Development of a Rapid Measurement Method for Analysis of the NOx Conversion Process Based on Quantum Cascade Laser Absorption Spectroscopy
Abstract
1. Introduction
2. Experimental System and Methods
2.1. Theory
2.2. Experimental Setup
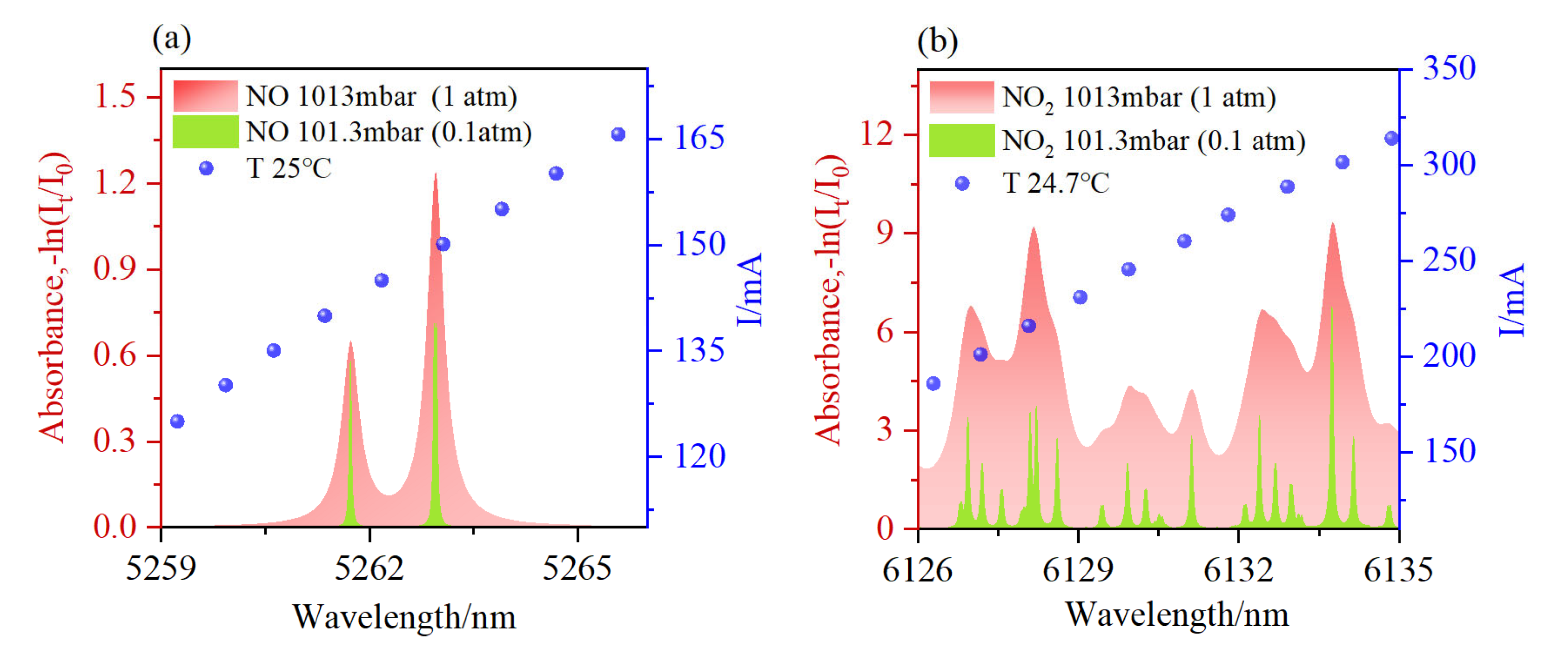
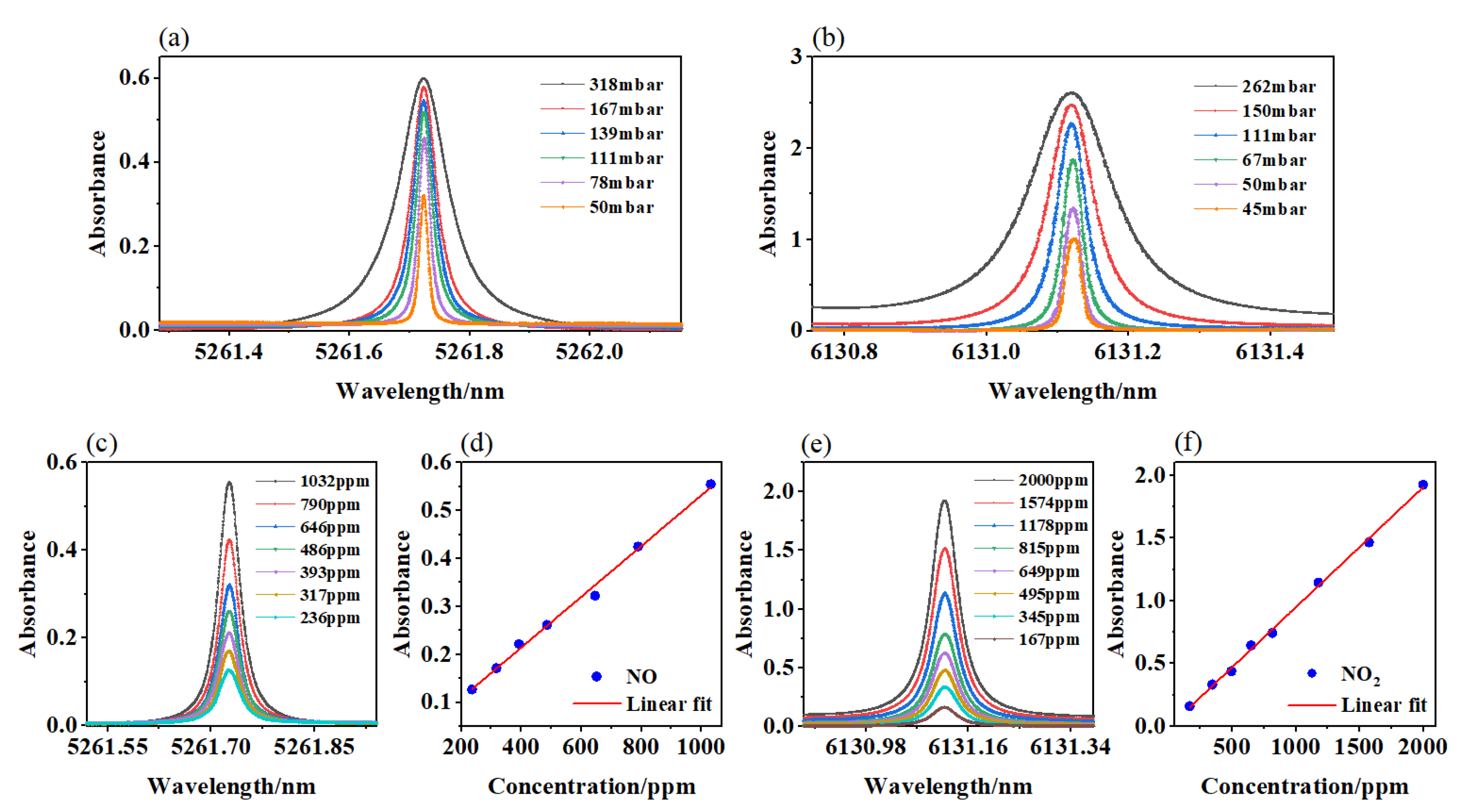
2.3. Transition Selection
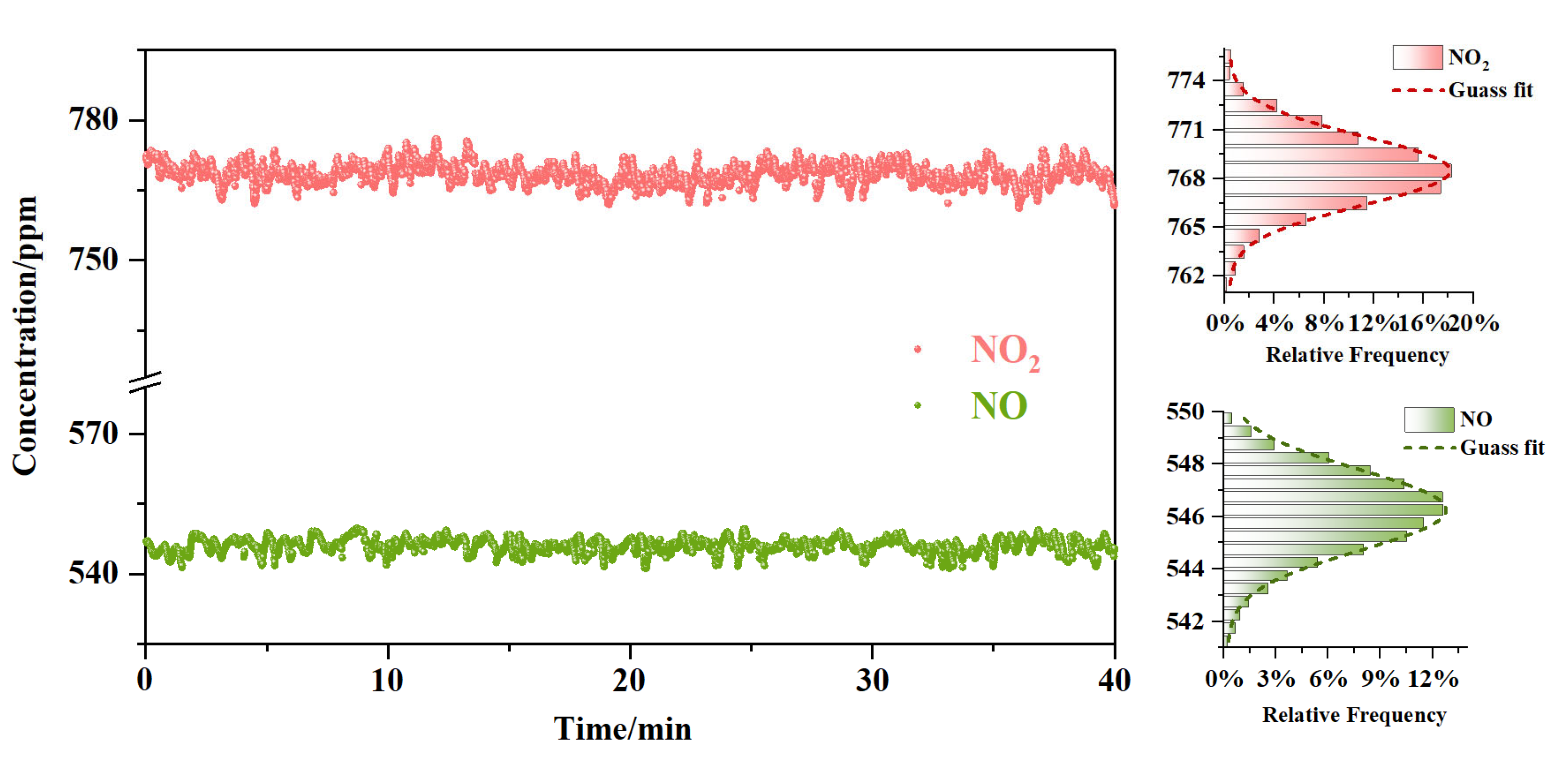
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Foy, B.; Lu, Z.; Streets, D.G. Satellite NO2 Retrievals Suggest China Has Exceeded Its NOx Reduction Goals from the Twelfth Five-Year Plan. Sci. Rep. 2016, 6, 35912. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Reiffs, A.; Parchatka, U.; Fischer, H. In Situ Measurements of Atmospheric CO and Its Correlation with NOx and O3 at a Rural Mountain Site. Metrol. Meas. Syst. 2015, 22, 25–38. [Google Scholar] [CrossRef]
- Van der A, R.J.; Mijling, B.; Ding, J.; Koukouli, M.E.; Liu, F.; Li, Q.; Mao, H.Q.; Theys, N. Cleaning up the Air: Effectiveness of Air Quality Policy for SO2 and NOx Emissions in China. Atmos. Chem. Phys. 2017, 17, 1775–1789. [Google Scholar] [CrossRef]
- Lal, S.; Patil, R.S. Monitoring of Atmospheric Behaviour of NOx from Vehicular Traffic. Environ. Monit. Assess. 2001, 68, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.G.; Zhou, S.; Sun, X.Y.; Yu, B.L.; Li, J.S. Continuous Measurement of NO2 in Flue Gas Employing Cavity-Enhanced Spectroscopy Sensing System. Measurement 2022, 201, 111729. [Google Scholar] [CrossRef]
- Crutzen, P. The Role of NO and NO2 in the Chemistry of the Troposphere and Stratosphere. Annu. Rev. Earth Planet. Sci. 2003, 7, 443–472. [Google Scholar] [CrossRef]
