UWB-Modulated Microwave Imaging for Human Brain Functional Monitoring
Abstract
1. Introduction
2. Analytical Formulation
2.1. Illuminating–Focusing Geometry
2.2. RF UWB Scattering
2.3. UWB Inverse Mapping
2.4. Scattering Low-Frequency ( kHz) Action Potential Modulation Process
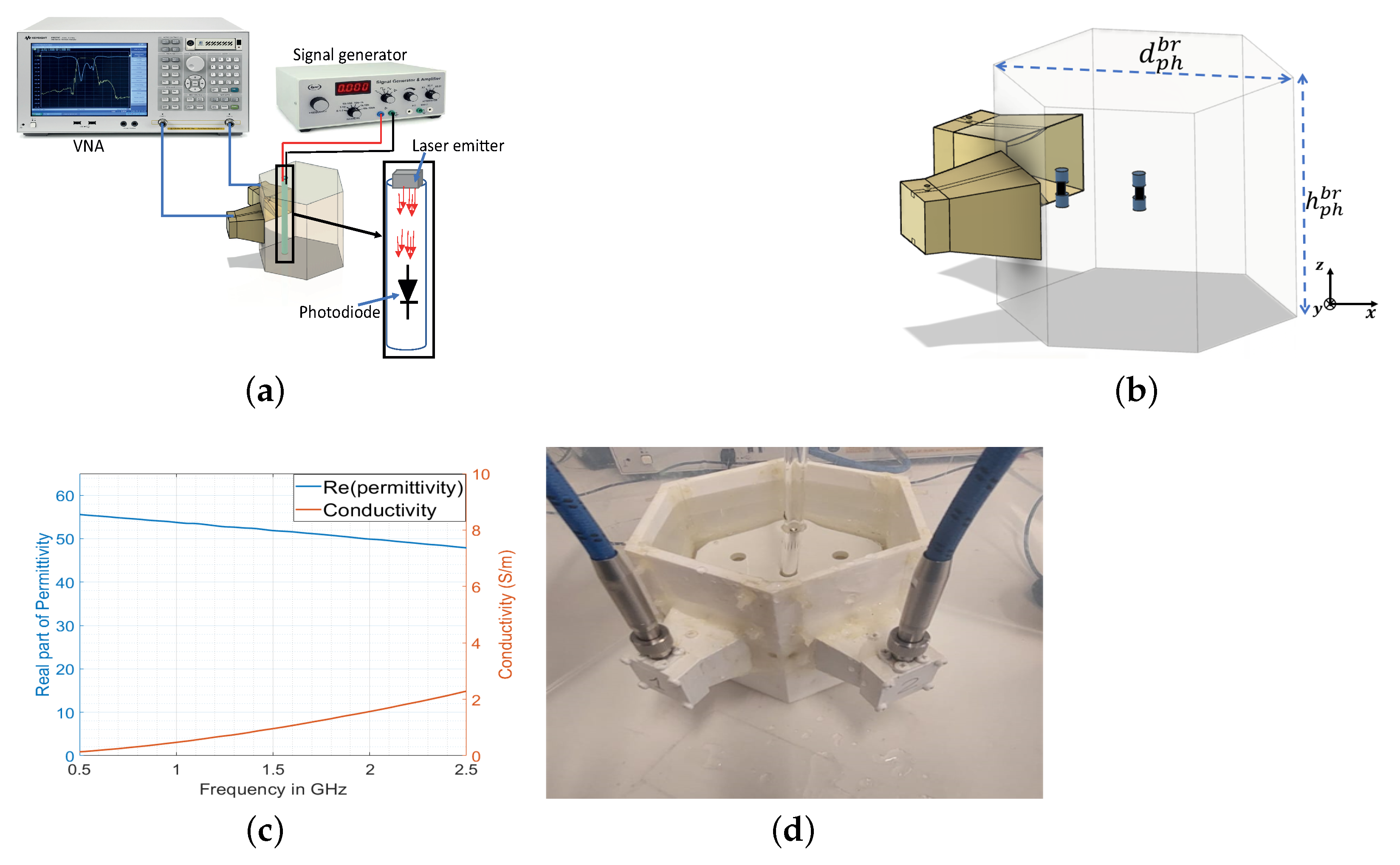
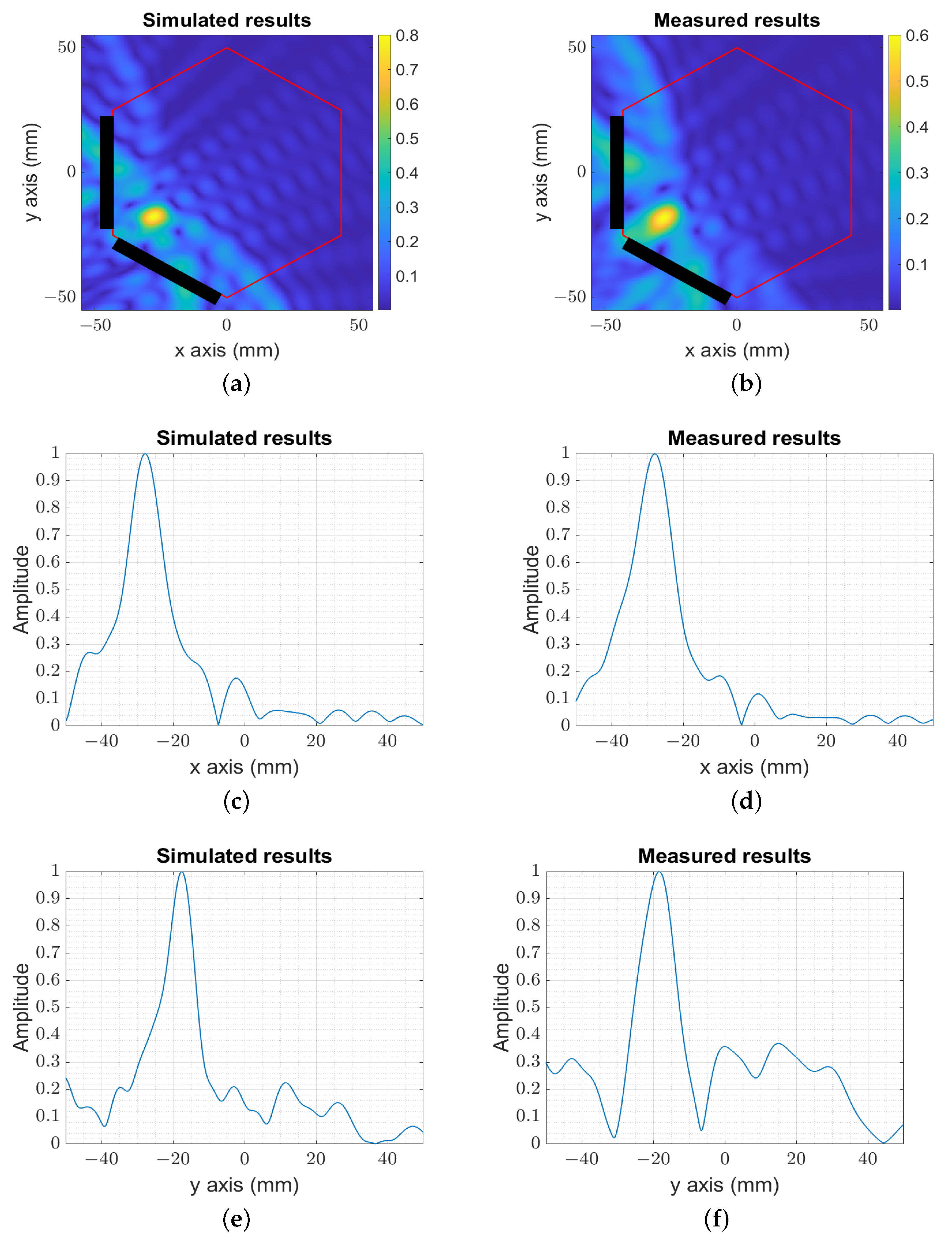
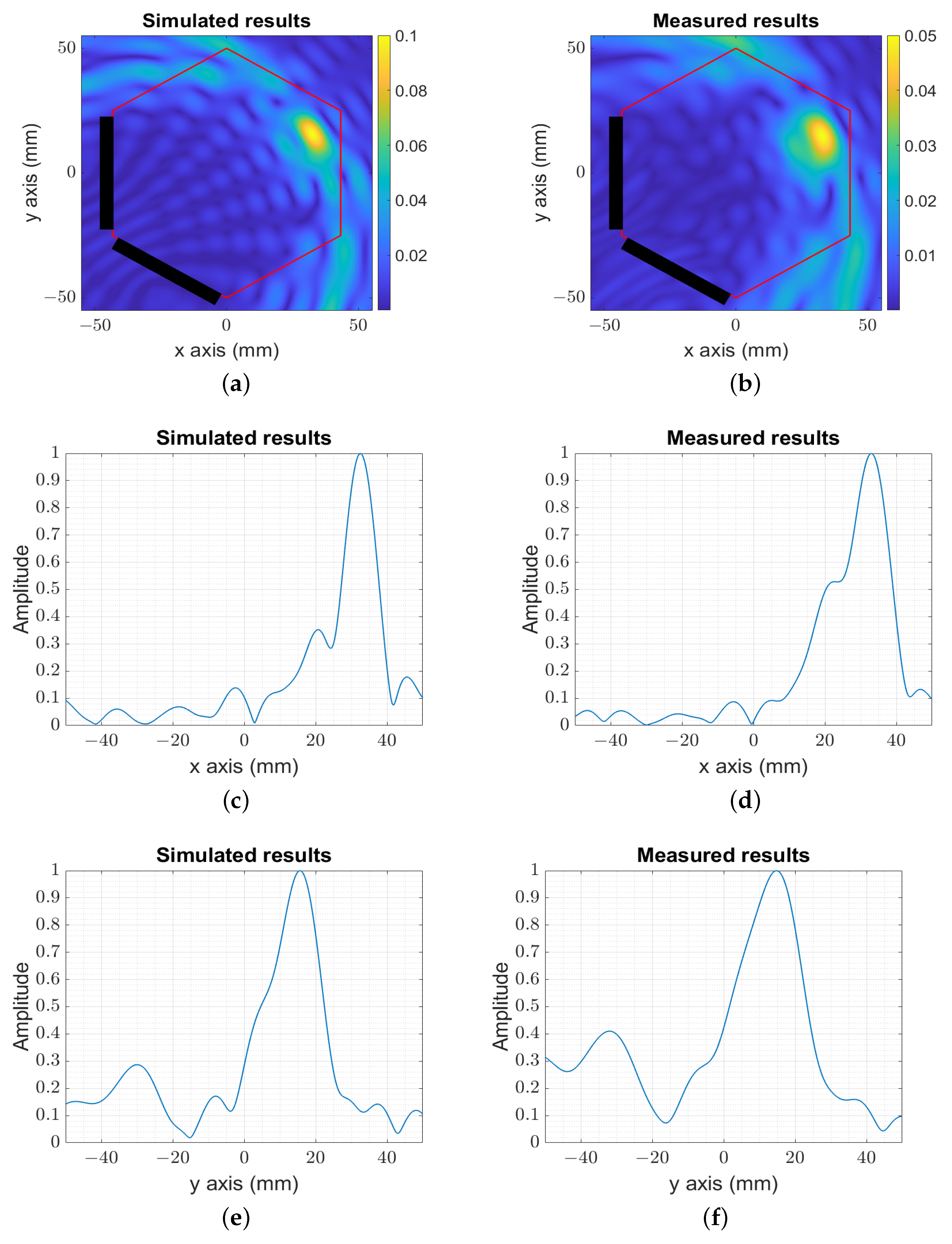
3. Numerical and Experimental Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larsen, L.E.; Jacobi, J.H. Medical Applications of Microwave Imaging; Technical Report; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 1985. [Google Scholar]
