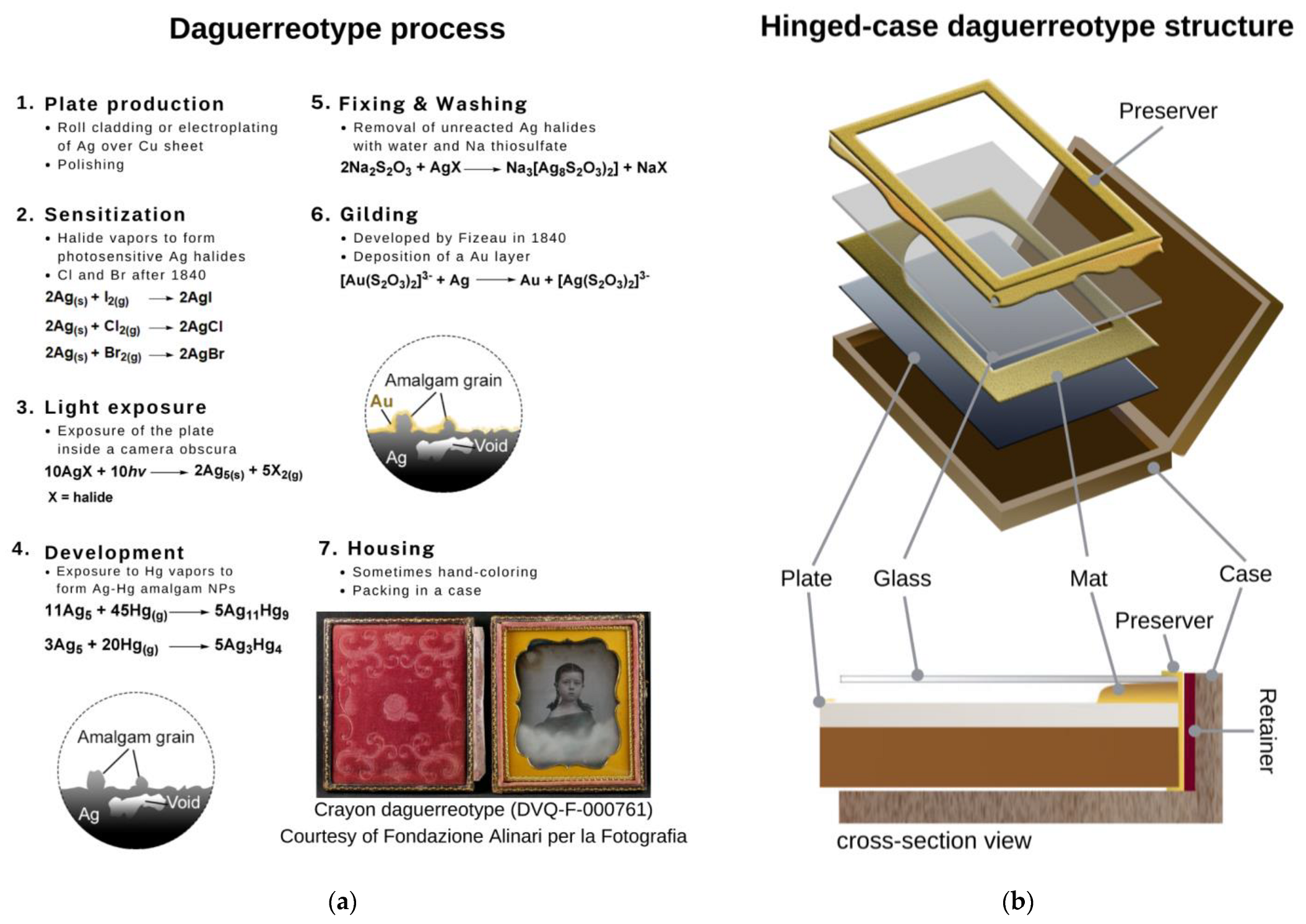
The Degradation of Daguerreotypes and the Relationship with Their Multi-Material Structure: A Multimodal Investigation
Abstract
1. Introduction
| Section | Degradation Product | Analytical Technique | Lit. |
|---|---|---|---|
| Glass | “Weeping” (sodium silicate gels) | SEM-EDS, X-ray Diffraction (XRD), FTIR-ATR (after sampling) | [19,24] |
| (Na, K) Sulfates | |||
| Formates | |||
| Mat and Preserver | Na/Cu formates and carbonates | Raman | [25,26] |
| Image Plate | Acanthite (Ag2S), silver sulfate, silver chloride, silver oxide | SEM-EDS, XRD, Raman, Synchrotron-based techniques, LIBS | [1,2] |
| Cu oxide, Cu sulfide |
2. Materials and Methods
2.1. Daguerreotypes Studied
2.2. Portable X-ray Fluorescence Spectrometry
2.3. Raman Microspectroscopy
2.4. Micro Reflection Fourier Transform Infrared Spectroscopy
2.5. Spectral-Domain Optical Coherence Tomography
3. Results
3.1. Characterization of the Multi-Material Structure: Plate, Mat, Preserver, and Cover Glass
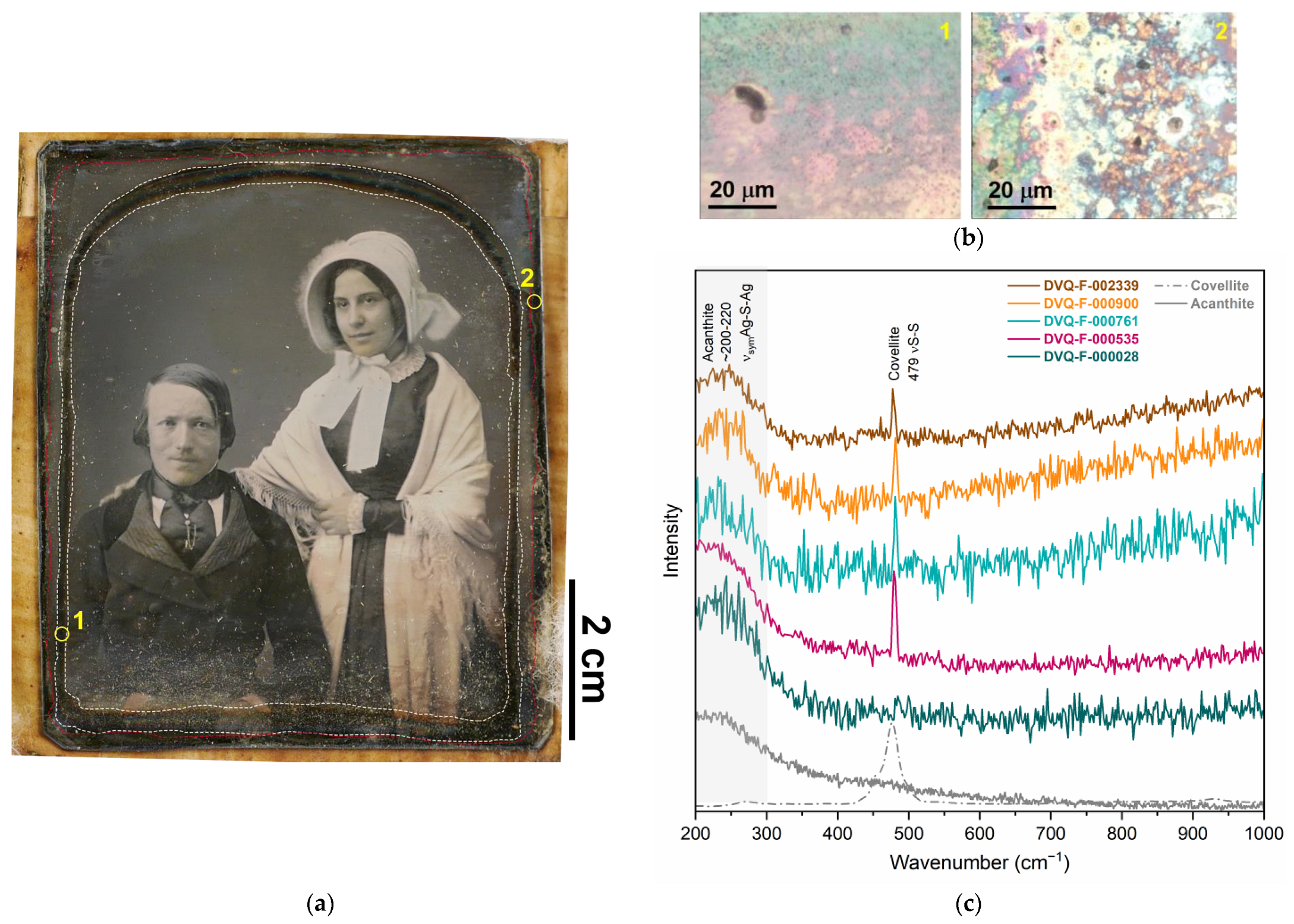
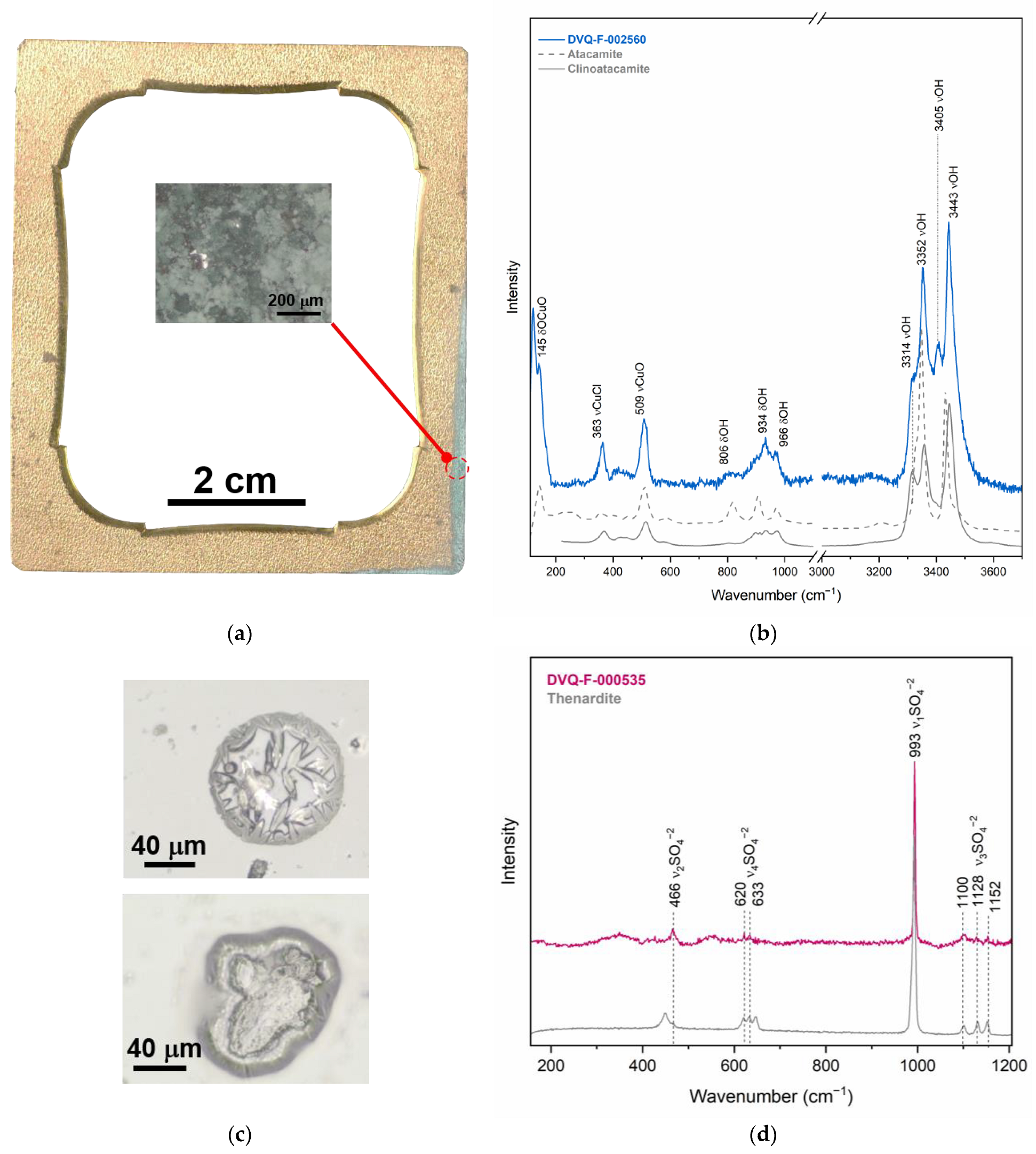
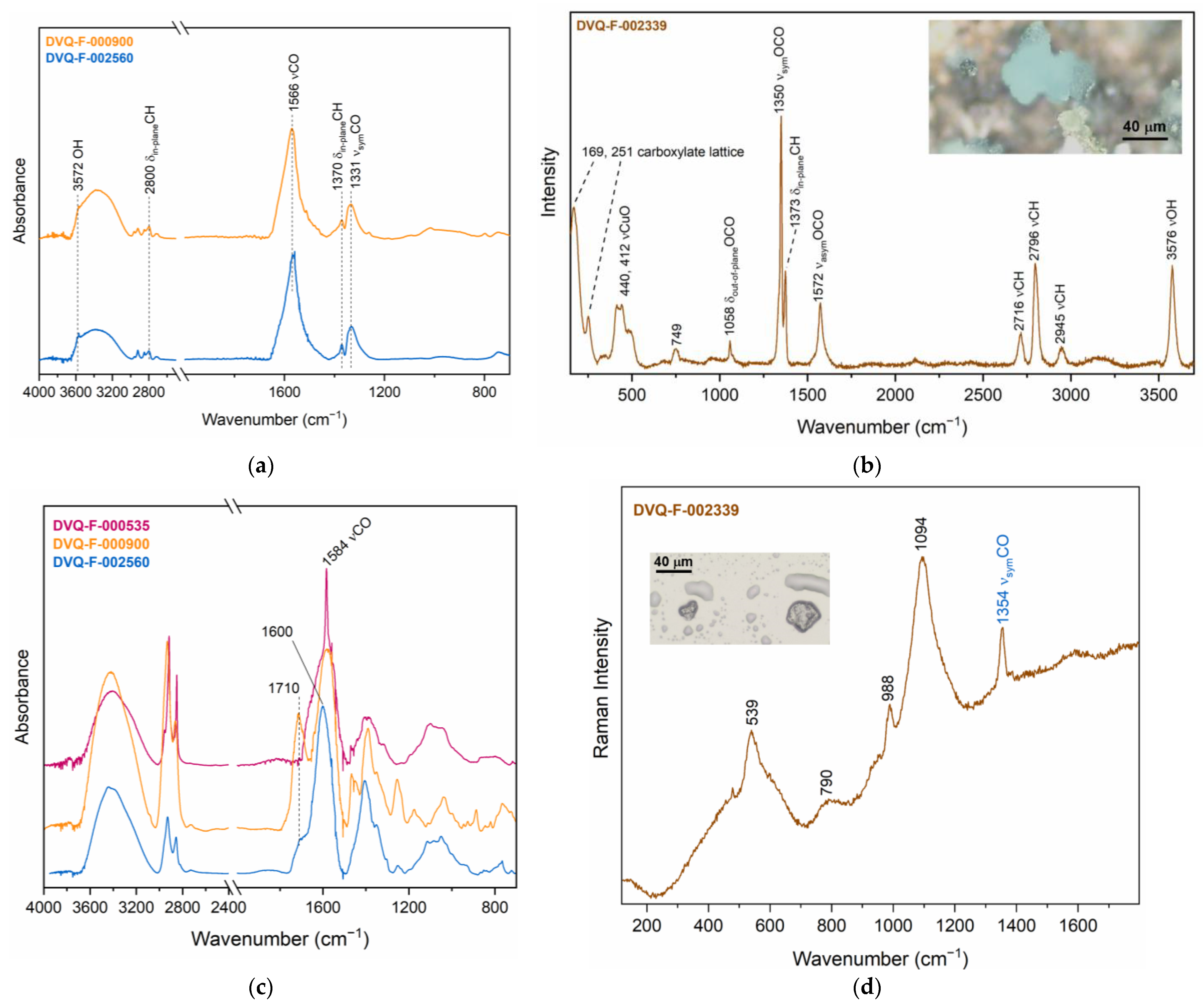
3.2. The Degradation of the Interrelated Elements
3.2.1. Corrosion of Single Elements
3.2.2. Interrelated Elements: Corrosion Induced by Materials Interaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barger, M.S.; White, W.B. The Daguerreotype: Nineteenth-Century Technology and Modern Science; Johns Hopkins University Press: Baltimore, MD, USA, 2000; ISBN 978-0-8018-6458-2. [Google Scholar]