- Monks, P.S.; Archibald, A.T.; Colette, A.; Cooper, O.; Coyle, M.; Derwent, R.; Fowler, D.; Granier, C.; Law, K.S.; Mills, G.E.; et al. Tropospheric Ozone and Its Precursors from the Urban to the Global Scale from Air Quality to Short-Lived Climate Forcer. Atmos. Chem. Phys. 2015, 15, 8889–8973. [Google Scholar] [CrossRef]
- Lelieveld, J.; Gromov, S.; Pozzer, A.; Taraborrelli, D. Global Tropospheric Hydroxyl Distribution, Budget and Reactivity. Atmos. Chem. Phys. 2016, 16, 12477–12493. [Google Scholar] [CrossRef]
- Wayne, R.P.; Barnes, I.; Biggs, P.; Burrows, J.P.; Canosa-Mas, C.E.; Hjorth, J.; Bras, G.L.; Moortgat, G.K.; Perner, D.; Poulet, G.; et al. The Nitrate Radical: Physics, Chemistry, and the Atmosphere. Atmos. Environ. Part A Gen. Top. 1991, 25, 1–203. [Google Scholar] [CrossRef]
- Lammel, G.; Grassl, H. Greenhouse Effect of NOx. Environ. Sci. Pollut. Res. 1995, 2, 40–45. [Google Scholar] [CrossRef]
- Ji, X.; Chen, G. Unified Account of Gas Pollutants and Greenhouse Gas Emissions: Chinese Transportation 1978–2004. Commun. Nonlinear Sci. Numer. Simul. 2010, 15, 2710–2722. [Google Scholar] [CrossRef]
- Papke, H.; Papen, H. Influence of Acid Rain and Liming on Fluxes of NO and NO2 from Forest Soil. Plant Soil 1998, 199, 131–139. [Google Scholar] [CrossRef]
- Mishra, J.; Mishra, K. Effect of SO2 and NO2 Generated Acidification on Nodulation of Alysicarpus monilifer and Phaseolus trilobus (Linn) in Alluvials of UP, India. Natl. Acad. Sci. Lett. 2007, 30, 165–168. [Google Scholar]
