Graphene Nanoribbon Field Effect Transistor Simulations for the Detection of Sugar Molecules: Semi-Empirical Modeling
Abstract
1. Introduction
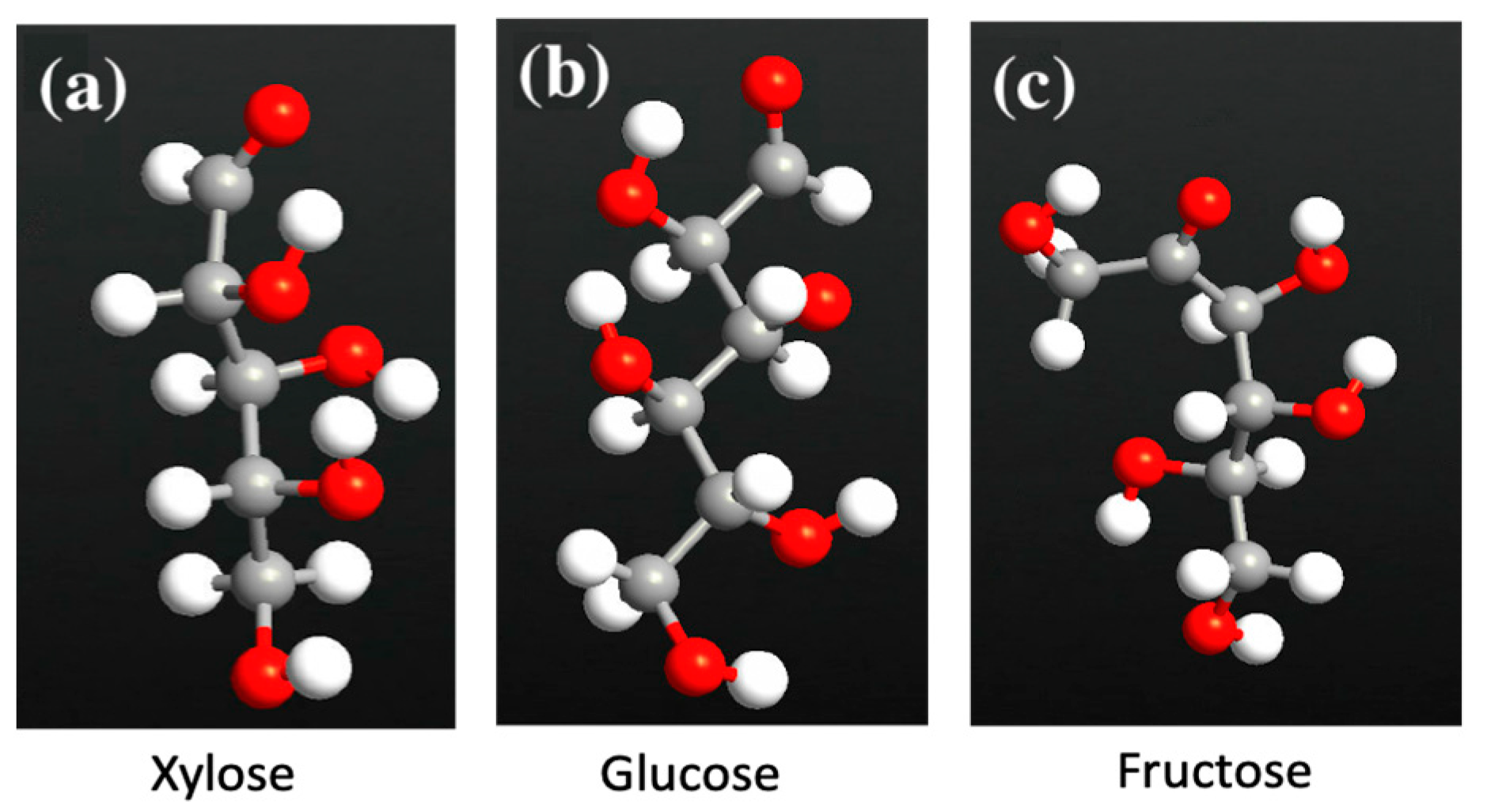
2. Materials and Methods
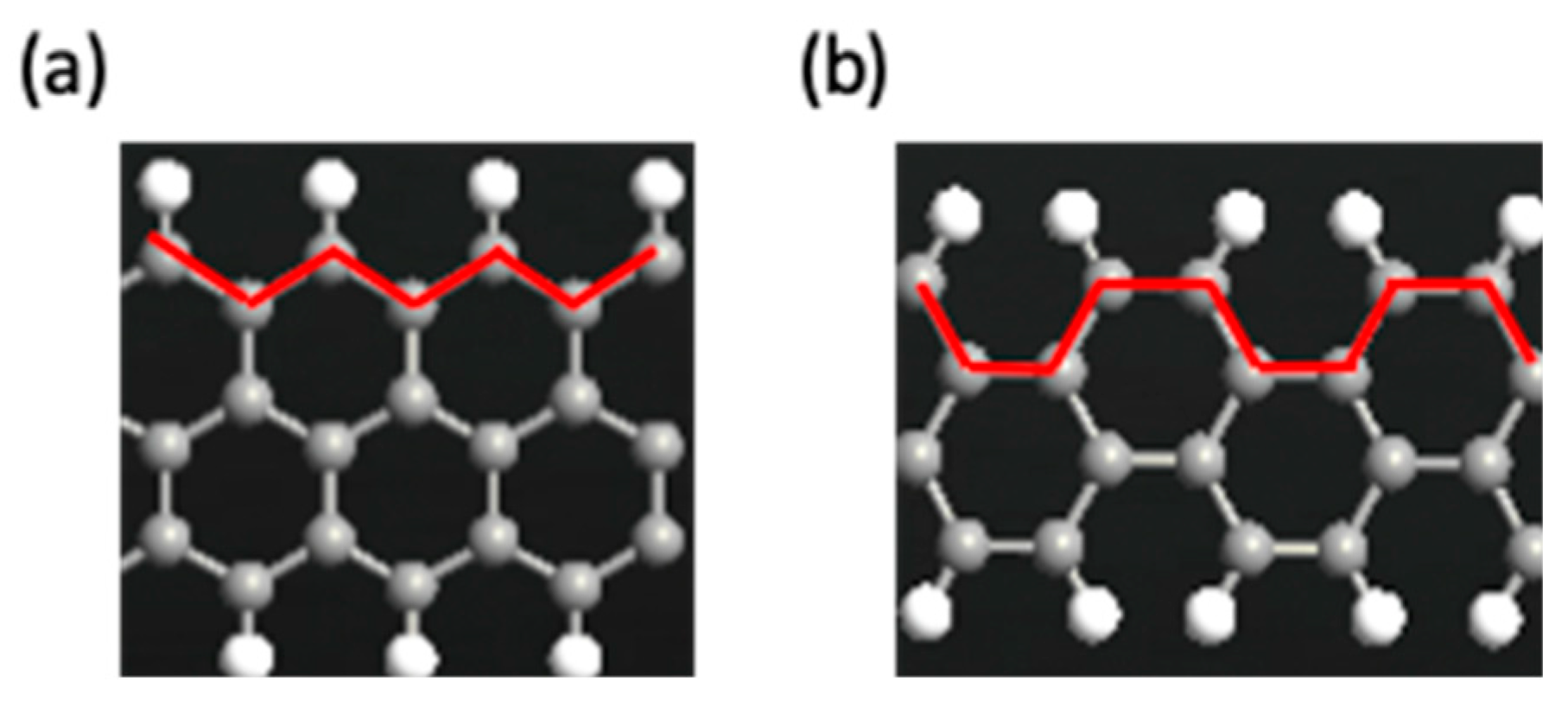
2.1. Graphene Nanoribbons (Armchair and Zigzag)
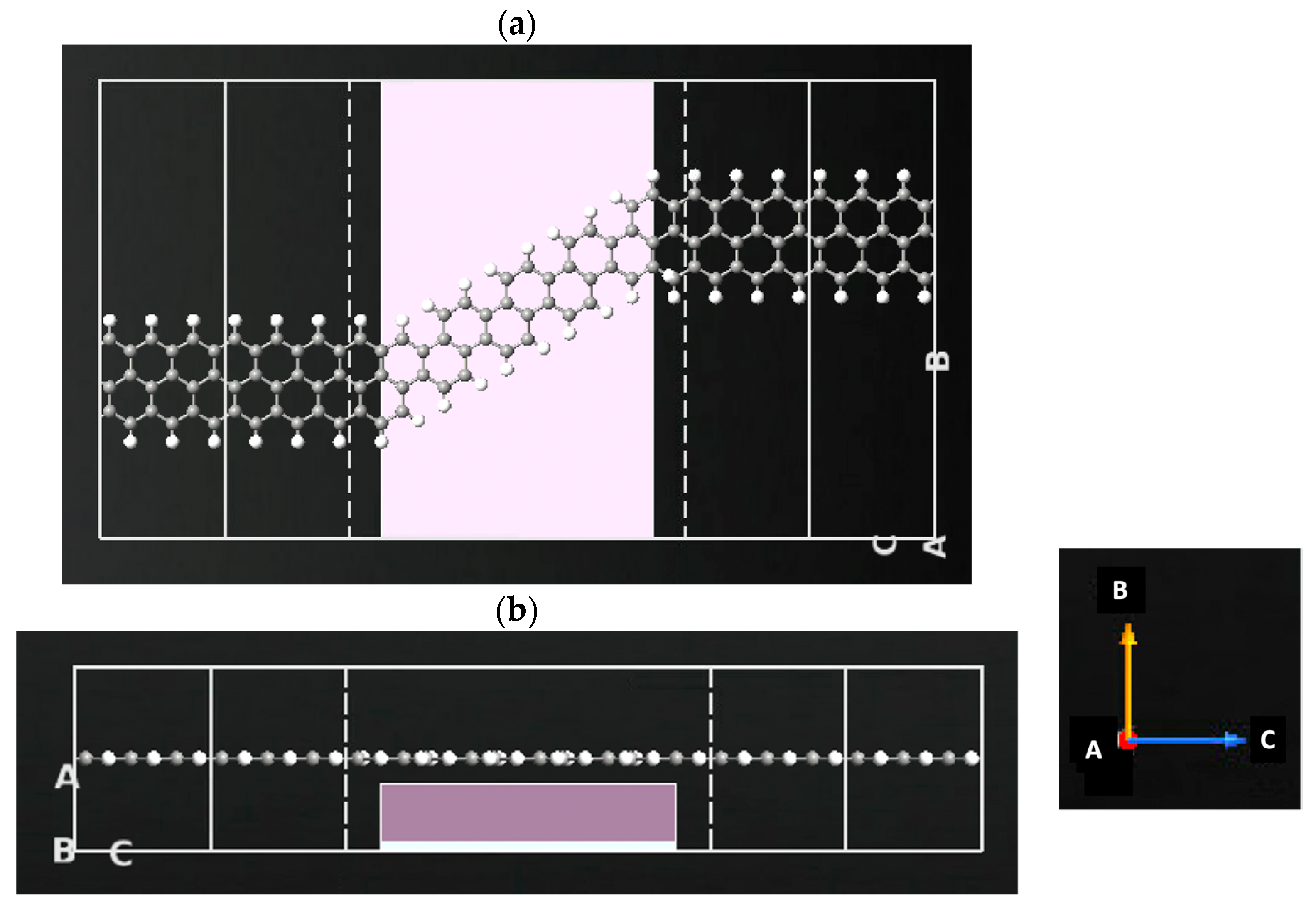
2.2. Sensor Setup and Configuration
2.3. Computational Method
3. Results and Discussion
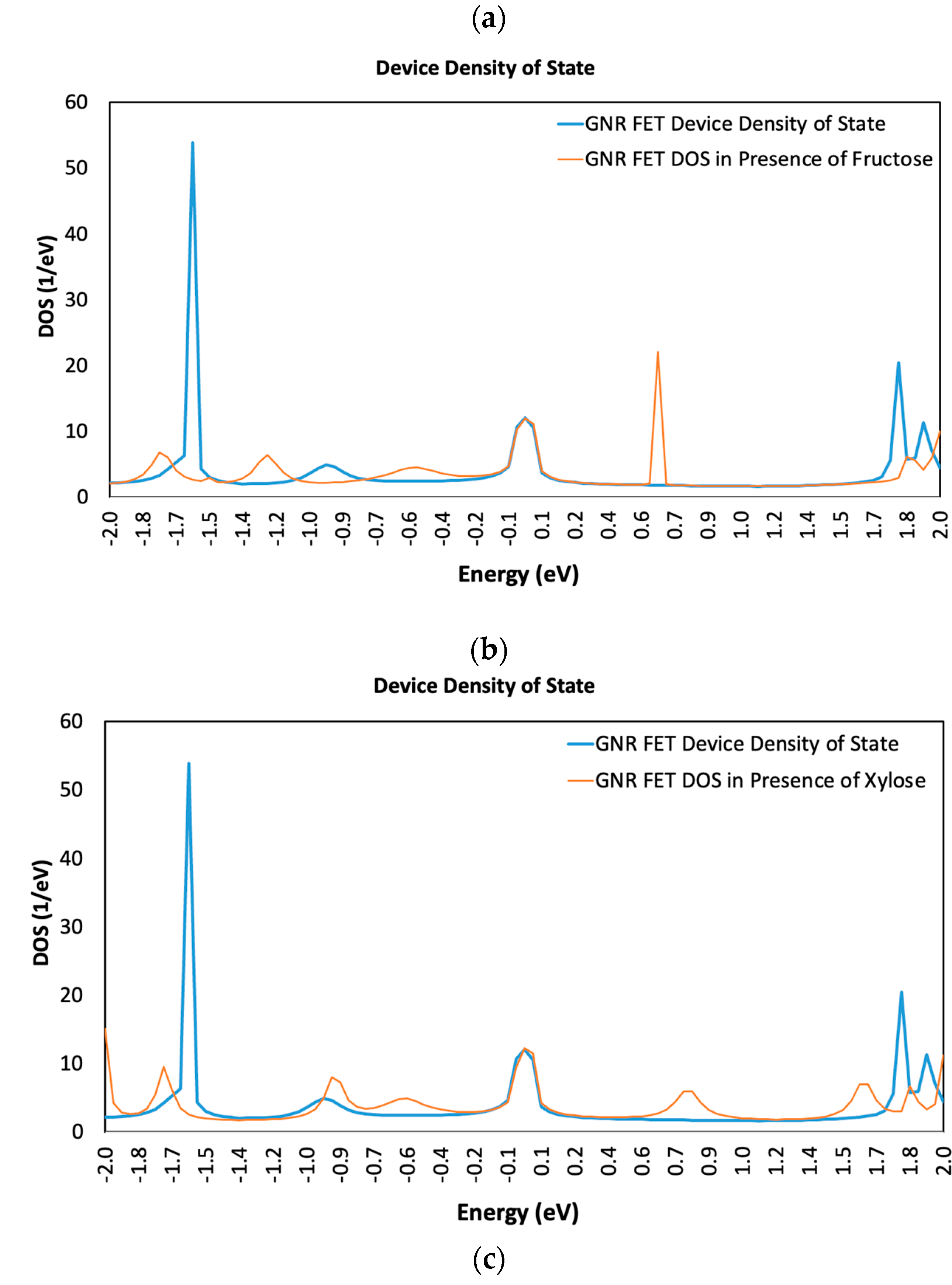
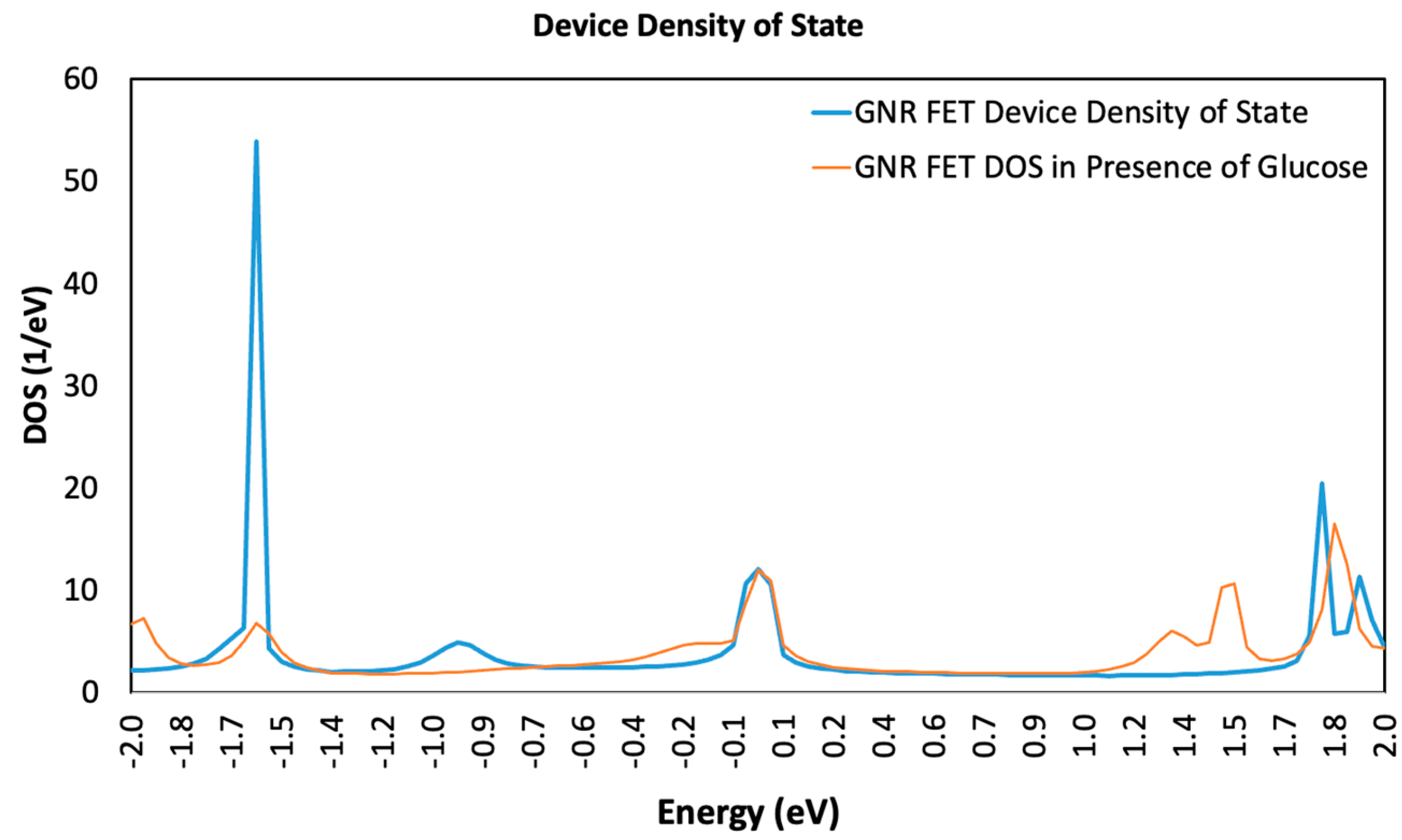
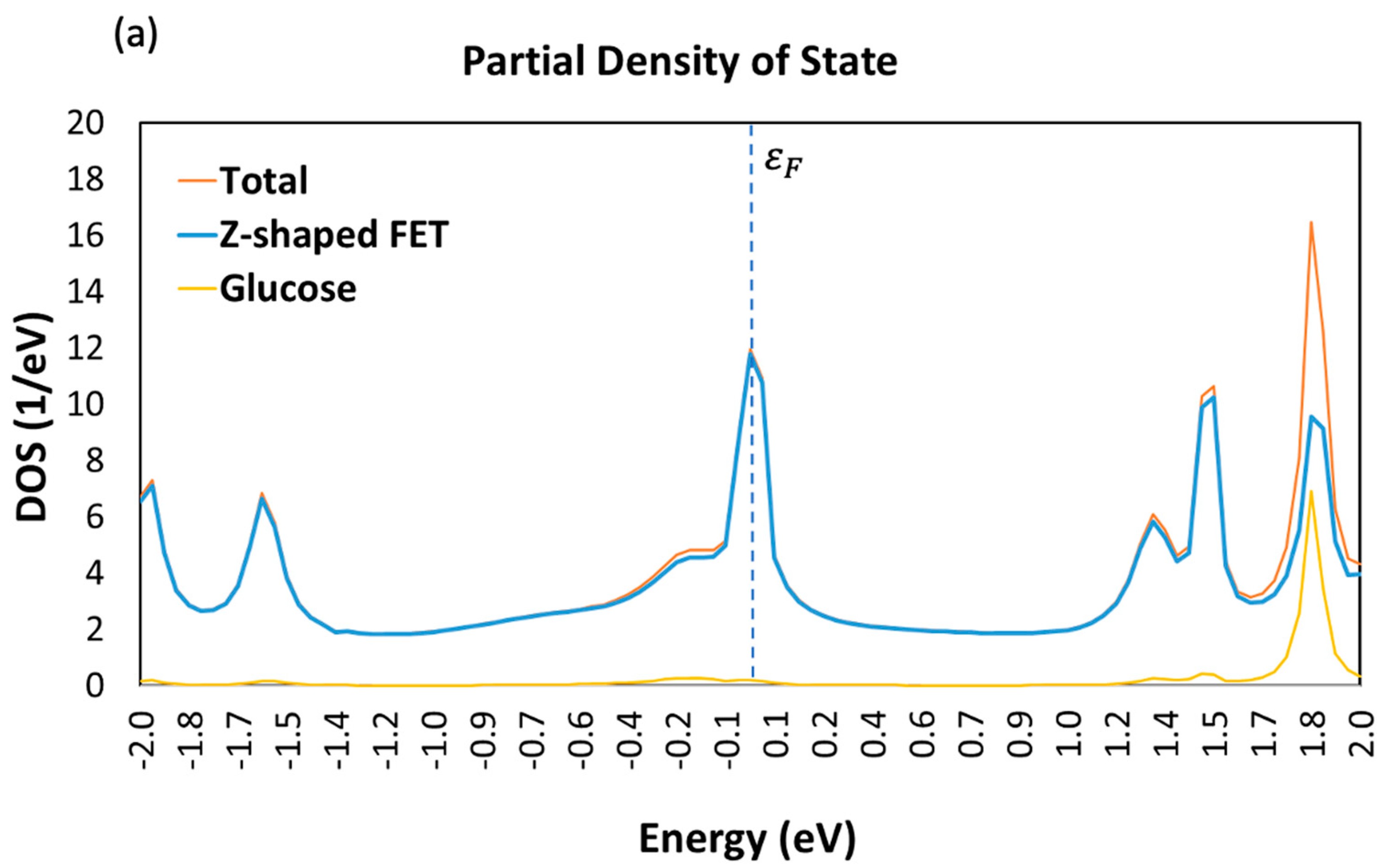
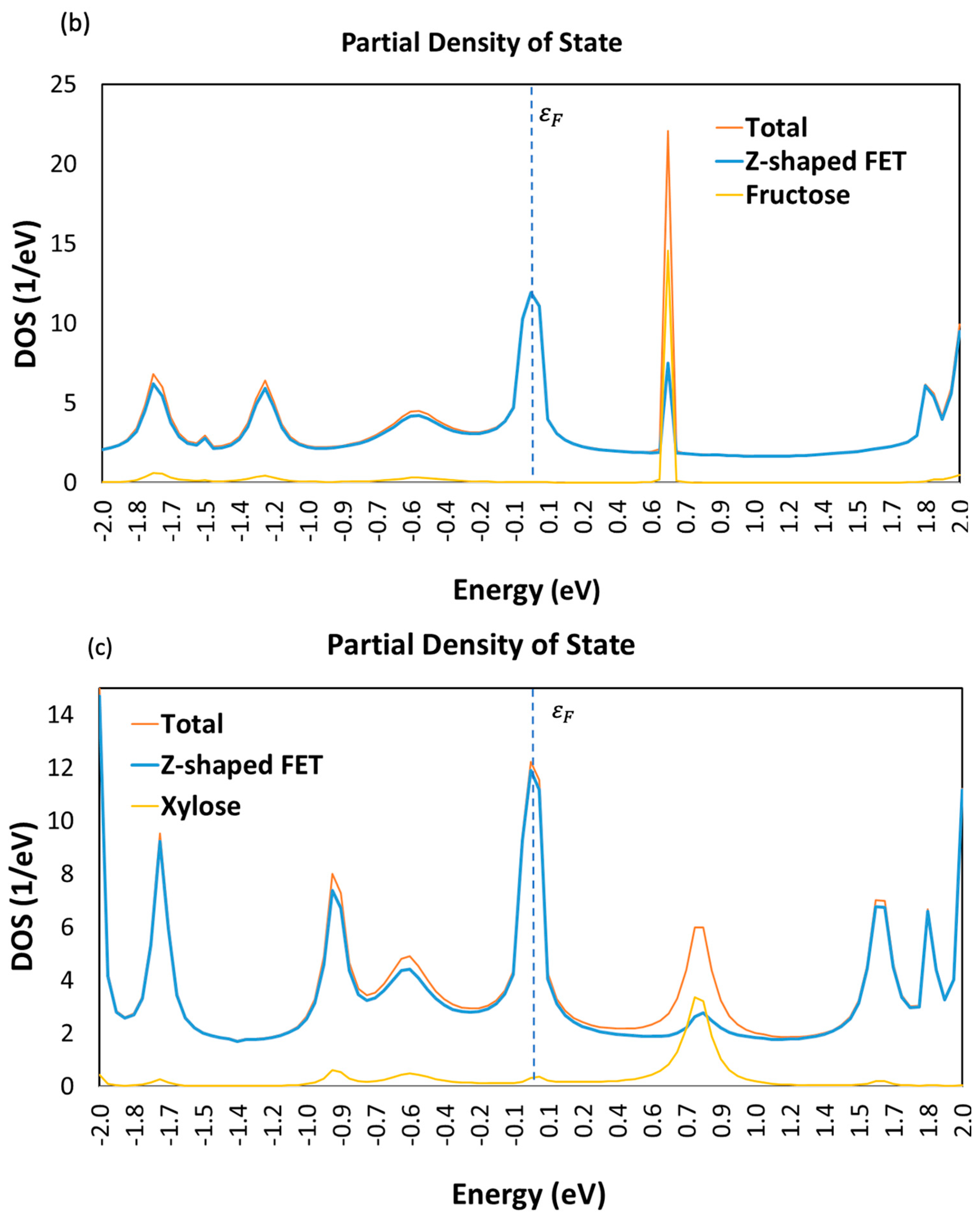
3.1. Device Density of State (DDOS)
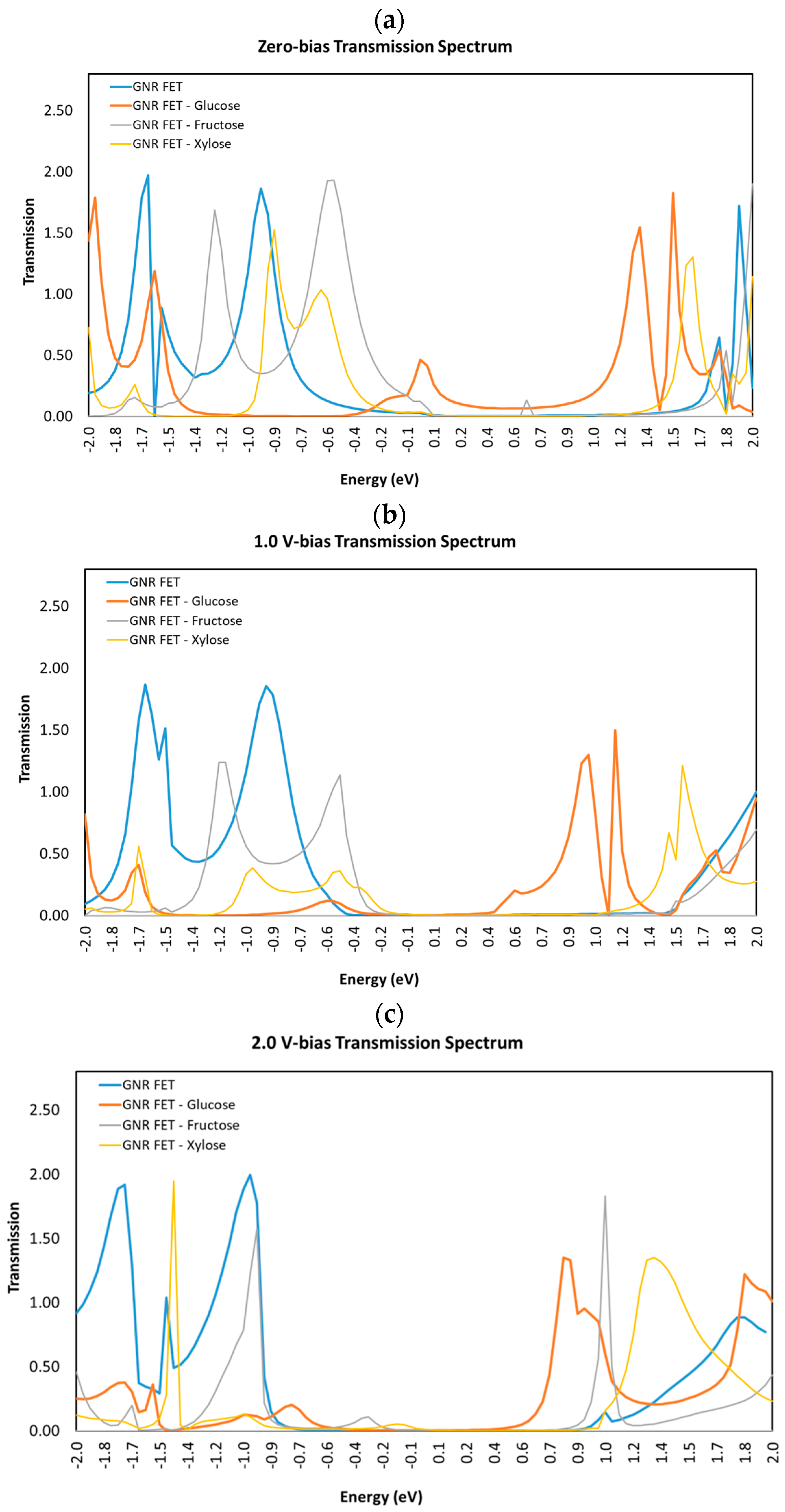
3.2. Transmission Spectra
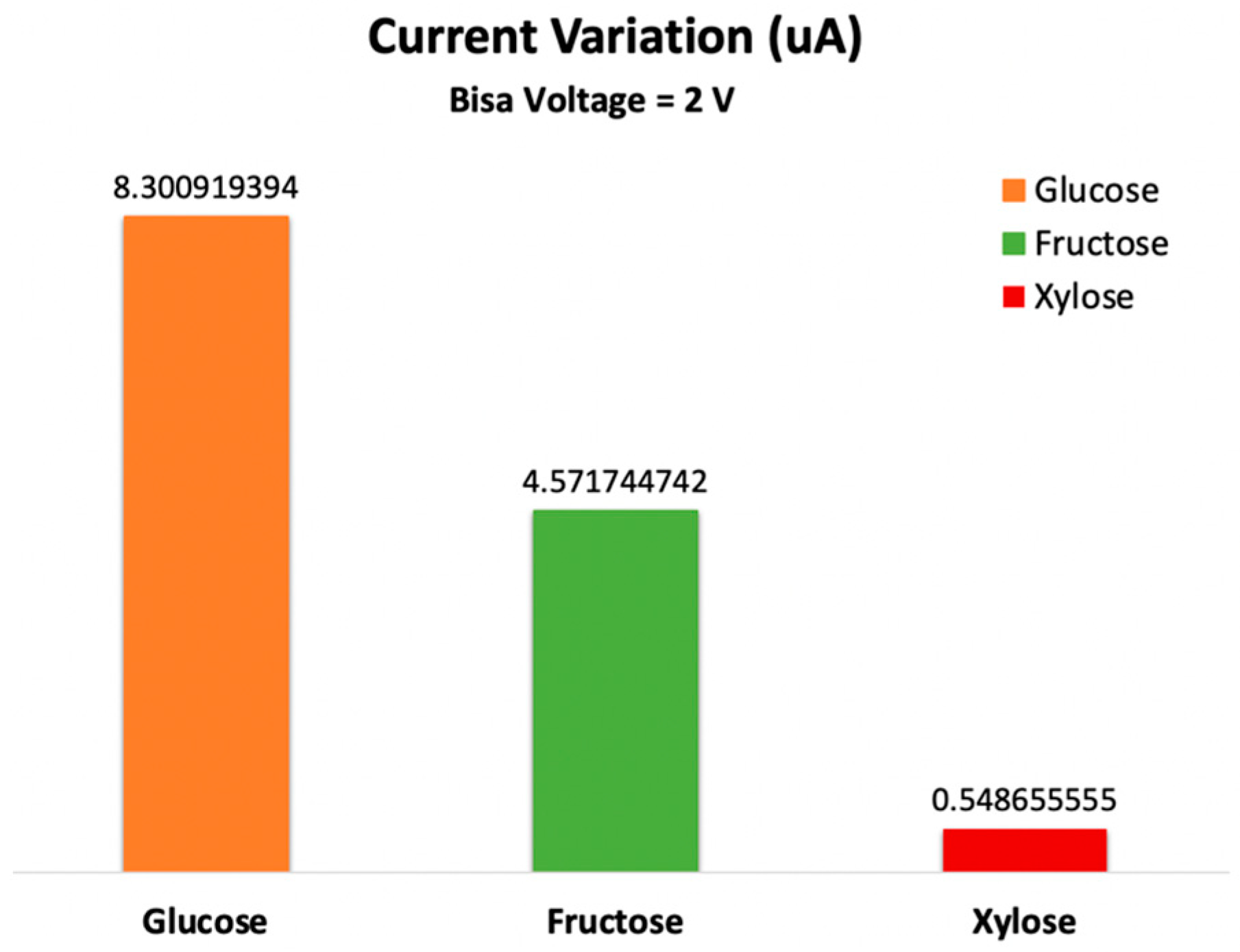
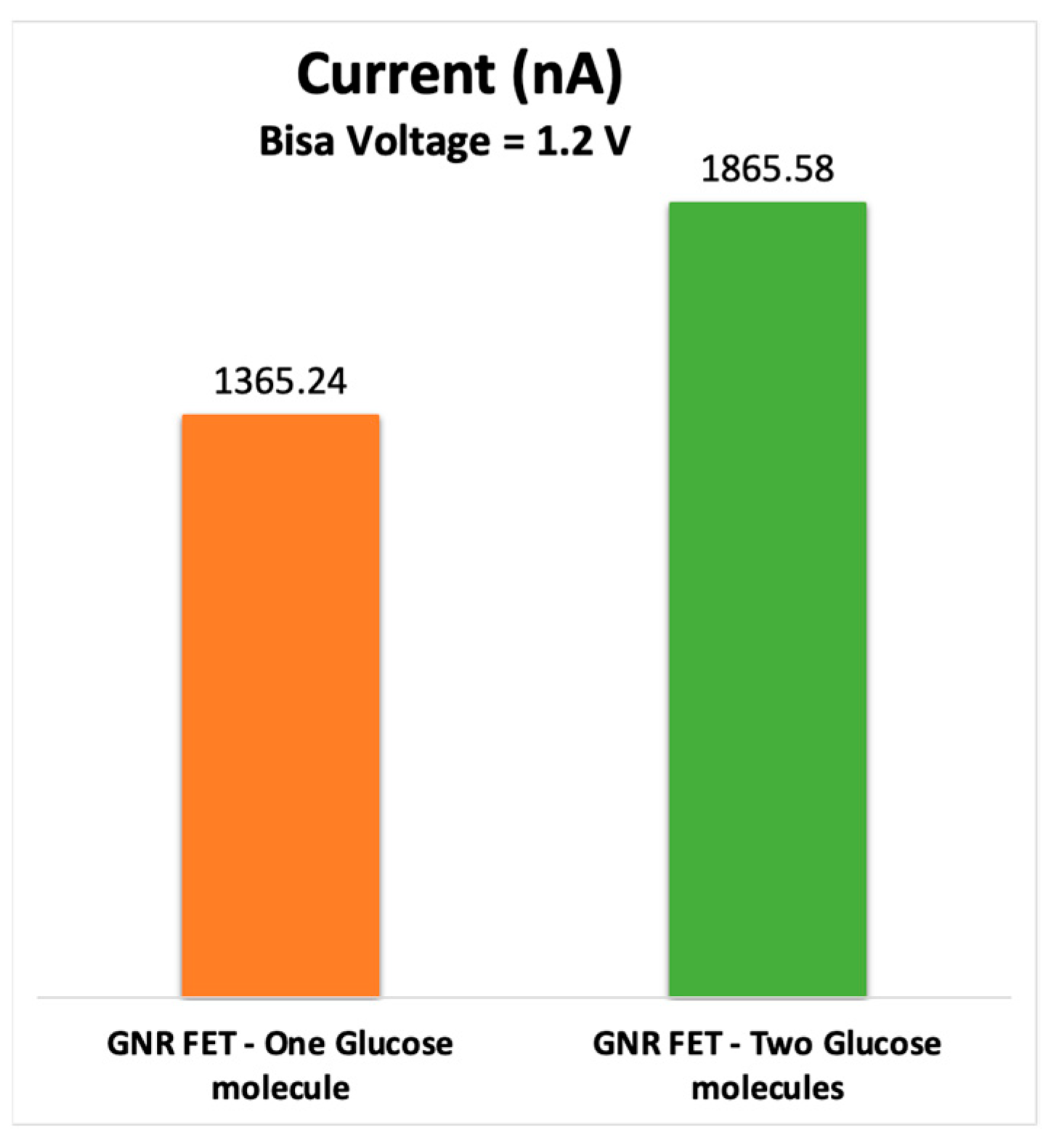
3.3. Current-Voltage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crespo-Rosa, J.R.; Foca, G.; Ulrici, A.; Pigani, L.; Zanfrognini, B.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Zanardi, C. Simultaneous Detection of Glucose and Fructose in Synthetic Musts by Multivariate Analysis of Silica-Based Amperometric Sensor Signals. Sensors 2021, 21, 4190. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Bai, T.; Zhang, L.; Ella-Menye, J.