- Chaudhary, S.; Mishra, R.; Swarup, A.; Thomas, J.M. Dielectric properties of normal & malignant human breast tissues at radiowave & microwave frequencies. Indian J. Biochem. Biophys. 1984, 21, 76–79. [Google Scholar]
- Jofre, L.; Hawley, M.S.; Broquetas, A.; de Los Reyes, E.; Ferrando, M.; Elias-Fuste, A.R. Medical imaging with a microwave tomographic scanner. IEEE Trans. Biomed. Eng. 1990, 37, 303–312. [Google Scholar] [CrossRef]
- Razzicchia, E.; Sotiriou, I.; Cano-Garcia, H.; Kallos, E.; Palikaras, G.; Kosmas, P. Feasibility study of enhancing microwave brain imaging using metamaterials. Sensors 2019, 19, 5472. [Google Scholar] [CrossRef]
- Guardiola Garcia, M.; Jofre Roca, L.; Capdevila Cascante, S.; Blanch Boris, S.; Romeu Robert, J. 3D UWB magnitude-combined tomographic imaging for biomedical applications. algorithm validation. Radioengineering 2011, 20, 366–372. [Google Scholar]
- Gilmore, C.; Zakaria, A.; Pistorius, S.; LoVetri, J. Microwave imaging of human forearms: Pilot study and image enhancement. Int. J. Biomed. Imaging 2013, 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Alwan, M.; Sadek, S.; Katbay, Z. Investigation of tumor using an antenna scanning system. In Proceedings of the 2014 Mediterranean Microwave Symposium (MMS2014), Marrakech, Morocco, 12–14 December 2014; pp. 1–4. [Google Scholar]
- Zhang, H.; Flynn, B.; Erdogan, A.T.; Arslan, T. Microwave imaging for brain tumour detection using an UWB Vivaldi Antenna array. In Proceedings of the 2012 Loughborough Antennas & Propagation Conference (LAPC), Loughborough, UK, 12–13 November 2012; pp. 1–4. [Google Scholar]
- Zhang, H. Microwave Imaging for Ultra-Wideband Antenna Based Cancer Detection. 2015. Available online: https://era.ed.ac.uk/bitstream/handle/1842/9958/Zhang2015.pdf?sequence=1&isAllowed=y (accessed on 25 March 2023).