- Centeno, S.A.; Schulte, F.; Kennedy, N.W.; Schrott, A.G. The Formation of Chlorine-Induced Alterations in Daguerreotype Image Particles: A High Resolution SEM-EDS Study. Appl. Phys. A 2011, 105, 55–63. [Google Scholar] [CrossRef]
- Ravines, P.; Li, L.; McElroy, R. An Electron Microscopy Study of the ImageMaking Process of the Daguerreotype, the 19th Century’s First Commercially Viable Photographic Process. J. Imaging Sci. Technol. 2016, 60, 030504-1–030504-10. [Google Scholar] [CrossRef]
- Schlather, A.E.; Gieri, P.; Robinson, M.; Centeno, S.A.; Manjavacas, A. Nineteenth-Century Nanotechnology: The Plasmonic Properties of Daguerreotypes. Proc. Natl. Acad. Sci. USA 2019, 116, 13791–13798. [Google Scholar] [CrossRef] [PubMed]
- Ljubić Tobisch, V.; Kautek, W. Highly Photosensitive Daguerreotypes and Their Reproduction: Physico-chemical Elucidation of Innovative Processes in Photography Developed around 1840 in Vienna. ChemPlusChem 2019, 84, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Ravines, P.; Nazarenko, A.Y. Characterizing the Image Forming Particles of Modern Ungilded Daguerreotypes Using XRD and SEM. Microsc. Microanal. 2019, 25, 1037–1051. [Google Scholar] [CrossRef]
- Cased Photographs. Including Daguerreotypes, Ambrotypes and Tintypes. In Photographic Materials Conservation Catalog; McElhone, J.P., Ed.; American Institute for Conservation of Historic and Artistic Works: Washington, DC, USA, 1998. [Google Scholar]
