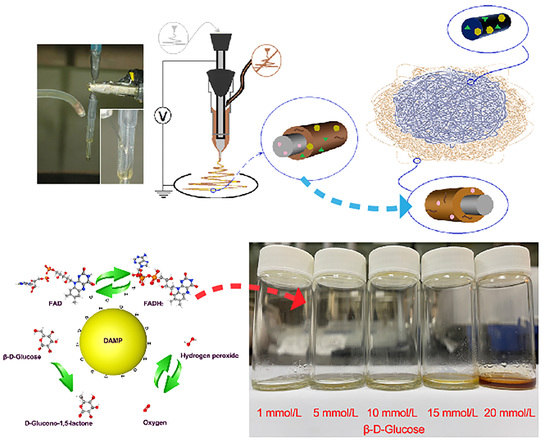
A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Core–Sheath and Hybrid Films
2.3. Characterization of Nanofiber Membrane Properties
2.3.1. Morphology and Physical State
2.3.2. Glucose Degradation Test
2.4. Glucose-Sensing Performance Detection
3. Results and Discussion
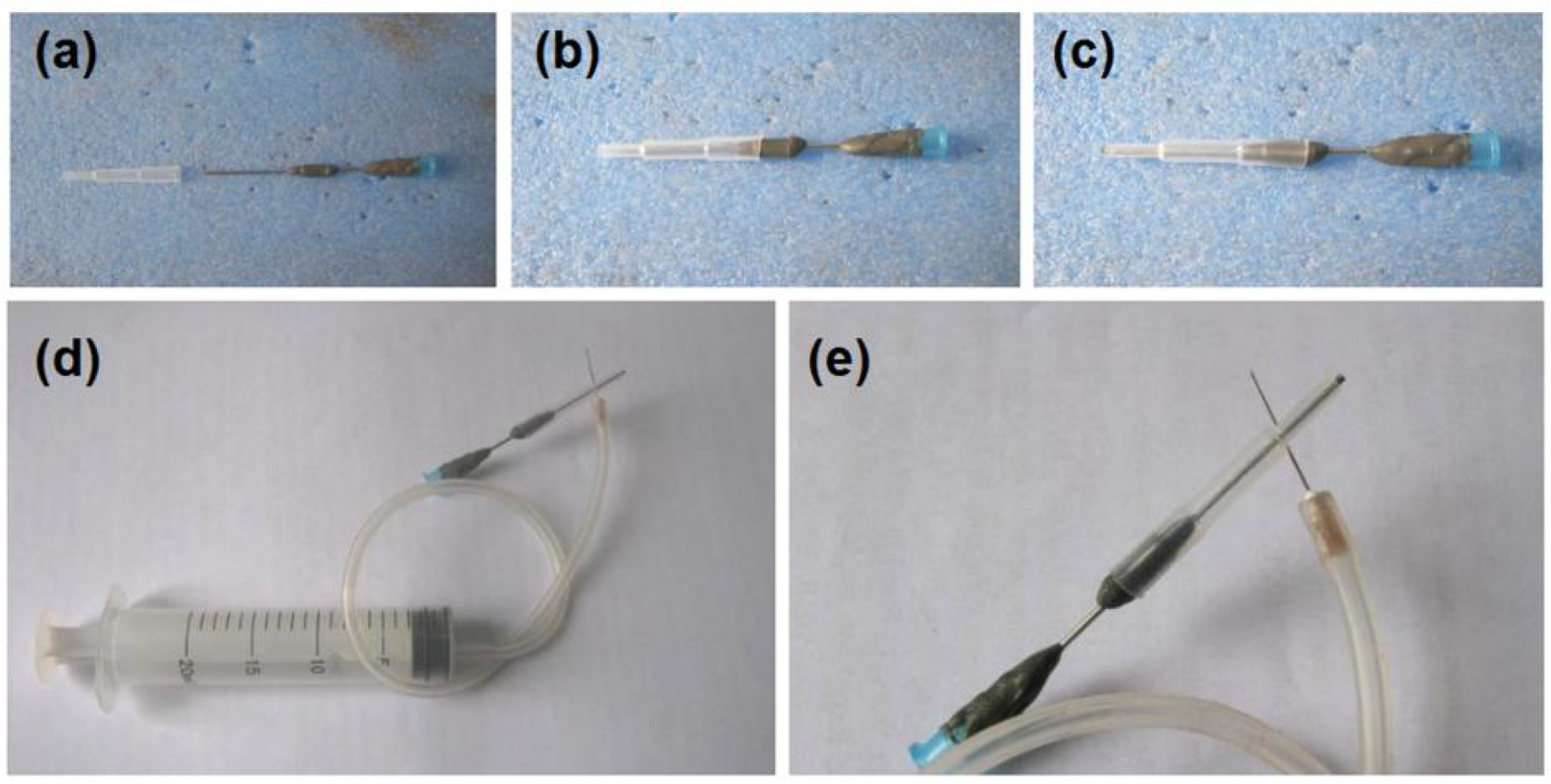
3.1. Implementation of Electrospinning
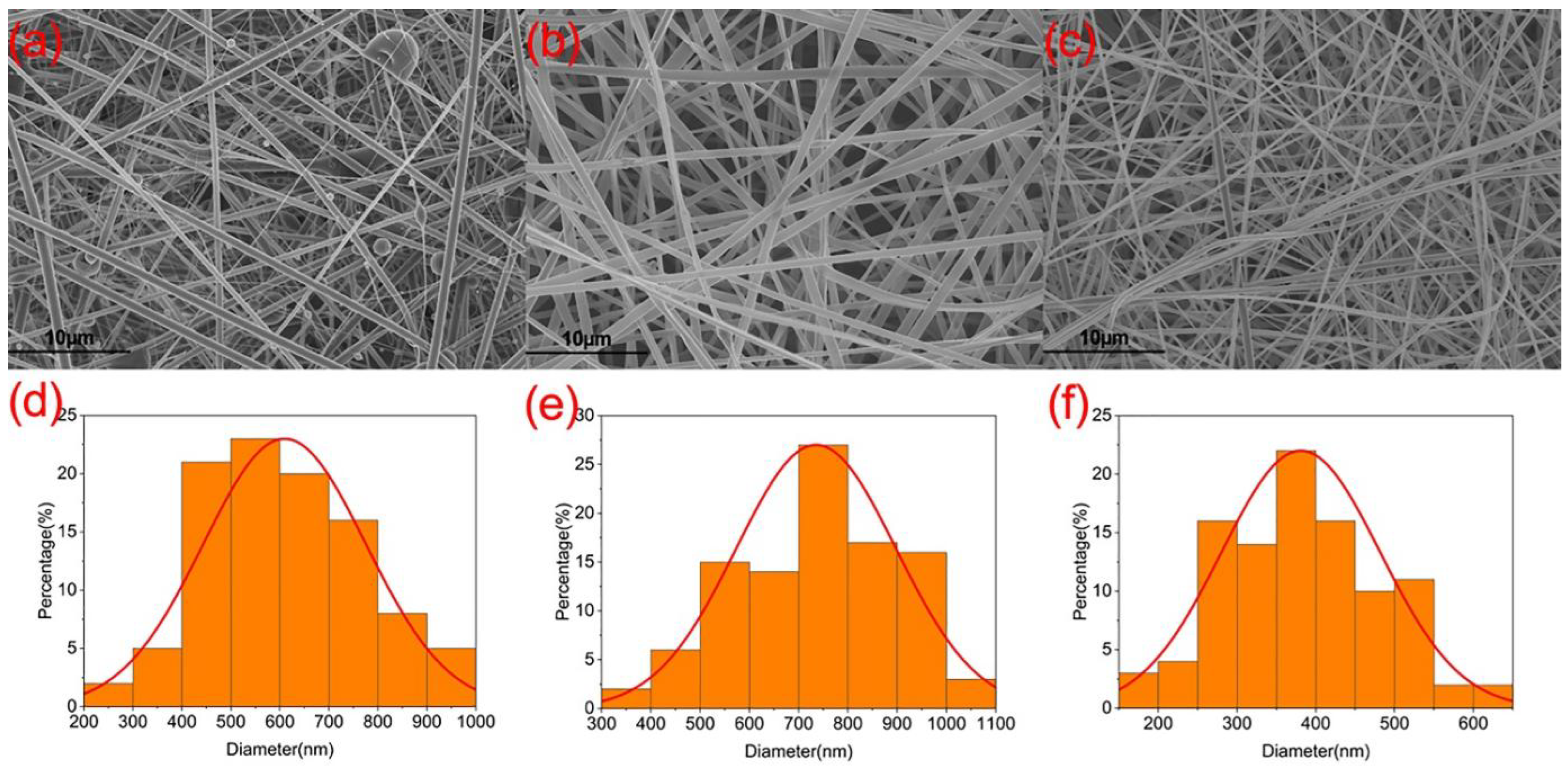
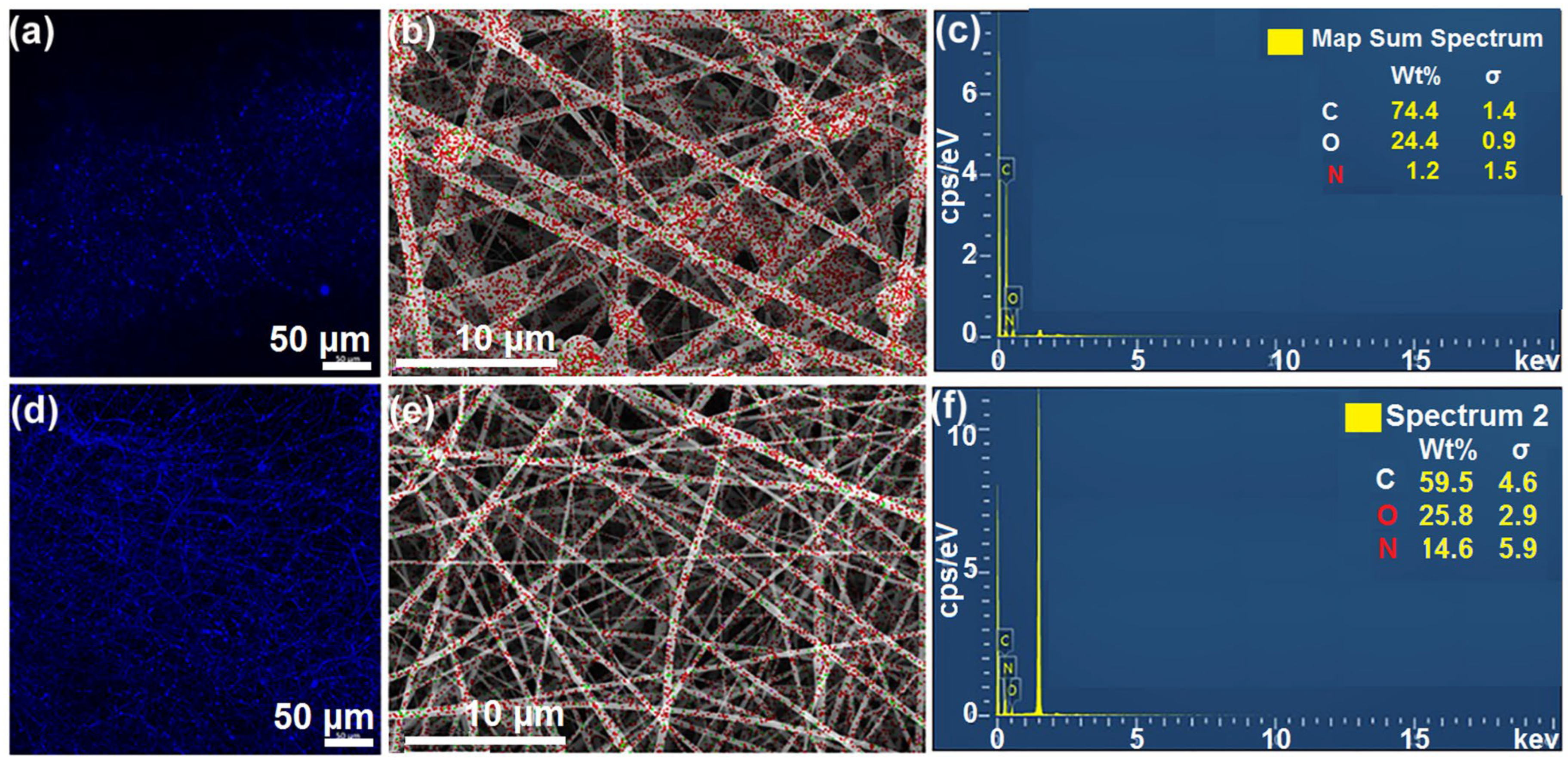
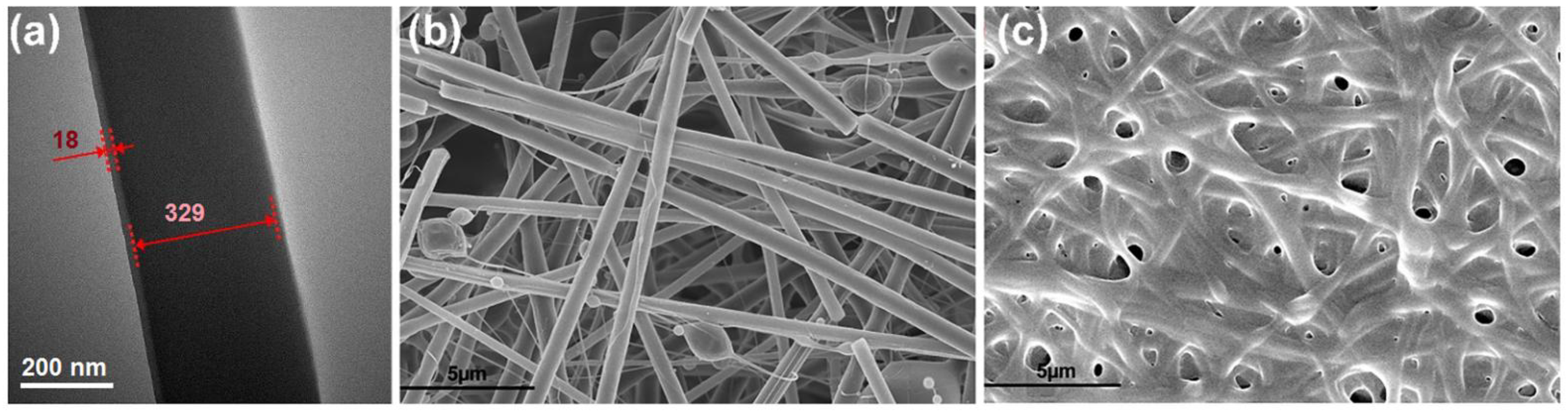
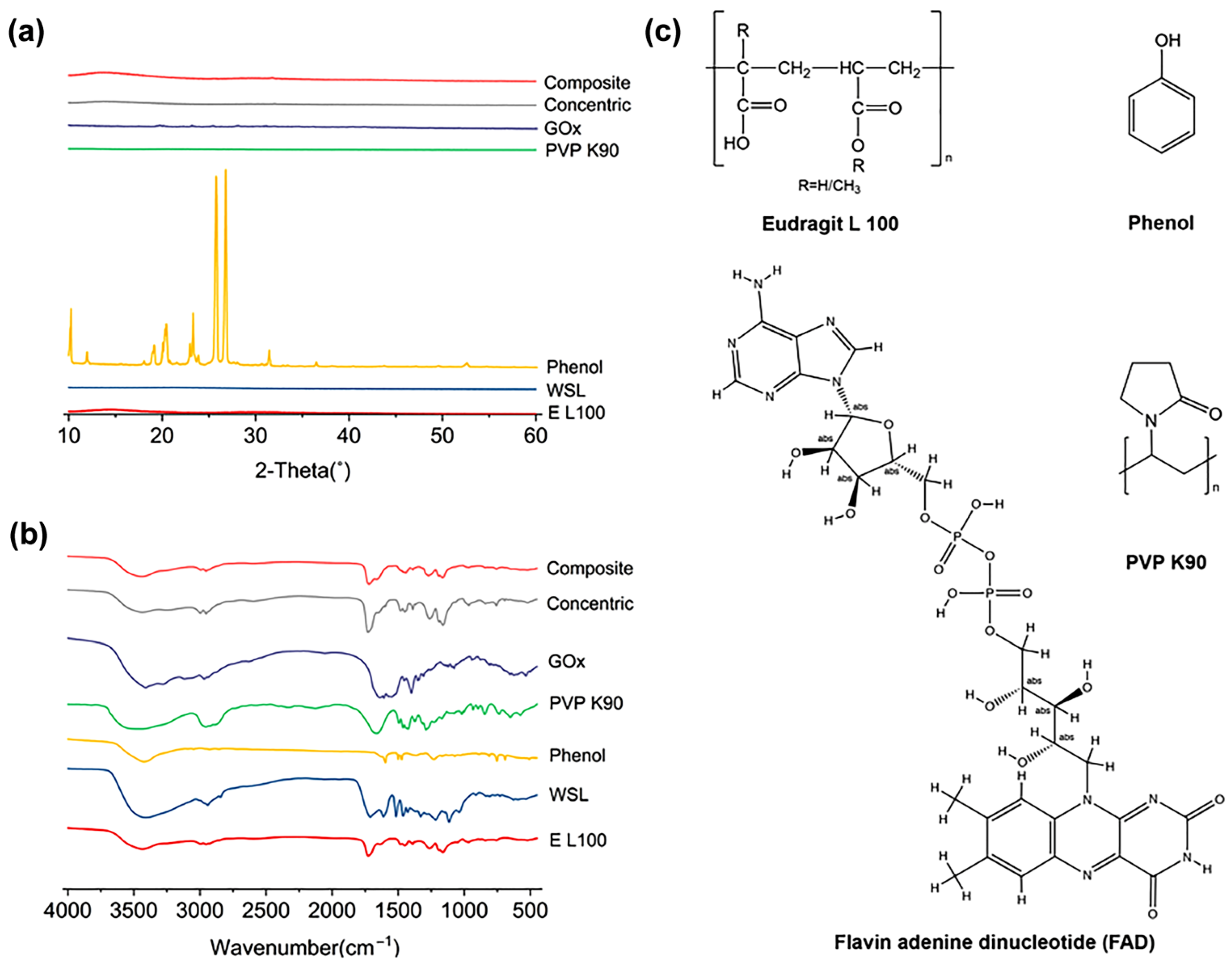
3.2. Morphology and Structure of Nanofibers and the Components’ State and Compatibility
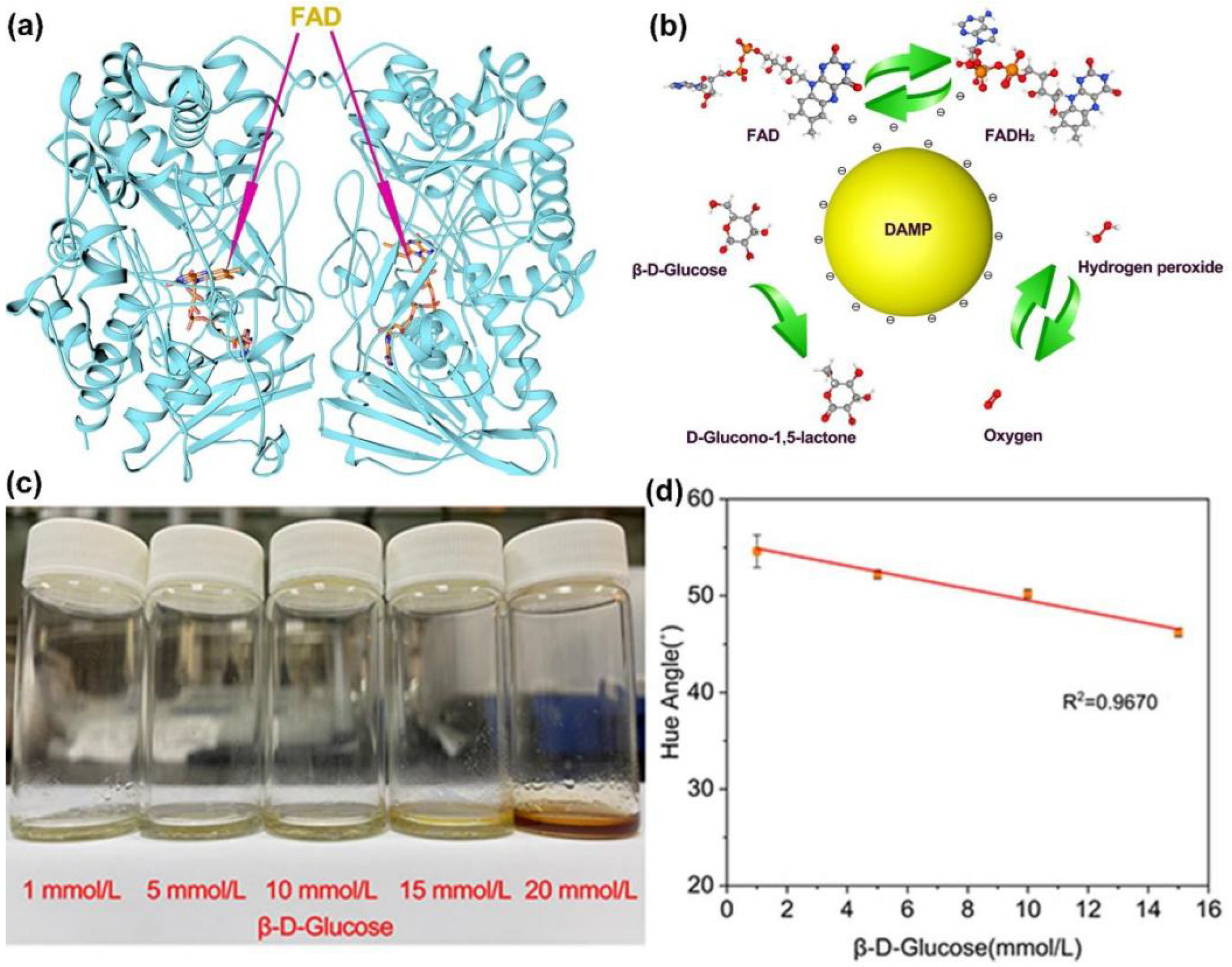
3.3. Glucose Degradation Test
3.4. Glucose-Sensing Performance Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maric, T.; Mikhaylov, G.; Khodakivskyi, P.; Bazhin, A.; Sinisi, R.; Bonhoure, N.; Yevtodiyenko, A.; Jones, A.; Muhunthan, V.; Abdelhady, G.; et al. Bioluminescent-Based Imaging and Quantification of Glucose Uptake in Vivo. Nat. Methods 2019, 16, 526. [Google Scholar] [CrossRef] [PubMed]
- McNichols, R.J.; Cote, G.L. Optical Glucose Sensing in Biological Fluids: An Overview. J. Biomed. Opt. 2000, 5, 5–16. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, P.; Hao, J.; Wang, Y.; Ohsaka, T.; Mao, L. Continuous and Simultaneous Electrochemical Measurements of Glucose, Lactate, and Ascorbate in Rat Brain Following Brain Ischemia. Anal. Chem. 2014, 86, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Rohaizad, N.; Nasir, M.Z.M.; Guan, J.; Pumera, M. Micromotor-Assisted Human Serum Glucose Biosensing. Anal. Chem. 2019, 91, 5660–5666. [Google Scholar] [CrossRef]
- Sun, X.; Chapin, B.M.; Metola, P.; Collins, B.; Wang, B.; James, T.D.; Anslyn, E. The Mechanisms of Boronate Ester Formation and Fluorescent Turn-on in Ortho-Aminomethylphenylboronic Acids. Nat. Chem. 2019, 11, 768–778. [Google Scholar] [CrossRef]
- Odaci, D.; Gacal, B.N.; Gacal, B.; Timur, S.; Yagci, Y. Fluorescence Sensing of Glucose Using Glucose Oxidase Modified by PVA-Pyrene Prepared via “Click” Chemistry. Biomacromolecules 2009, 10, 2928–2934. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.J.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. New York Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Gleiter, H. Nanostructured Materials: Basic Concepts and Microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Lee, K.-K.; Kim, J.-W.; Chung, K.-H.; Kim, S.-J.; Yun, W.-S.; Lee, C.-S. Effective Diagnosis of Foot-And-Mouth Disease Virus (FMDV) Serotypes O and A Based on Optical and Electrochemical Dual-Modal Detection. Biomolecules 2021, 11, 841. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Li, X.; Niu, X.; Chen, Y.; Yuan, K.; He, W.; Yang, S.; Tang, T.; Yu, D.-G. Electrospraying Micro-Nano Structures on Chitosan Composite Coatings for Enhanced Antibacterial Effect. Prog. Org. Coat. 2023, 174, 107310. [Google Scholar] [CrossRef]