- Saiz-Lopez, A.; Notario, A.; Albaladejo, J.; McFiggans, G. Seasonal Variation of NOx Loss Processes Coupled to the HNO3 Formation in a Daytime Urban Atmosphere: A Model Study. Water Air Soil Pollut. 2007, 182, 197–206. [Google Scholar] [CrossRef]
- Reed, C.; Evans, M.J.; Di Carlo, P.; Lee, J.D.; Carpenter, L.J. Interferences in Photolytic NO2 Measurements: Explanation for an Apparent Missing Oxidant? Atmos. Chem. Phys. 2016, 16, 4707–4724. [Google Scholar] [CrossRef]
- Yau, Y.Y.Y.; Geeraert, N.; Baker, D.M.; Thibodeau, B. Elucidating Sources of Atmospheric NOX Pollution in a Heavily Urbanized Environment Using Multiple Stable Isotopes. Sci. Total Environ. 2022, 832, 154781. [Google Scholar] [CrossRef]
- De Castro, A.; Meneses, J.; Briz, S.; Lopez, F. Nondispersive Infrared Monitoring of NO Emissions in Exhaust Gases of Vehicles. Rev. Sci. Instrum. 1999, 70, 3156–3159. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Ju, L.; Lin, L.; Chao, Y.; Su, H.W. Research of NDIR Applied on Measuring NOx Emission from In-Use Diesel Vehicles. Environ. Monit. China 2019, 35, 28–33. [Google Scholar]
- Saiz-Lopez, A.; Adame, J.A.; Notario, A.; Poblete, J.; Bolívar, J.P.; Albaladejo, J. Year-Round Observations of NO, NO2, O3, SO2, and Toluene Measured with a DOAS System in the Industrial Area of Puertollano, Spain. Water Air Soil Pollut. 2009, 200, 277–288. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Xia, H.; Dong, F.Z.; Pang, T.; Wu, B. Simultaneous and On-Line Detection of Multiple Gas Concentration with Tunable Diode Laser Absorption Spectroscopy. Opt. Precis. Eng. 2013, 21, 2771. [Google Scholar] [CrossRef]