- Quintero Balbas, D.; Cattaneo, B.; Cagnini, A.; Belluzzo, P.; Innocenti, S.; Rossi, S.; Fontana, R.; Striova, J. The Colors of the Butterfly Wings: Non-Invasive Microanalytical Studies of Hand-Coloring Materials in 19th-Century Daguerreotypes. Heritage 2022, 5, 4306–4324. [Google Scholar] [CrossRef]
- Marquis, E.A.; Chen, Y.; Kohanek, J.; Dong, Y.; Centeno, S.A. Exposing the Sub-Surface of Historical Daguerreotypes and the Effects of Sulfur-Induced Corrosion. Corros. Sci. 2015, 94, 438–444. [Google Scholar] [CrossRef]
- Kozachuk, M.S.; Sham, T.K.; Martin, R.R.; Nelson, A.J.; Coulthard, I. Exploring Tarnished Daguerreotypes with Synchrotron Light: XRF and Μ-XANES Analysis. Herit. Sci. 2018, 6, 12. [Google Scholar] [CrossRef]
- Barger, M.S.; Messier, R.; White, W.B. Nondestructive Assessment of Daguerreotype Image Quality by Diffuse Reflectance Spectroscopy. Stud. Conserv. 1984, 29, 84–86. [Google Scholar]
- Selwyn, L.; McKinnon, W.R. Silver and Acid-Thiourea Silver Dips: Rinsing and Aging Monitored by Electrochemistry. Stud. Conserv. 2021, 66, 98–112. [Google Scholar] [CrossRef]
- Daffner, L.A.; Kushel, D.; Messinger, J.M., II. Investigation of a Surface Tarnish Found on 19th-Century Daguerreotypes. J. Am. Inst. Conserv. 1996, 35, 9–21. [Google Scholar] [CrossRef]