- Xie, D.; Zhou, X.; Xiao, B.; Duan, L.; Zhu, Z. Mucus-Penetrating Silk Fibroin-Based Nanotherapeutics for Efficient Treatment of Ulcerative Colitis. Biomolecules 2022, 12, 1263. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-Decorated BixTiyOz/TiO2 Flexible Carbon Nanofibers and Their Applications on Degradating of Organic Pollutants under Solar Radiation. J. Mater. Sci. Technol. 2022, 150, 114–123. [Google Scholar] [CrossRef]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Yu, D.G.; Zhao, P. The key elements for biomolecules to biomaterials and to bioapplications. Biomolecules 2022, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional Electrospun Nanocomposites for Efficient Oxygen Reduction Reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Zhao, P.; Bai, Y.; Cui, W.; Shen, S.; Liu, Y.; Wang, Z.; Yu, D.G. Insight into the Superior Piezophotocatalytic Performance of BaTiO3//ZnO Janus Nanofibrous Heterostructures in the Treatment of Multi-Pollutants from water. Appl. Cata. B Environ. 2023, 330, 122623. [Google Scholar] [CrossRef]
- Xu, J.-F.; Chen, Y.-Z.; Wu, D.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Photoresponsive Hydrogen-Bonded Supramolecular Polymers Based on a Stiff Stilbene Unit. Angew. Chem. Int. Ed. 2013, 52, 9738–9742. [Google Scholar] [CrossRef]
- Gu, Y.X.; Chen, D.R.; Jiao, M.L. Synthesis and Electrochemical Properties of Nanostructured LiCoO2 Fibers as Cathode Materials for Lithium-Ion Batteries. J. Phys. Chem. B 2005, 109, 17901–17906. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, W.; Formo, E.; Sun, Y.; Xia, Y. Ceramic Nanofibers Fabricated by Electrospinning and Their Applications in Catalysis, Environmental Science, and Energy Technology. Polym. Adv. Technol. 2011, 22, 326–338. [Google Scholar] [CrossRef]
- Luo, C.J.; Stride, E.; Edirisinghe, M. Mapping the Influence of Solubility and Dielectric Constant on Electrospinning Polycaprolactone Solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Sarkar, S.; Deevi, S.; Tepper, G. Biased AC Electrospinning of Aligned Polymer Nanofibers. Macromol. Rapid Commun. 2007, 28, 1034–1039. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D.-G. Electrospun Nanofibers for Periodontal Treatment: A Recent Progress. Int. J. Nanomed. 2022, 17, 4137–4162. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Hu, B.; Yao, Q.-F.; Wang, K.; Yu, S.-H. Large-Scale Synthesis of Flexible Free-Standing SERS Substrates with High Sensitivity: Electrospun PVA Nanofibers Embedded with Controlled Alignment of Silver Nanoparticles. ACS Nano 2009, 3, 3993–4002. [Google Scholar] [CrossRef]