- Kan, R.F.; Liu, W.Q.; Zhang, Y.J.; Liu, J.G.; Dong, F.Z.; Gao, S.H.; Wang, M.; Chen, J. Absorption Measurements of Ambient Methane with Tunable Diode Laser. Acta Phys. Sin. 2005, 54, 1927. [Google Scholar]
- Cui, R.; Dong, L.; Wu, H.; Ma, W.; Xiao, L.; Jia, S.; Chen, W.; Tittel, F.K. Three-Dimensional Printed Miniature Fiber-Coupled Multipass Cells with Dense Spot Patterns for Ppb-Level Methane Detection Using a Near-IR Diode Laser. Anal. Chem. 2020, 92, 13034–13041. [Google Scholar] [CrossRef] [PubMed]
- Faist, J.; Capasso, F.; Sivco, D.L.; Sirtori, C.; Hutchinson, A.L.; Cho, A.Y. Quantum Cascade Laser. Science 1994, 264, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Hofstetter, D.; Aellen, T.; Faist, J.; Oesterle, U.; Ilegems, M.; Gini, E.; Melchior, H. Continuous Wave Operation of a Mid-Infrared Semiconductor Laser at Room Temperature. Science 2002, 295, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Tredicucci, A.; Beltram, F.; Beere, H.E.; Linfield, E.H.; Davies, A.G.; Ritchie, D.A.; Iotti, R.C.; Rossi, F. Terahertz Semiconductor-Heterostructure Laser. Nature 2002, 417, 156–159. [Google Scholar] [CrossRef]
- McManus, J.B.; Shorter, J.H.; Nelson, D.D.; Zahniser, M.S.; Glenn, D.E.; McGovern, R.M. Pulsed Quantum Cascade Laser Instrument with Compact Design for Rapid, High Sensitivity Measurements of Trace Gases in Air. Appl. Phys. B 2008, 92, 387. [Google Scholar] [CrossRef]
- Yang, R.; Hill, C.J.; Wong, C.M. Recent Progress in Development of Mid-IR Interband Cascade Lasers. In Proceedings of the Integrated Optoelectronic Devices 2004, San Jose, CA, USA, 26–29 January 2004; Volume 5365, pp. 218–227. [Google Scholar]