- Vicenzi, E.P.; Landin, T.; Herzing, A.A. Examination of a 19th Century Daguerreotype Photograph Using High Resolution Scanning Transmission Electron Microscopy for 2D and 3D Nanoscale Imaging and Analysis. Microsc. Microanal. 2014, 20, 2000–2001. [Google Scholar] [CrossRef]
- Ravines, P.; Wiegandt, R.; Wichern, C.M. Surface Characterisation of Daguerreotypes with the Optical Metrological Technique of Confocal Microscopy. Surf. Eng. 2008, 24, 138–146. [Google Scholar] [CrossRef]
- Kozachuk, M.S.; Avilés, M.O.; Martin, R.R.; Potts, B.; Sham, T.-K.; Lagugné-Labarthet, F. Imaging the Surface of a Hand-Colored 19th Century Daguerreotype. Appl. Spectrosc. 2018, 72, 1215–1224. [Google Scholar] [CrossRef]
- Anglos, D.; Melesanaki, K.; Zafiropulos, V.; Gresalfi, M.J.; Miller, J.C. Laser-Induced Breakdown Spectroscopy for the Analysis of 150-Year-Old Daguerreotypes. Appl. Spectrosc. 2002, 56, 423–432. [Google Scholar] [CrossRef]
- Centeno, S.A.; Meller, T.; Kennedy, N.; Wypyski, M. The Daguerreotype Surface as a SERS Substrate: Characterization of Image Deterioration in Plates from the 19th Century Studio of Southworth & Hawes. J. Raman Spectrosc. 2008, 39, 914–921. [Google Scholar] [CrossRef]
- Thickett, D.; Pretzel, B. FTIR Surface Analysis for Conservation. Herit. Sci. 2020, 8, 5. [Google Scholar] [CrossRef]
- Kozachuk, M.S.; Sham, T.-K.; Martin, R.R.; Nelson, A.J.; Coulthard, I.; Smieska, L.; Woll, A.R. Recovering Past Reflections: X-ray Fluorescence Imaging of Electrocleaned 19th Century Daguerreotypes. Heritage 2019, 2, 568–586. [Google Scholar] [CrossRef]