- Liu, D.; Li, W.; Li, L.; Ling, H.; You, T. Facile Preparation of Ni Nanowire Embedded Nitrogen and Sulfur Dual-Doped Carbon Nanofibers and Its Superior Catalytic Activity toward Urea Oxidation. J. Colloid Interface Sci. 2018, 529, 337–344. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-Drug Delivery Systems: Pros and Cons, Challenges, and Future Directions. Biomater. Sci. 2023, 11, 37–61. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Yan, C.; Liu, H.; Yu, D.-G. Advances in the Application of Electrospun Drug-Loaded Nanofibers in the Treatment of Oral Ulcers. Biomolecules 2022, 12, 1254. [Google Scholar] [CrossRef]
- Wang, M.; Ge, R.L.; Zhang, F.; Yu, D.G.; Liu, Z.P.; Li, X.; Shen, H.; Williams, G.R. Electrospun Fibers with Blank Surface and Inner Drug Gradient for Improving Sustained Release. Biomater. Adv. 2023, 2, 213404. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.-G.; Ge, R. Electrospun Nanofiber-Based Glucose Sensors for Glucose Detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef]
- Karimi Afshar, S.; Abdorashidi, M.; Dorkoosh, F.A.; Akbari Javar, H. Electrospun Fibers: Versatile Approaches for Controlled Release Applications. Int. J. Polym. Sci. 2022, 2022, 9116168. [Google Scholar] [CrossRef]
- Du, Y.; Yu, D.-G.; Yi, T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors 2023, 11, 208. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Liu, P.; Zhang, Y.; Song, W.; Yu, D.-G.; Lu, X. Electrospun Healthcare Nanofibers from Medicinal Liquor of Phellinus Igniarius. Adv. Compos. Hybrid Mater. 2022, 5, 3045–3056. [Google Scholar] [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Porous Nanofibers: Pore-Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers 2022, 14, 3990. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; He, W.; Xiong, M.; Niu, X.; Li, X.; Yu, D.G. A Review on Fabrication and Application of Tunable Hybrid Micro–Nano Array Surfaces. Adv. Mater. Interf. 2023, 10, 2202160. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.-G.; Wang, K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of Electrospun Polyvinylidene Fluoride-Based Nanofibers, Their Piezoelectric Properties, and Several Fantastic Applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, Q.; Song, W.; Xu, L.; Zhang, K.; Zhou, T. Advanced technique-based combination of innovation education and safety education in higher education. J. Chem. Edu. 2023, 100, 507–516. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Yu, D.-G. Recent Progress in Electrospun Nanofibers and Their Applications in Heavy Metal Wastewater Treatment. Front. Chem. Sci. Eng. 2022, 17, 1–27. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered Spindles of Little Molecules Around Electrospun Nanofibers for Biphasic Drug Release. Adv. Fiber Mater. 2022, 4, 305–317. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Y.; Huang, C.; Wang, Y.; Ji, Y.; Du, Y.; Xu, L.; Yu, D.-G.; Bligh, S.W.A. Recent Progress of Electrospun Herbal Medicine Nanofibers. Biomolecules 2023, 13, 184. [Google Scholar] [CrossRef]