- Bahramiabarghouei, H.; Porter, E.; Santorelli, A.; Gosselin, B.; Popović, M.; Rusch, L.A. Flexible 16 antenna array for microwave breast cancer detection. IEEE Trans. Biomed. Eng. 2015, 62, 2516–2525. [Google Scholar] [CrossRef]
- Zerrad, F.e.; Taouzari, M.; Makroum, E.M.; Aoufi, J.E.; Qanadli, S.D.; Karaaslan, M.; Al-Gburi, A.J.A.; Zakaria, Z. Microwave Imaging Approach for Breast Cancer Detection Using a Tapered Slot Antenna Loaded with Parasitic Components. Materials 2023, 16, 1496. [Google Scholar] [CrossRef]
- Westerlund, U.; Moe, M.C.; Varghese, M.; Berg-Johnsen, J.; Ohlsson, M.; Langmoen, I.A.; Svensson, M. Stem cells from the adult human brain develop into functional neurons in culture. Exp. Cell Res. 2003, 289, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Teplov, I.Y.; Tuleukhanov, S.; Zinchenko, V. Regulation of Action Potential Frequency and Amplitude by T-type Ca2+ Channel During Spontaneous Synchronous Activity of Hippocampal Neurons. Biophysics 2018, 63, 566–575. [Google Scholar] [CrossRef]
- Tovar, K.R.; Bridges, D.C.; Wu, B.; Randall, C.; Audouard, M.; Jang, J.; Hansma, P.K.; Kosik, K.S. Recording action potential propagation in single axons using multi-electrode arrays. bioRxiv 2017, 126425. [Google Scholar] [CrossRef]
- Sharma, M.; Gardner, A.T.; Strathman, H.J.; Warren, D.J.; Silver, J.; Walker, R.M. Acquisition of neural action potentials using rapid multiplexing directly at the electrodes. Micromachines 2018, 9, 477. [Google Scholar] [CrossRef]
- Tseng, K.C.; Lin, B.S.; Liao, L.D.; Wang, Y.T.; Wang, Y.L. Development of a wearable mobile electrocardiogram monitoring system by using novel dry foam electrodes. IEEE Syst. J. 2013, 8, 900–906. [Google Scholar] [CrossRef]
- Ren, K.; Ma, R.; Nikkhah, M.R.; Eggleston, S.; Ren, Y.J.; Behdad, N. Contact ECG Recording Using Copper and E-Textile Based Flexible Dry Electrodes. Prog. Electromagn. Res. M 2021, 101, 47–58. [Google Scholar] [CrossRef]
- Obeid, D.; Sadek, S.; Zaharia, G.; Zein, G.E. Noncontact heartbeat detection at 2.4, 5.8, and 60 GHz: A comparative study. Microw. Opt. Technol. Lett. 2009, 51, 666–669. [Google Scholar] [CrossRef]
- El-Samad, S.; Obeid, D.; Zaharia, G.; Sadek, S.; El Zein, G. Remote heartbeat detection using microwave system from four positions of a normally breathing patient. Int. J. Commun. Antenna Propag. 2016, 6. [Google Scholar] [CrossRef]
- Obeid, D.; Zaharia, G.; Sadek, S.; El Zein, G. ECG vs. single-antenna system for heartbeat activity detection. In Proceedings of the 4th International Symposium on Applied Sciences in Biomedical and Communication Technologies, Barcelona, Spain, 26–29 October 2011; pp. 1–5. [Google Scholar]
- Jiang, X.; Geng, Z.; Li, X.; Peng, L.; Kang, B.; Zheng, C. Microwave transmission approach for dynamic dielectric detection at brain functional site. In Proceedings of the 2017 IEEE MTT-S International Microwave Symposium (IMS), Honololu, HI, USA, 4–9 June 2017; pp. 1235–1238. [Google Scholar]
- Wang, J.K.; Jiang, X.; Peng, L.; Li, X.M.; An, H.J.; Wen, B.J. Detection of neural activity of brain functional site based on microwave scattering principle. IEEE Access 2019, 7, 13468–13475. [Google Scholar] [CrossRef]
- Palacios, C.; Jofre, M.; Jofre, L.; Romeu, J.; Jofre-Roca, L. Superheterodyne microwave system for the detection of bioparticles with coplanar electrodes on a microfluidic platform. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Jofre, M.; Jofre, L.; Jofre-Roca, L. On the wireless microwave sensing of bacterial membrane potential in microfluidic-actuated platforms. Sensors 2021, 21, 3420. [Google Scholar] [CrossRef]