-R.; Liu, S.; Nowinski, A.K.; Jiang, S.; Yu, Q. Sensitive and Fast Detection of Fructose in Complex Media via Symmetry Breaking and Signal Amplification Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2014, 86, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Lin, S.; Du, D.; Lin, Y. Integrated immunochromatographic strip with glucometer readout for rapid quantification of phosphorylated proteins. Anal. Chim. Acta 2017, 964, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhao, G.; Dou, W. Portable and quantitative point-of-care monitoring of Escherichia coli O157: H7 using a personal glucose meter based on immunochromatographic assay. Biosens. Bioelectron. 2018, 107, 266–271. [Google Scholar] [CrossRef]
- Das, A.; Cui, X.; Chivukula, V.; Iyer, S.S. Detection of enzymes, viruses, and bacteria using glucose meters. Anal. Chem. 2018, 90, 11589–11598. [Google Scholar] [CrossRef]
- Gu, C.; Chen, X.; Liu, H. Portable, quantitative, and sequential monitoring of copper ions and pyrophosphate based on a DNAzyme-Fe3O4 nanosystem and glucometer readout. Anal. Bioanal. Chem. 2021, 413, 6941–6949. [Google Scholar] [CrossRef]
- Mitro, N.; Mak, P.A.; Vargas, L.; Godio, C.; Hampton, E.; Molteni, V.; Kreusch, A.; Saez, E. The nuclear receptor LXR is a glucose sensor. Nature 2007, 445, 219–223. [Google Scholar] [CrossRef]
- Kerzenmacher, S.; Ducrée, J.; Zengerle, R.; Von Stetten, F. Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J. Power Sources 2008, 182, 1–17. [Google Scholar] [CrossRef]