- Guler, E.; Nur Hazar-Yavuz, A.; Tatar, E.; Morid Haidari, M.; Sinemcan Ozcan, G.; Duruksu, G.; Pedro, F.; Graça, M.; Kalaskar, D.M.; Gunduz, O.; et al. Oral Empagliflozin-Loaded Tri-layer Core-Sheath Fibers Fabricated Using Tri-axial Electrospinning: Enhanced In Vitro and In Vivo Antidiabetic Performance. Int. J. Pharm. 2023, 635, 122716. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Ji, Y.; Ding, Y.; Huang, C.; He, H.; Yu, D.-G. Electrospun self-emulsifying core-shell nanofibers for effective delivery of paclitaxel. Front. Bioeng. Biotechnol. 2023, 11, 1112338. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Enhancement of AFB1 Removal Efficiency via Adsorption/Photocatalysis Synergy Using Surface-Modifified Electrospun PCL-g-C3N4/CQDs Membranes. Biomolecules 2023, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hou, J.; Yu, D.G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, G.; Wang, J.; Zhang, J. Free-Standing, Welded Mesoporous Carbon Nanofibers as Anode for High-Rate Performance Li-Ion Batteries. J. Electroanal. Chem. 2017, 795, 26–31. [Google Scholar] [CrossRef]
- Li, H.; Zhu, C.; Xue, J.; Ke, Q.; Xia, Y. Enhancing the Mechanical Properties of Electrospun Nanofiber Mats through Controllable Welding at the Cross Points. Macromol. Rapid Commun. 2017, 38, 1600723. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent Advances in Electrospun Metal-Oxide Nanofiber Based Interfaces for Electrochemical Biosensing. RSC Adv. 2016, 6, 94595–94616. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-Based (Bio)Sensors for Food and Agricultural Applications: A Review. Trac.-Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Tripathy, S.; Gangwar, R.; Supraja, P.; Rao, A.V.S.S.N.; Vanjari, S.R.K.; Singh, S.G. Graphene Doped Mn2O3 Nanofibers as a Facile Electroanalytical DNA Point Mutation Detection Platform for Early Diagnosis of Breast/Ovarian Cancer. Electroanalysis 2018, 30, 2110–2120. [Google Scholar] [CrossRef]