- Sobanski, N.; Tuzson, B.; Scheidegger, P.; Looser, H.; Hüglin, C.; Emmenegger, L. A High-Precision Mid-Infrared Spectrometer for Ambient HNO3 Measurements. Sensors 2022, 22, 9158. [Google Scholar] [CrossRef]
- Genner, A.; Martín-Mateos, P.; Moser, H.; Lendl, B. A Quantum Cascade Laser-Based Multi-Gas Sensor for Ambient Air Monitoring. Sensors 2020, 20, 1850. [Google Scholar] [CrossRef]
- Sobanski, N.; Tuzson, B.; Scheidegger, P.; Looser, H.; Kupferschmid, A.; Iturrate, M.; Pascale, C.; Hüglin, C.; Emmenegger, L. Advances in High-Precision NO2 Measurement by Quantum Cascade Laser Absorption Spectroscopy. Appl. Sci. 2021, 11, 1222. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, N.; Shen, C.; Zhang, L.; He, T.; Yu, B.; Li, J. An Adaptive Kalman Filtering Algorithm Based on Back-Propagation (BP) Neural Network Applied for Simultaneously Detection of Exhaled CO and N2O. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117332. [Google Scholar] [CrossRef]
- Nelson, D.; McManus, J.; Herndon, S.; Shorter, J.; Zahniser, M.; Blaser, S.; Hvozdara, L.; Muller, A.; Giovannini, M.; Faist, J. Characterization of a Near-Room-Temperature, Continuous-Wave Quantum Cascade Laser for Long-Term, Unattended Monitoring of Nitric Oxide in the Atmosphere. Opt. Lett. 2006, 31, 2012–2014. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 Molecular Spectroscopic Database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Yu, Y.J.; Sanchez, N.P.; Yi, F.; Zheng, C.T.; Ye, W.; Wu, H.P.; Griffin, R.J.; Tittel, F.K. Dual Quantum Cascade Laser-Based Sensor for Simultaneous NO and NO2 Detection Using a Wavelength Modulation-Division Multiplexing Technique. Appl. Phys. B 2017, 123, 164. [Google Scholar] [CrossRef]