- Al-Mhanna, N.M.; Huebner, H.; Buchholz, R. Analysis of the sugar content in food products by using gas chromatography mass spectrometry and enzymatic methods. Foods 2018, 7, 185. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal. Chem. 2012, 84, 4174–4178. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Dalgård, C.; Möller, S.; Kyvik, K.O. Heritability of curve patterns in oral glucose tolerance test. Twin Res. Hum. Genet. 2020, 23, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Lee, A.-R. Recent developments in blood glucose sensors. J. Food Drug Anal. 2015, 23, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kwon, L.; Long, K.; Wan, Y.; Yu, H.; Cunningham, B. Medical diagnostics with mobile devices: Comparison of intrinsic and extrinsic sensing. Biotechnol. Adv. 2016, 34, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Keenan, D.B.; Mastrototaro, J.J.; Voskanyan, G.; Steil, G.M. Delays in minimally invasive continuous glucose monitoring devices: A review of current technology. J. Diabetes Sci. Technol. 2009, 3, 1207–1214. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef]
- Mishra, A.K.; Jarwal, D.K.; Mukherjee, B.; Kumar, A.; Ratan, S.; Tripathy, M.R.; Jit, S. Au nanoparticles modified CuO nanowire electrode based non-enzymatic glucose detection with improved linearity. Sci. Rep. 2020, 10, 11451. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Ptitsyn, A.; McLamore, E.S.; Claussen, J.C. Nanomaterial-mediated Biosensors for Monitoring Glucose. J. Diabetes Sci. Technol. 2014, 8, 403–411. [Google Scholar] [CrossRef]
- Kwak, Y.H.; Choi, D.S.; Kim, Y.N.; Kim, H.; Yoon, D.H.; Ahn, S.-S.; Yang, J.-W.; Yang, W.S.; Seo, S. Flexible glucose sensor using CVD-grown graphene-based field effect transistor. Biosens. Bioelectron. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Scandurra, A.; Ruffino, F.; Sanzaro, S.; Grimaldi, M.G. Laser and thermal dewetting of gold layer onto graphene paper for non-enzymatic electrochemical detection of glucose and fructose. Sens. Actuators B Chem. 2019, 301, 127113. [Google Scholar] [CrossRef]
- Juſík, T.; Podešva, P.; Farka, Z.; Kováſ, D.; Skládal, P.; Foret, F. Nanostructured gold deposited in gelatin template applied for electrochemical assay of glucose in serum. Electrochim. Acta 2016, 188, 277–285. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Kwen, H.-D.; Choi, S.-H. Fabrication of Nonenzymatic Glucose Sensors Based on Multiwalled Carbon Nanotubes with Bimetallic Pt-M (M = Ru and Sn) Catalysts by Radiolytic Deposition. J. Sens. 2012, 2012, 784167. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Shamkhalichenar, H.; Choi, J.-W. Inkjet-Printed and Paper-Based Electrochemical Sensors. Appl. Sci. 2018, 8, 288. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Merkoçi, A. Graphene oxide as an optical biosensing platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Wu, G.; Meyyappan, M.; Lai, K.W. Simulation of Graphene Field-Effect Transistor Biosensors for Bacterial Detection. Sensors 2018, 18, 1715. [Google Scholar] [CrossRef]
- Oshin, O.; Kireev, D.; Hlukhova, H.; Idachaba, F.; Akinwande, D.; Atayero, A. Graphene-Based Biosensor for Early Detection of Iron Deficiency. Sensors 2020, 20, 3688. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Gelovani, J.G.; Qamhieh, N.; Ayesh, A.I. COVID-19 Detection via Silicon Nanowire Field-Effect Transistor: Setup and Modeling of Its Function. Nanomaterials 2022, 12, 2638. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Qamhieh, N.; Al Murshidi, B.; Palakkott, A.R.; Gelovani, J.G. Real-time COVID-19 detection via graphite oxide-based field-effect transistor biosensors decorated with Pt/Pd nanoparticles. Sci. Rep. 2022, 12, 18155. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Ayesh, A.I. Detection of DNA Bases via Field Effect Transistor of Graphene Nanoribbon with a Nanopore: Semi-empirical Modeling. IEEE Trans. NanoBioscience 2021, 21, 347–357. [Google Scholar] [CrossRef]