- Goltz, D.; Hill, G. Hyperspectral Imaging of Daguerreotypes. Restaur. Int. J. Preserv. Libr. Arch. Mater. 2012, 33, 1–16. [Google Scholar] [CrossRef]
- Ravines, P.; Baum, K.G.; Cox, N.A.; Welch, S.; Helguera, M. Multimodality Imaging of Daguerreotypes and Development of a Registration Program for Image Evaluation. J. Am. Inst. Conserv. 2014, 53, 19–32. [Google Scholar] [CrossRef]
- Tang, X.; Ardis, P.A.; Messing, R.; Brown, C.M.; Nelson, R.C.; Ravines, P.; Wiegandt, R. Digital Analysis and Restoration of Daguerreotypes; Stork, D.G., Coddington, J., Bentkowska-Kafel, A., Eds.; SPIE: San Jose, CA, USA, 2010; p. 75310A. [Google Scholar]
- Barger, M.S.; Smith, D.K.; White, W.B. Characterization of Corrosion Products on Old Protective Glass, Especially Daguerreotype Cover Glasses. J. Mater. Sci. 1989, 24, 1343–1356. [Google Scholar] [CrossRef]
- Eggert, G.; Bührer, A.; Barbier, B.; Euler, H. When Glass and Metal Corrode Together, II: A Black Forest Schäppel and Further Occurrences of Socoformacite; Roemich, H., Ed.; ICOM: Corning, NY, USA, 2010; Volume Glass and Ceramics Conservation, pp. 174–180. [Google Scholar]
- Fischer, A.; Eggert, G.; Dinnebier, R.; Runčevski, T. When Glass and Metal Corrode Together, V: Sodium Copper Formate. Stud. Conserv. 2018, 63, 342–355. [Google Scholar] [CrossRef]
- Catalogo Immagini Archivio Fotografico—FAF Toscana. Available online: https://www.alinari.it/en/explore-unicum-objects (accessed on 24 October 2022).