- González-López, G.; Jofre Roca, L.; Amorós García de Valdecasas, S.; Rodríguez-Leor, O.; Gálvez-Montón, C.; Bayés-Genís, A.; O’Callaghan, J. Resonance-Based microwave technique for body implant sensing. Sensors 2019, 19, 4828. [Google Scholar] [CrossRef]
- Dostrovsky, J.O.; Hutchison, W.D.; Lozano, A.M. The globus pallidus, deep brain stimulation, and Parkinson’s disease. Neuroscientist 2002, 8, 284–290. [Google Scholar]
- Akazzim, Y.; El Mrabet, O.; Romeu, J.; Jofre-Roca, L. Multi-Element UWB Probe Optimization for Medical Microwave Imaging. Sensors 2022, 23, 271. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Jofre, L.; Garrido, A.; Gonzalez, G.; Ding, Y.; Aguasca, A.; O’Callaghan, J.; Romeu, J. 3-D Printed UWB Microwave Bodyscope for Biomedical Measurements. IEEE Antennas Wirel. Propag. Lett. 2019, 18, 626–630. [Google Scholar] [CrossRef]
- Shlivinski, A.; Heyman, E.; Kastner, R. Antenna characterization in the time domain. IEEE Trans. Antennas Propag. 1997, 45, 1140–1149. [Google Scholar] [CrossRef]
- Bolomey, J.C.; Capdevila, S.; Jofre, L.; Romeu, J. Electromagnetic modeling of RFID-modulated scattering mechanism. Application to tag performance evaluation. Proc. IEEE 2010, 98, 1555–1569. [Google Scholar] [CrossRef]
- Madanan, G.; Krishna, D.D. Time Domain Performance Evaluation of UWB Antennas. In Innovations in Ultra-WideBand Technologies; IntechOpen: London, UK, 2020. [Google Scholar]
- Jofre, L.; Broquetas, A.; Romeu, J.; Blanch, S.; Toda, A.P.; Fabregas, X.; Cardama, A. UWB tomographic radar imaging of penetrable and impenetrable objects. Proc. IEEE 2009, 97, 451–464. [Google Scholar] [CrossRef]
- Jofre, L.; Toda, A.P.; Montana, J.M.J.; Carrascosa, P.C.; Romeu, J.; Blanch, S.; Cardama, A. UWB short-range bifocusing tomographic imaging. IEEE Trans. Instrum. Meas. 2008, 57, 2414–2420. [Google Scholar] [CrossRef]
- Rokunuzzaman, M.; Ahmed, A.; Baum, T.; Rowe, W.S. Microwave power penetration enhancement inside an inhomogeneous human head. Sci. Rep. 2021, 11, 21793. [Google Scholar] [CrossRef]
- Mohammed, B.J.; Abbosh, A.M. Realistic head phantom to test microwave systems for brain imaging. Microw. Opt. Technol. Lett. 2014, 56, 979–982. [Google Scholar] [CrossRef]




| Backscattered Modulation Index @ 1GHz | Analytical Validation of the Microtag | Experimental Validation of the Microtag | Real Microorganism |
|---|---|---|---|
| Photodiode WL-SDCB SMT | Membrane | ||
| ≃0.045 | ≃0.040 | ≃0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akazzim, Y.; Jofre, M.; El Mrabet, O.; Romeu, J.; Jofre-Roca, L. UWB-Modulated Microwave Imaging for Human Brain Functional Monitoring. Sensors 2023, 23, 4374. https://doi.org/10.3390/s23094374
Akazzim Y, Jofre M, El Mrabet O, Romeu J, Jofre-Roca L. UWB-Modulated Microwave Imaging for Human Brain Functional Monitoring. Sensors. 2023; 23(9):4374. https://doi.org/10.3390/s23094374
Chicago/Turabian StyleAkazzim, Youness, Marc Jofre, Otman El Mrabet, Jordi Romeu, and Luis Jofre-Roca. 2023. "UWB-Modulated Microwave Imaging for Human Brain Functional Monitoring" Sensors 23, no. 9: 4374. https://doi.org/10.3390/s23094374
APA StyleAkazzim, Y., Jofre, M., El Mrabet, O., Romeu, J., & Jofre-Roca, L. (2023). UWB-Modulated Microwave Imaging for Human Brain Functional Monitoring. Sensors, 23(9), 4374. https://doi.org/10.3390/s23094374