- Prasongkit, J.; Grigoriev, A.; Pathak, B.; Ahuja, R.; Scheicher, R.H. Transverse Conductance of DNA Nucleotides in a Graphene Nanogap from First Principles. Nano Lett. 2011, 11, 1941–1945. [Google Scholar] [CrossRef] [PubMed]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Koel, A.; Rang, T. Simulations of Propane and Butane Gas Sensor Based on Pristine Armchair Graphene Nanoribbon. IOP Conf. Ser. Mater. Sci. Eng. 2018, 362, 012001. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, B.; Yu, J.; Zheng, F.; Zang, J.; Wu, J.; Gu, B.-L.; Liu, F.; Duan, W. Intrinsic current− voltage characteristics of graphene nanoribbon transistors and effect of edge doping. Nano Lett. 2007, 7, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, J.; Soria, F. Accurate and transferable extended H\″uckel-type tight-binding parameters. Phys. Rev. B 2000, 61, 7965–7971. [Google Scholar] [CrossRef]
- Nikolai, A.P.; Eugene, F.K.; Sergey, A.V.; Oleg, N.B.; Sergey, V.R. Electronic band structure and magnetic states of zigzag graphene nanoribbons: Quantum chemical calculations. J. Nanophotonics 2012, 6, 061712. [Google Scholar] [CrossRef]
- Owens, F.J. Electronic and magnetic properties of armchair and zigzag graphene nanoribbons. J. Chem. Phys. 2008, 128, 194701. [Google Scholar] [CrossRef]