- Mane, P.P.; Ambekar, R.S.; Kasubramanian, B. Electrospun Nanofiber-Based Cancer Sensors: A Review. Int. J. Pharm. 2020, 583, 119364. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Glucose Sensors Based on Electrospun Nanofibers: A Review. Anal. Bioanal. Chem. 2016, 408, 1285–1306. [Google Scholar] [CrossRef] [PubMed]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Manesh, K.M.; Kim, H.T.; Santhosh, P.; Gopalan, A.I.; Lee, K.-P. A Novel Glucose Biosensor Based on Immobilization of Glucose Oxidase into Multiwall Carbon Nanotubes-Polyelectrolyte-Loaded Electrospun Nanofibrous Membrane. Biosens. Bioelectron. 2008, 23, 771–779. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Zhang, Z.; Bian, H.; Yong, Q. Procuring the Nano-Scale Lignin in Prehydrolyzate as Ingredient to Prepare Cellulose Nanofibril Composite Film with Multiple Functions. Cellulose 2020, 27, 9355–9370. [Google Scholar] [CrossRef]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Xiong, Y.; Liu, X.; Nsabimana, A.; Bo, X.; Guo, L. Bimetallic MCo (M=Cu, Fe, Ni, and Mn)Nanoparticles Doped-Carbon Nanofibers Synthetized by Electrospinning for Nonenzymatic Glucose Detection. Sens. Actuators B Chem. 2015, 207, 614–622. [Google Scholar] [CrossRef]
- Rios, N.S.; Pinheiro, M.P.; dos Santos, J.C.S.; Fonseca, T.d.S.; Lima, L.D.; de Mattos, M.C.; Freire, D.M.G.; da Silva Junior, I.J.; Rodriguez-Aguado, E.; Goncalves, L.R.B. Strategies of Covalent Immobilization of a Recombinant Candida Antarctica Lipase B on Pore-Expanded SBA-15 and Its Application in the Kinetic Resolution of (R,S)-Phenylethyl Acetate. J. Mol. Catal. B-Enzym. 2016, 133, 246–258. [Google Scholar] [CrossRef]
- Polyak, P.; Urban, E.; Nagy, G.N.; Vertessy, B.G.; Pukanszky, B. The Role of Enzyme Adsorption in the Enzymatic Degradation of an Aliphatic Polyester. Enzyme Microb. Technol. 2019, 120, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z. Protein Adsorption: A Quest for a Universal Mechanism. Curr. Opin. Colloid Interface Sci. 2019, 41, 50–65. [Google Scholar] [CrossRef]
- Hu, W.; Liu, M.; Yang, X.; Zhang, C.; Zhou, H.; Xie, W.; Fan, L.; Nie, M. Modification of Chitosan Grafted with Collagen Peptide by Enzyme Crosslinking. Carbohydr. Polym. 2019, 206, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Drout, R.J.; Robison, L.; Farha, O.K. Catalytic Applications of Enzymes Encapsulated in Metal-Organic Frameworks. Coord. Chem. Rev. 2019, 381, 151–160. [Google Scholar] [CrossRef]
- Furuno, K.; Suzuki, K.; Sakai, S. Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery. Biomolecules 2022, 12, 1638. [Google Scholar] [CrossRef]
- Adhikari, B.-R.; Schraft, H.; Chen, A. A High-Performance Enzyme Entrapment Platform Facilitated by a Cationic Polymer for the Efficient Electrochemical Sensing of Ethanol. Analyst 2017, 142, 2595–2602. [Google Scholar] [CrossRef]
