 [68] | 322 individuals aged visited by the GP in the previous 2 years for vestibular symptoms. Age > 50 years. Experimental group: 98 individuals. Control group: 120 individuals. | Experimental group: “Stand alone internet-based VR” 6 weeks of: daily sessions of 6 online VR exercises independently provided by the “Balance Retraining” site (10 min × 2 times a day) without support from the therapist. Different weekly sessions of online VR without therapist support. Information and advice on anxiety control strategies. Weekly email to remind you to access the site. Control group: “Usual care”: standard level of care provided by their doctor, with access to every available treatment between primary and secondary care after referral. | Primary Outcome:
| Primary Outcome (3 months): Stand-Alone VR vs. usual care
Stand-Alone VR vs. usual care
| Stand-alone and blended internet-based VR are clinically effective and safe interventions to treat adults aged 50 and older with a chronic vestibular syndrome. |
| Geraghty et al., 2017 [67] | 296 individuals visited the GP for vertigo in the last 2 years, and with still present vertigo which worsens with movement of the head. Age > 50 years. Experimental group:160 individuals. Control group: 136 individuals. | Experimental group: 6 weeks of online VR provided by the site “Balance Retraining” without therapist support.—information and advice on anxiety control strategies. Control group: “Usual Care”: subjects receive usual UK primary care, i.e., reassurance, symptom relief (e.g., medication for nausea) and sometimes education. | Primary Outcome:
| Primary Outcome (3 months):
| Internet-based vestibular rehabilitation reduces dizziness and dizziness-related disability in older primary care patients without requiring clinical support. |
| Smaerup et al., 2016 [72] | 63 individuals who completed the inpatient rehabilitation program. Individuals with peripheral, central or mixed stable vestibular disorder. ≥65 years. Experimental group: 32 individuals Control group: 31 individuals | Experimental group: Intervention provided by the “Mitii” website through a PC connected to the Internet and a webcam. Daily exercise program of 20/30′, at least once a day, with sequences of games. The site sends information on the duration of the treatment to the hospital therapist, who contacts the patient in the event of a 7-day absence from the program. The therapist prompts participants to continue with exercise sessions even after they finish. Twelve-week follow-up. Control group: Standard care: after hospital discharge, printed instructions are given for continuing the exercises at home. Exercise program of 20/30′. Twelve-week follow-up. | Primary outcome:
| Primary outcome (12 weeks):
| Elderly vestibular dysfunction patients exercising at home seem to maintain their functional level, level of dizziness, and quality of life three months following discharge from hospital. In this specific setup, no greater effect was found by introducing a computer-assisted training program, when compared to standard home training guided by printed instruction. |
| Smaerup et al., 2015 [71] | 63 individuals underwent in-hospital rehabilitation 2 times a week for 16 weeks, with diagnosis peripheral, central or mixed stable vestibular disorder. Age ≥ 65 years. Experimental group: 32 individuals. Control gropu:31 individuals. | Experimental group: Intervention provided by the “Mitii” website through a PC connected to the Internet and a webcam. Daily exercise program of 20/30′ with sequences of games for 16 weeks. The site sends information on the duration of the treatment to the hospital therapist, who contacts the patient in the event of a 7-day absence from the program. The therapist calls once a month to adjust the duration, speed, and difficulty of the exercises based on progress. The patient is also undergoing rehabilitation in hospital 2 times/week for 16 weeks. Control group: Delivered a paper program of the exercises to be performed, of 20/30′ at least once a day, for 16 weeks. | Primary Outcome:
| Primary outcome (16 weeks):
| A computer-assisted program to support the home training of elderly patients with vestibular dysfunction did not improve rehabilitation more than printed instructions did. |
| Yardley et al., 2012 [69] | 337 individuals with dizziness in the last 2 years. Age ≥ 18 years. Experimental group 112 individuals. Experimental group: 113 individuals. Control group: 112 individuals. | Experimental group: “Book self-management and telephone support” group, with exercise sessions conducted through a validated booklet, of 5–10′ twice a day for 12 weeks, plus 3 telephone support sessions at baseline, first and third week. Follow up at 12 weeks and 1 year. Experimental group: “Book self-management” group with exercise sessions carried out through a validated booklet, of 5–10′ twice a day for 12 weeks. Follow up at 12 weeks and 1 year. Control group: “Routine care” group, treated with reassurance and symptom reduction (e.g., drugs). Follow up at 12 weeks and at 1 year. | Primary outcome:
| Primary outcome (12 weeks): Book self-management and telephone support vs. routine care
Book self-management and telephone support vs. routine care
| Booklet-based vestibular rehabilitation for chronic dizziness is a simple and cost-effective means of improving patient-reported outcomes in primary care. |
| Yardley et al., 2004 [73] | 170 individuals with vertigo in the last two years. Age ≥ 60 years. Experimental group: 83 individuals. Control group: 87 individuals. | Experimental group: “Vestibular rehabilitation group” with exercise sessions carried out through a booklet, plus two telephone support sessions in the first and third week. Follow up at 12 weeks and 6 months. Control group: “Usual medical care group” treated with reassurance and symptom reduction (e.g., drugs). Follow up at 12 weeks and at 6 months. | Primary Outcome:
| Primary Outcome (3 months):
| Booklet based vestibular rehabilitation for chronic dizziness is a simple and cost-effective means of improving patient reported outcomes in primary care. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillo, D.; Zitti, M.; Cieślik, B.; Vania, S.; Zangarini, S.; Bargellesi, S.; Kiper, P. Effectiveness of Telerehabilitation in Dizziness: A Systematic Review with Meta-Analysis. Sensors 2024, 24, 3028. https://doi.org/10.3390/s24103028
Grillo D, Zitti M, Cieślik B, Vania S, Zangarini S, Bargellesi S, Kiper P. Effectiveness of Telerehabilitation in Dizziness: A Systematic Review with Meta-Analysis. Sensors. 2024; 24(10):3028. https://doi.org/10.3390/s24103028
Chicago/Turabian StyleGrillo, Davide, Mirko Zitti, Błażej Cieślik, Stefano Vania, Silvia Zangarini, Stefano Bargellesi, and Pawel Kiper. 2024. "Effectiveness of Telerehabilitation in Dizziness: A Systematic Review with Meta-Analysis" Sensors 24, no. 10: 3028. https://doi.org/10.3390/s24103028
APA StyleGrillo, D., Zitti, M., Cieślik, B., Vania, S., Zangarini, S., Bargellesi, S., & Kiper, P. (2024). Effectiveness of Telerehabilitation in Dizziness: A Systematic Review with Meta-Analysis. Sensors, 24(10), 3028. https://doi.org/10.3390/s24103028