- Xie, S.-Y.; Li, X.-B. Metallic Graphene Nanoribbons. Nano-Micro Lett. 2021, 13, 53. [Google Scholar] [CrossRef]
- Narendar, V.; Gupta, S.K.; Saxena, S. First Principle Study of Doped Graphene for FET Applications. Silicon 2018, 11, 277–286. [Google Scholar] [CrossRef]
- Chang, P.-H.; Liu, H.; Nikolic, B.K. First-principles vs. semi-empirical modeling of global and local electronic transport properties of graphene nanopore-based sensors for DNA sequencing. J. Comput. Electron. 2014, 13, 847–856. [Google Scholar] [CrossRef]
- Saha, K.K.; Drndić, M.; Nikolić, B.K. DNA Base-Specific Modulation of Microampere Transverse Edge Currents through a Metallic Graphene Nanoribbon with a Nanopore. Nano Lett. 2012, 12, 50–55. [Google Scholar] [CrossRef]
- Stokbro, K.; Petersen, D.; Smidstrup, S.; Blom, A.; Ipsen, M.; Kaasbjerg, K. Semi-Empirical Model for Nano-Scale Device Simulations. Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Li, G.; Song, Y. Construction and properties analysis of ZAZ graphene nanoribbons transistors. J. Phys. Conf. Ser. 2022, 2313, 012015. [Google Scholar] [CrossRef]
- Gupta, M.; Gaur, N.; Kumar, P.; Singh, S.; Jaiswal, N.K.; Kondekar, P. Tailoring the electronic properties of a Z-shaped graphene field effect transistor via B/N doping. Phys. Lett. A 2015, 379, 710–718. [Google Scholar] [CrossRef]