- Herricks, T.E.; Kim, S.H.; Kim, J.; Li, D.; Kwak, J.H.; Grate, J.W.; Kim, S.H.; Xia, Y.N. Direct Fabrication of Enzyme-Carrying Polymer Nanofibers by Electrospinning. J. Mater. Chem. 2005, 15, 3241–3245. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, K.; Liu, Y.; Han, B.; Yu, D.-G. Piezoelectric Enhancement of Piezoceramic Nanoparticle-Doped PVDF/PCL Core-Sheath Fibers. Nanomaterials 2023, 13, 1243. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Wang, M.-L.; Yu, D.-G.; Annie Bligh, S.W. Progress in Preparing Electrospun Janus Fibers and Their Applications. App. Mater. Today 2023, 31, 101766. [Google Scholar] [CrossRef]
- Song, W.; Tang, Y.; Qian, C.; Kim, B.J.; Liao, Y.; Yu, D.G. Electrospinning Spinneret: A Bridge Between the Visible World and the Invisible Nanostructures. Innovation 2023, 4, 100381. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ge, R.; Zhao, P.; Williams, G.R.; Yu, D.-G.; Annie Bligh, S.W. Exploring wettability difference-driven wetting by utilizing electrospun chimeric Janus microfiber comprising cellulose acetate and polyvinylpyrrolidone. Mater. Des. 2023, 226, 111652. [Google Scholar] [CrossRef]
- Yu, D.-G.; Du, Y.; Chen, J.; Song, W.; Zhou, T. A Correlation Analysis between Undergraduate Students’ Safety Behaviors in the Laboratory and Their Learning Efficiencies. Behav. Sci. 2023, 13, 127. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef]
- Hameed, A.; Rehman, T.U.; Rehan, Z.A.; Noreen, R.; Iqbal, S.; Batool, S.; Qayyum, M.A.; Ahmed, T.; Farooq, T. Development of Polymeric Nanofibers Blended with Extract of Neem (Azadirachta Indica), for Potential Biomedical Applications. Front. Mater. 2022, 9, 1042304. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, Y.; Qin, S.; Zhang, Y.; Liu, J.; Zhang, J.; Feng, C.; Zhao, W. Fluorine Substitution Tunes the Nanofiber Chirality of Supramolecular Hydrogels to Promote Cell Adhesion and Proliferation. Adv. Fiber Mater. 2022, 5, 377–387. [Google Scholar] [CrossRef]
- Monirul Islam, M.; Hemmanahalli Ramesh, V.; Durga Bhavani, P.; Goudanavar, P.S.; Naveen, N.R.; Ramesh, B.; Fattepur, S.; Narayanappa Shiroorkar, P.; Habeebuddin, M.; Meravanige, G.; et al. Optimization of Process Parameters for Fabrication of Electrospun Nanofibers Containing Neomycin Sulfate and Malva Sylvestris Extract for a Better Diabetic Wound Healing. Drug Deliv. 2022, 29, 3370–3383. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Deng, C.; Wang, R.; Zhang, H. Protocol for Atmospheric Water Harvesting Using in Situ Polymerization Honeycomb Hygroscopic Polymers. STAR Protoc. 2022, 3, 101780. [Google Scholar] [CrossRef] [PubMed]
- Sapountzi, E.; Braiek, M.; Vocanson, F.; Chateaux, J.-F.; Jaffrezic-Renault, N.; Lagarde, F. Gold Nanoparticles Assembly on Electrospun Poly(Vinyl Alcohol)/Poly(Ethyleneimine)/Glucose Oxidase Nanofibers for Ultrasensitive Electrochemical Glucose Biosensing. Sens. Actuators B Chem. 2017, 238, 392–401. [Google Scholar] [CrossRef]