- Kosterev, A.; Wysocki, G.; Bakhirkin, Y.; So, S.; Lewicki, R.; Fraser, M.; Tittel, F.; Curl, R.F. Application of Quantum Cascade Lasers to Trace Gas Analysis. Appl. Phys. B 2008, 90, 165–176. [Google Scholar] [CrossRef]
- Ventrillard, I.; Gorrotxategi-Carbajo, P.; Romanini, D. Part per Trillion Nitric Oxide Measurement by Optical Feedback Cavity-Enhanced Absorption Spectroscopy in the Mid-Infrared. Appl. Phys. B 2017, 123, 180. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, Z.; Yang, S.; Sun, P.; Wu, B.; Xia, H.; Yu, R. Development of a Rapid Measurement Method for Analysis of the NOx Conversion Process Based on Quantum Cascade Laser Absorption Spectroscopy. Sensors 2023, 23, 3885. https://doi.org/10.3390/s23083885
Yang X, Zhang Z, Yang S, Sun P, Wu B, Xia H, Yu R. Development of a Rapid Measurement Method for Analysis of the NOx Conversion Process Based on Quantum Cascade Laser Absorption Spectroscopy. Sensors. 2023; 23(8):3885. https://doi.org/10.3390/s23083885
Chicago/Turabian StyleYang, Xi, Zhirong Zhang, Shuang Yang, Pengshuai Sun, Bian Wu, Hua Xia, and Runqing Yu. 2023. "Development of a Rapid Measurement Method for Analysis of the NOx Conversion Process Based on Quantum Cascade Laser Absorption Spectroscopy" Sensors 23, no. 8: 3885. https://doi.org/10.3390/s23083885
APA StyleYang, X., Zhang, Z., Yang, S., Sun, P., Wu, B., Xia, H., & Yu, R. (2023). Development of a Rapid Measurement Method for Analysis of the NOx Conversion Process Based on Quantum Cascade Laser Absorption Spectroscopy. Sensors, 23(8), 3885. https://doi.org/10.3390/s23083885