- QuantumATK. Transmission Spectrum. Available online: https://docs.quantumatk.com/manual/Types/TransmissionSpectrum/TransmissionSpectrum.html (accessed on 26 February 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasfi, A.; Al Hamarna, A.; Al Shehhi, O.M.H.; Al Ameri, H.F.M.; Awwad, F. Graphene Nanoribbon Field Effect Transistor Simulations for the Detection of Sugar Molecules: Semi-Empirical Modeling. Sensors 2023, 23, 3010. https://doi.org/10.3390/s23063010
Wasfi A, Al Hamarna A, Al Shehhi OMH, Al Ameri HFM, Awwad F. Graphene Nanoribbon Field Effect Transistor Simulations for the Detection of Sugar Molecules: Semi-Empirical Modeling. Sensors. 2023; 23(6):3010. https://doi.org/10.3390/s23063010
Chicago/Turabian StyleWasfi, Asma, Ahmed Al Hamarna, Omar Mohammed Hasani Al Shehhi, Hazza Fahad Muhsen Al Ameri, and Falah Awwad. 2023. "Graphene Nanoribbon Field Effect Transistor Simulations for the Detection of Sugar Molecules: Semi-Empirical Modeling" Sensors 23, no. 6: 3010. https://doi.org/10.3390/s23063010
APA StyleWasfi, A., Al Hamarna, A., Al Shehhi, O. M. H., Al Ameri, H. F. M., & Awwad, F. (2023). Graphene Nanoribbon Field Effect Transistor Simulations for the Detection of Sugar Molecules: Semi-Empirical Modeling. Sensors, 23(6), 3010. https://doi.org/10.3390/s23063010