- Narron, R.H.; Chang, H.; Jameel, H.; Park, S. Soluble Lignin Recovered from Biorefinery Pretreatment Hydrolyzate Characterized by Lignin-Carbohydrate Complexes. ACS Sustain. Chem. Eng. 2017, 5, 10763–10771. [Google Scholar] [CrossRef]
- Giummarella, N.; Zhang, L.; Henriksson, G.; Lawoko, M. Structural Features of Mildly Fractionated Lignin Carbohydrate Complexes (LCC) from Spruce. RSC Adv. 2016, 6, 42120–42131. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevicius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Haouz, A.; Twist, C.; Zentz, C.; Tauc, P.; Alpert, B. Dynamic and Structural Properties of Glucose Oxidase Enzyme. Eur. Biophy. J. 1998, 27, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, B.; Dong, H.; Zhang, Y.; Zhang, Y.; Chen, R.; Yang, Z.; Huang, C.; Jiang, Q. Coupling Biocompatible Au Nanoclusters and Cellulose Nanofibrils to Prepare the Antibacterial Nanocomposite Films. Front. Bioeng. Biotech. 2020, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Ou, X.M.; Tang, F.Q.; Jiang, L. Effect of Nanometer Particles on the Adsorbability and Enzymatic Activity of Glucose Oxidase. Colloid. Surf. B 1996, 7, 173–179. [Google Scholar] [CrossRef]
- Wang, X.; Feng, C. Chiral Fiber Supramolecular Hydrogels for Tissue Engineering. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2022, 15, e1847. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, L.; Chen, Y.; Shi, J.; Zhang, Z.; Wang, Y.; Wu, F.; Jiang, X.; Yang, W.; Zhang, L.; et al. Reactive Metal Boride Nanoparticles Trap Lipopolysaccharide and Peptidoglycan for Bacteria-Infected Wound Healing. Nat. Commun. 2022, 13, 7353. [Google Scholar] [CrossRef]
- Zhu, H.; Xing, C.; Dou, X.; Zhao, Y.; Peng, Y.; Feng, C.; Fang, Y. Chiral Hydrogel Accelerates Re-Epithelization in Chronic Wounds via Mechanoregulation. Adv. Healthc. Mater. 2022, 11, 2201032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, H.; Bu, W. A Forward Vision for Chemodynamic Therapy: Issues and Opportunities. Angew. Chem. Int. Ed. 2023, 62, e202210415. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Gao, J.; He, J.; Zhang, H.; Ding, J. Stereo Coverage and Overall Stiffness of Biomaterial Arrays Underly Parts of Topography Effects on Cell Adhesion. ACS Appl. Mater. Interf. 2023, 15, 46142–46155. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, S.; Zhao, P.; Wang, H.; Ni, D.; Li, H.; Jiang, X.; Wu, Y.; Meng, Y.; Yao, Z.; et al. Chemical Factory-Guaranteed Enhanced Chemodynamic Therapy for Orthotopic Liver Cancer. Adv. Sci. 2022, 9, 2201232. [Google Scholar] [CrossRef]