- Chiesa, G.; Gosio, P. Daguerreotype Hallmarks, 1st ed.; Gabriele Chiesa & Paolo Gosio: Brescia, Italy, 2020. [Google Scholar]
- Brocchieri, J.; Scialla, E.; Sabbarese, C. Estimation of Ag Coating Thickness by Different Methods Using a Handheld XRF Instrument. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021, 486, 73–84. [Google Scholar] [CrossRef]
- Robinet, L.; Coupry, C.; Eremin, K.; Hall, C. The Use of Raman Spectrometry to Predict the Stability of Historic Glasses. J. Raman Spectrosc. 2006, 37, 789–797. [Google Scholar] [CrossRef]
- Newbury, B.; Stephenson, B.; Almer, J.; Notis, M.; Cargill, G.S.; Stephenson, G.B.; Haeffner, D. Synchrotron Applications in Archaeometallurgy: Analysis of High Zinc Brass Astrolabes. Powder Diffr. 2004, 19, 12–15. [Google Scholar] [CrossRef][Green Version]
- Prati, S.; Sciutto, G.; Bonacini, I.; Mazzeo, R. New Frontiers in Application of FTIR Microscopy for Characterization of Cultural Heritage Materials. Top. Curr. Chem. 2016, 374, 26. [Google Scholar] [CrossRef]
- Stark, A.; Filice, F.; Noël, J.J.; Martin, R.R.; Sham, T.-K.; Finfrock, Y.Z.; Heald, S.M. Retrieving Tarnished Daguerreotype Content Using X-ray Fluorescence Imaging—Recent Observations on the Effect of Chemical and Electrochemical Cleaning Methods. Heritage 2021, 4, 1605–1615. [Google Scholar] [CrossRef]
- de Caro, T.; Caschera, D.; Ingo, G.M.; Calandra, P. Micro-Raman Innovative Methodology to Identify Ag-Cu Mixed Sulphides as Tarnishing Corrosion Products: Micro-Raman Innovative Methodology. J. Raman Spectrosc. 2016, 47, 852–859. [Google Scholar] [CrossRef]
- Martina, I.; Wiesinger, R.; Schreiner, M. Micro-Raman Investigations of Early Stage Silver Corrosion Products Occurring in Sulfur Containing Atmospheres. J. Raman Spectrosc. 2013, 44, 770–775. [Google Scholar] [CrossRef]
- Saleh, G.; Xu, C.; Sanvito, S. Silver Tarnishing Mechanism Revealed by Molecular Dynamics Simulations. Angew. Chem. Int. Ed. 2019, 58, 6017–6021. [Google Scholar] [CrossRef]
- Elvira Tonelli I Dagherrotipi. In Il Restauro Della Fotografia. MATERIALI Fotografici e Cinematografici, Analogici e Digitali; Cattaneo, B., Ed.; Nardini Editore: Florence, Italy, 2012; pp. 63–78. [Google Scholar]
- Krivovichev, S.V.; Hawthorne, F.C.; Williams, P.A. Structural Complexity and Crystallization: The Ostwald Sequence of Phases in the Cu2(OH)3Cl System (Botallackite–Atacamite–Clinoatacamite). Struct. Chem. 2017, 28, 153–159. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Fdez-Ortiz de Vallejuelo, S.; Veneranda, M.; Lama, E.; Castro, K.; Arana, G.; Larrañaga, A.; Madariaga, J.M. The Raman Spectra of the Na2SO4-K2SO4 System: Applicability to Soluble Salts Studies in Built Heritage. J. Raman Spectrosc. 2019, 50, 175–183. [Google Scholar] [CrossRef]
- Aulinas, M.; Garcia-Valles, M.; Gimeno, D.; Fernandez-Turiel, J.L.; Ruggieri, F.; Pugès, M. Weathering Patinas on the Medieval (S. XIV) Stained Glass Windows of the Pedralbes Monastery (Barcelona, Spain). Environ. Sci. Pollut. Res. 2009, 16, 443–452. [Google Scholar] [CrossRef]
- Eggert, G.; Haseloff, S.; Euler, H.; Barbier, B. When Glass and Metal Corrode Together, III: The Formation of Dicoppertrihydroxyformate; Bridgland, J., Ed.; ICOMM: Lisbon, Portugal, 2011; Volume Preprints, p. 901. [Google Scholar]