- Chen, K.; Chou, W.; Liu, L.; Cui, Y.; Xue, P.; Jia, M. Electrochemical Sensors Fabricated by Electrospinning Technology: An Overview. Sensors 2019, 19, 3676. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, X.; Lv, M.; Wang, X.; Zhao, P.; Zhang, M.; Lv, G.; Wu, J.; Liu, Y.; Yang, Y.; et al. Reductive-Damage-Induced Intracellular Maladaptation for Cancer Electronic Interference Therapy. Chem 2022, 8, 866–879. [Google Scholar] [CrossRef]
- Asghar, N.; Mustafa, G.; Jabeen, N.; Dawood, A.; Rida; Jabeen, Z.; Malik, Q.H.; Khan, M.A.; Khan, M.U. Fabrication of Efficient and Non-Enzymatic Electrochemical Sensors for the Detection of Sucrose. Sensors 2023, 23, 2008. [Google Scholar] [CrossRef]
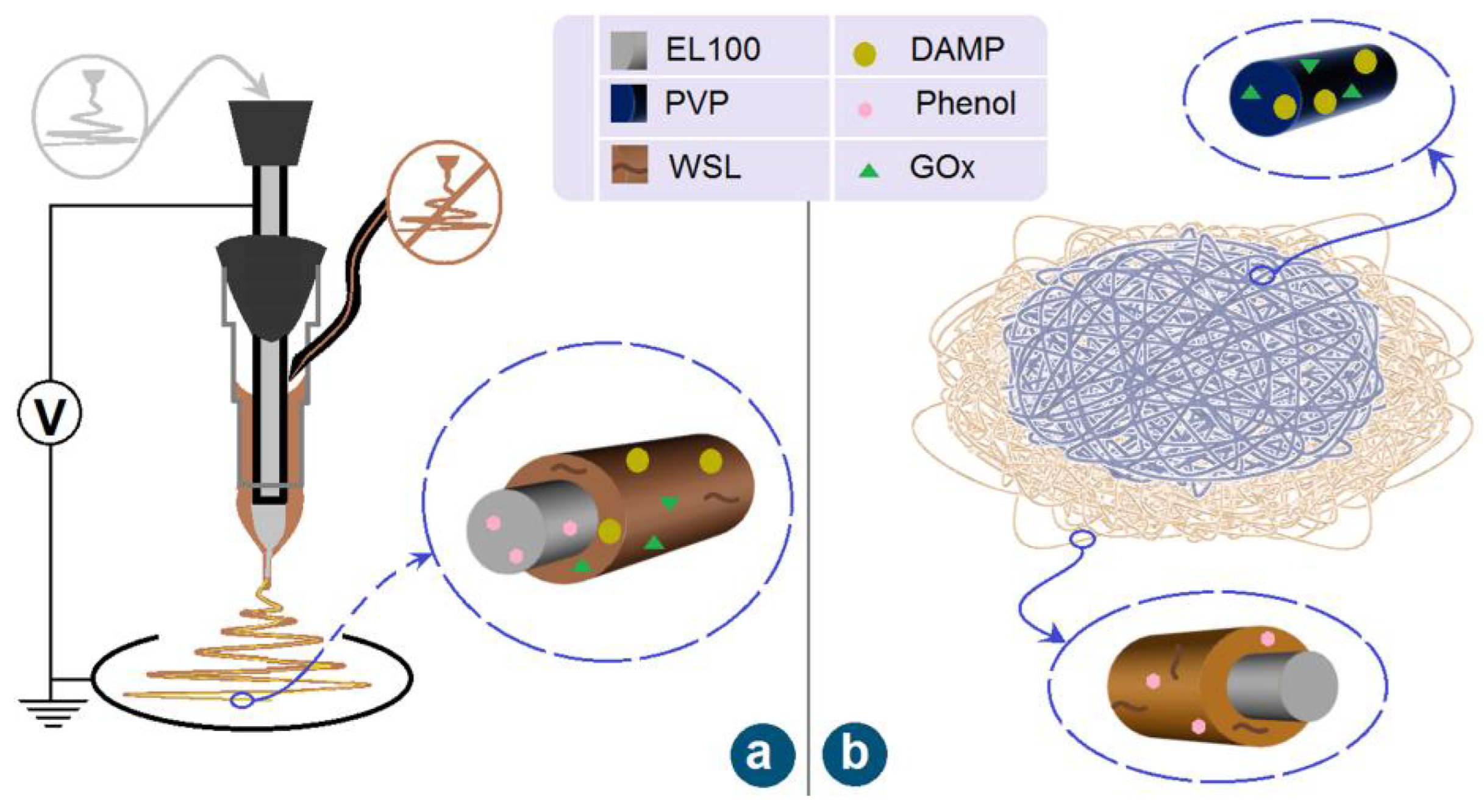


| Film No. | Structure | Working Process | Working Fluid | Experimental Conditions a |
|---|---|---|---|---|
| F1 | Core–sheath nanofibers | Coaxial | Core fluid: 1.5 g Eudragit L 100 (EL100) was dissolved in 10 mL of anhydrous ethanol, and 2 mL of phenol was added. Sheath fluid: 0.15 g WSL was dissolved in 3 mL of deionized water; 150 mg GOx was added to 2 mL DAMP water dispersion solution. The two solutions were mixed as sheath fluid. | V = 10 kV Fc = 0.5 mL/h Fs = 0.2 mL/h D = 15 cm |
| F2 | Hybrids of core–sheath and monolithic fibers layers | Coaxial electrospinning | Core fluid: 1.5 g EL100 in 10 mL anhydrous ethanol. Sheath fluid: 0.15 g WSL was dissolved in 3 mL anhydrous ethanol, and later 0.6 mL of phenol was added. The two solutions were mixed as sheath fluid. | V = 10 kV Fc = 0.5 mL/h Fs = 0.2 mL/h D = 15 cm |
| Single-fluid blending | Working fluid: 0.8 g PVP K90 was dissolved in 10 mL of deionized water; 150 mg GOx was added to 2 mL DAMP water dispersion. The two solutions were mixed homogeneously for spinning. | V = 8 kV F = 0.5 mL/h D = 15 cm |
| Component (%) | Hydroxyl Group (mmol/g) | |||||
|---|---|---|---|---|---|---|
| Lignin | Xylan | Dextran | Aliphatic Series | Condensed Phenolic Moieties | Non-Condensed Phenols | |
| WSL | 84.5 ± 0.2 | 5.6 ± 1.3 | 2.1 ± 10.6 | 2.8 ± 0.1 | 2.6 ± 0.2 | 1.3 ± 0.1 |
| 84.5 ± 0.2 | 5.6 ± 1.3 | 2.1 ± 10.6 | 2.8 ± 0.1 | 2.6 ± 0.2 | 1.3 ± 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Yang, Z.; Kang, S.; Yu, D.-G.; Chen, X.; Shao, J. A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose. Sensors 2023, 23, 3685. https://doi.org/10.3390/s23073685
Du Y, Yang Z, Kang S, Yu D-G, Chen X, Shao J. A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose. Sensors. 2023; 23(7):3685. https://doi.org/10.3390/s23073685
Chicago/Turabian StyleDu, Yutong, Zili Yang, Shixiong Kang, Deng-Guang Yu, Xiren Chen, and Jun Shao. 2023. "A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose" Sensors 23, no. 7: 3685. https://doi.org/10.3390/s23073685
APA StyleDu, Y., Yang, Z., Kang, S., Yu, D.-G., Chen, X., & Shao, J. (2023). A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose. Sensors, 23(7), 3685. https://doi.org/10.3390/s23073685