- Veiga, A.; Teixeira, D.M.; Candeias, A.J.; Mirão, J.; Rodrigues, P.S.; Teixeira, J.G. On the Chemical Signature and Origin of Dicoppertrihydroxyformate (Cu2(OH)3HCOO) Formed on Copper Miniatures of 17th and 18th Centuries. Microsc. Microanal. 2016, 22, 1007–1017. [Google Scholar] [CrossRef]
- Marchetti, A.; Beltran, V.; Nuyts, G.; Borondics, F.; De Meyer, S.; Van Bos, M.; Jaroszewicz, J.; Otten, E.; Debulpaep, M.; De Wael, K. Novel Optical Photothermal Infrared (O-PTIR) Spectroscopy for the Noninvasive Characterization of Heritage Glass-Metal Objects. Sci. Adv. 2022, 8, eabl6769. [Google Scholar] [CrossRef]
- Tétreault, J.; Cano, E.; van Bommel, M.; Scott, D.; Dennis, M.; Barthés-Labrousse, M.-G.; Minel, L.; Robbiola, L. Corrosion of Copper and Lead by Formaldehyde, Formic and Acetic Acid Vapours. Stud. Conserv. 2003, 48, 237–250. [Google Scholar] [CrossRef]
- Poli, T.; Piccirillo, A.; Nervo, M.; Chiantore, O. Aging of Natural Resins in Presence of Pigments: Metal Soap and Oxalate Formation. In Metal Soaps in Art; Casadio, F., Keune, K., Noble, P., Van Loon, A., Hendriks, E., Centeno, S.A., Osmond, G., Eds.; Cultural Heritage Science; Springer International Publishing: Cham, Switzerland, 2019; pp. 141–152. ISBN 978-3-319-90616-4. [Google Scholar]
- Poli, T.; Piccirillo, A.; Zoccali, A.; Conti, C.; Nervo, M.; Chiantore, O. The Role of Zinc White Pigment on the Degradation of Shellac Resin in Artworks. Polym. Degrad. Stab. 2014, 102, 138–144. [Google Scholar] [CrossRef]
- Rodrigues, B.; Santos, Â.; Melo, M.J.; Otero, V.; Vilarigues, M. Magic Lantern Glass Slides Materials and Techniques: The First Multi-Analytical Study. Heritage 2019, 2, 2513–2530. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Gros, D.; Wyss, K.; Scherrer, N.C.; Zumbühl, S.; Marone, F. Faded Shine…. The Degradation of Brass Powder in Two Nineteenth Century Paintings. Herit. Sci. 2015, 3, 24. [Google Scholar] [CrossRef]
- Fischer, A.; Eggert, G.; Stelzner, J. When Glass and Metal Corrode Together, VI: Chalconatronite. Stud. Conserv. 2020, 65, 152–159. [Google Scholar] [CrossRef]
- Trentelman, K.; Stodulski, L.; Scott, D.; Back, M.; Stock, S.; Strahan, D.; Drews, A.R.; O’Neill, A.; Weber, W.H.; Chen, A.E.; et al. The Characterization of a New Pale Blue Corrosion Product Found on Copper Alloy Artifacts. Stud. Conserv. 2002, 47, 217–227. [Google Scholar]
- Gibson, L.T.; Watt, C.M. Acetic and Formic Acids Emitted from Wood Samples and Their Effect on Selected Materials in Museum Environments. Corros. Sci. 2010, 52, 172–178. [Google Scholar] [CrossRef]
- Eggert, G.; Fischer, A. The Formation of Formates: A Review of Metal Formates on Heritage Objects. Herit. Sci. 2021, 9, 26. [Google Scholar] [CrossRef]
- Robinet, L.; Eremin, K.; Cobo del Arco, B.; Gibson, L.T. A Raman Spectroscopic Study of Pollution-Induced Glass Deterioration. J. Raman Spectrosc. 2004, 35, 662–670. [Google Scholar] [CrossRef]
- San Andrés, M.; de la Roja, J.M.; Baonza, V.G.; Sancho, N. Verdigris Pigment: A Mixture of Compounds. Input from Raman Spectroscopy. J. Raman Spectrosc. 2010, 41, 1468–1476. [Google Scholar] [CrossRef]
- Jeziorowski, H.; Moser, B. Characterization of Formate Complexes Formed as Corrosion Products on Copper by Raman Spectroscopy. Microchim. Acta 1989, 98, 101–107. [Google Scholar] [CrossRef]



| Code * | Subject | Plate Dimensions (cm2) | Historical Information [27,28] |
|---|---|---|---|
 DVQ-F-000028 DVQ-F-000028 | Portrait of Zelinda Tallini in Galli | 10.7 × 8.1 | ca. 1846–1848, Claude Porraz (Bologna, Italy). |
 DVQ-F-000535 DVQ-F-000535 | Couple portrait | 7.60 × 6.40 | Before 1845. Unidentified author. |
 DVQ-F-000761 DVQ-F-000761 | Portrait of a girl | 8.4 × 7.2 | After 1850. Unidentified author. Hallmark: Asterisk J P Doublé 40. Probably a French plate maker (production from ca. 1850–1858). |
 DVQ-F-000900 DVQ-F-000900 | Portrait of a woman with a child | 7.9 × 6.9 | ca. 1840–1850. Unidentified author. |
 DVQ-F-001667 DVQ-F-001667 | Portrait of a young woman | 11.0 × 8.5 | ca. 1855, Désiré François Millet (active in London, UK). |
 DVQ-F-002339 DVQ-F-002339 | Male portrait | 14.0 × 10.7 | 1847–1860, James E. McClees and Washington Lafayette Germon studio (Philadelphia, PA, USA). |
 DVQ-F-002560 DVQ-F-002560 | Male portrait in half-length | 7.7 × 6.9 | Before 1845. Unidentified author. |
 DVQ-F-002694 DVQ-F-002694 | Portrait of a seated man with a cane | 8.2 × 7.1 | After 1850. Unidentified author. |
 DVQ-F-002766 DVQ-F-002766 | Female portrait | 7.9 × 6.4 | ca. 1841–1851. Antoine François-Jean Claudet (active in London, UK). |
| Daguerreotype Element | Degradation Product | Analytical Technique | Factors Involved |
|---|---|---|---|
| Cover glass (analyzed with XRF, μ-Raman) | Na sulfate (Na2SO4) | μ-Raman | S-containing pollutants |
| Formates | μ-Raman | VOCs from the case and relative humidity | |
| Mat (analyzed with XRF, OCT) | Atacamite (Cu2Cl(OH)3), clinoatacamite (Cu2(OH)3Cl) | μ-Raman | Chlorine contamination |
| Cu formate, cuprite (CuO) | μ-Raman, μ-rFTIR | VOCs from the case and relative humidity | |
| Cu carboxylates | μ-rFTIR | Resin–metal interaction, relative humidity, VOCs from the case? | |
| Image plate (analyzed with XRF, μ-Raman, OCT) | Acanthite (Ag2S) | μ-Raman | S-containing pollutants |
| Covellite (CuS) | μ-Raman | S-containing pollutants | |
| Atacamite (Cu2Cl(OH)3) | μ-Raman | Degradation products from the mat | |
| Cu formate, Cu acetate | μ-Raman, OCT | Degradation products from glass, VOCs from the case, relative humidity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero Balbas, D.; Cattaneo, B.; Cagnini, A.; Belluzzo, P.; Rossi, S.; Fontana, R.; Striova, J. The Degradation of Daguerreotypes and the Relationship with Their Multi-Material Structure: A Multimodal Investigation. Sensors 2023, 23, 4341. https://doi.org/10.3390/s23094341
Quintero Balbas D, Cattaneo B, Cagnini A, Belluzzo P, Rossi S, Fontana R, Striova J. The Degradation of Daguerreotypes and the Relationship with Their Multi-Material Structure: A Multimodal Investigation. Sensors. 2023; 23(9):4341. https://doi.org/10.3390/s23094341
Chicago/Turabian StyleQuintero Balbas, Diego, Barbara Cattaneo, Andrea Cagnini, Paolo Belluzzo, Sandra Rossi, Raffaella Fontana, and Jana Striova. 2023. "The Degradation of Daguerreotypes and the Relationship with Their Multi-Material Structure: A Multimodal Investigation" Sensors 23, no. 9: 4341. https://doi.org/10.3390/s23094341
APA StyleQuintero Balbas, D., Cattaneo, B., Cagnini, A., Belluzzo, P., Rossi, S., Fontana, R., & Striova, J. (2023). The Degradation of Daguerreotypes and the Relationship with Their Multi-Material Structure: A Multimodal Investigation. Sensors, 23(9), 4341. https://doi.org/10.3390/s23094341









