A Laboratory Evaluation of the New Automated Pollen Sensor Beenose: Pollen Discrimination Using Machine Learning Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrument Description
2.2. Instrument Calibration
2.3. Pollen Samples and Laboratory Measurements
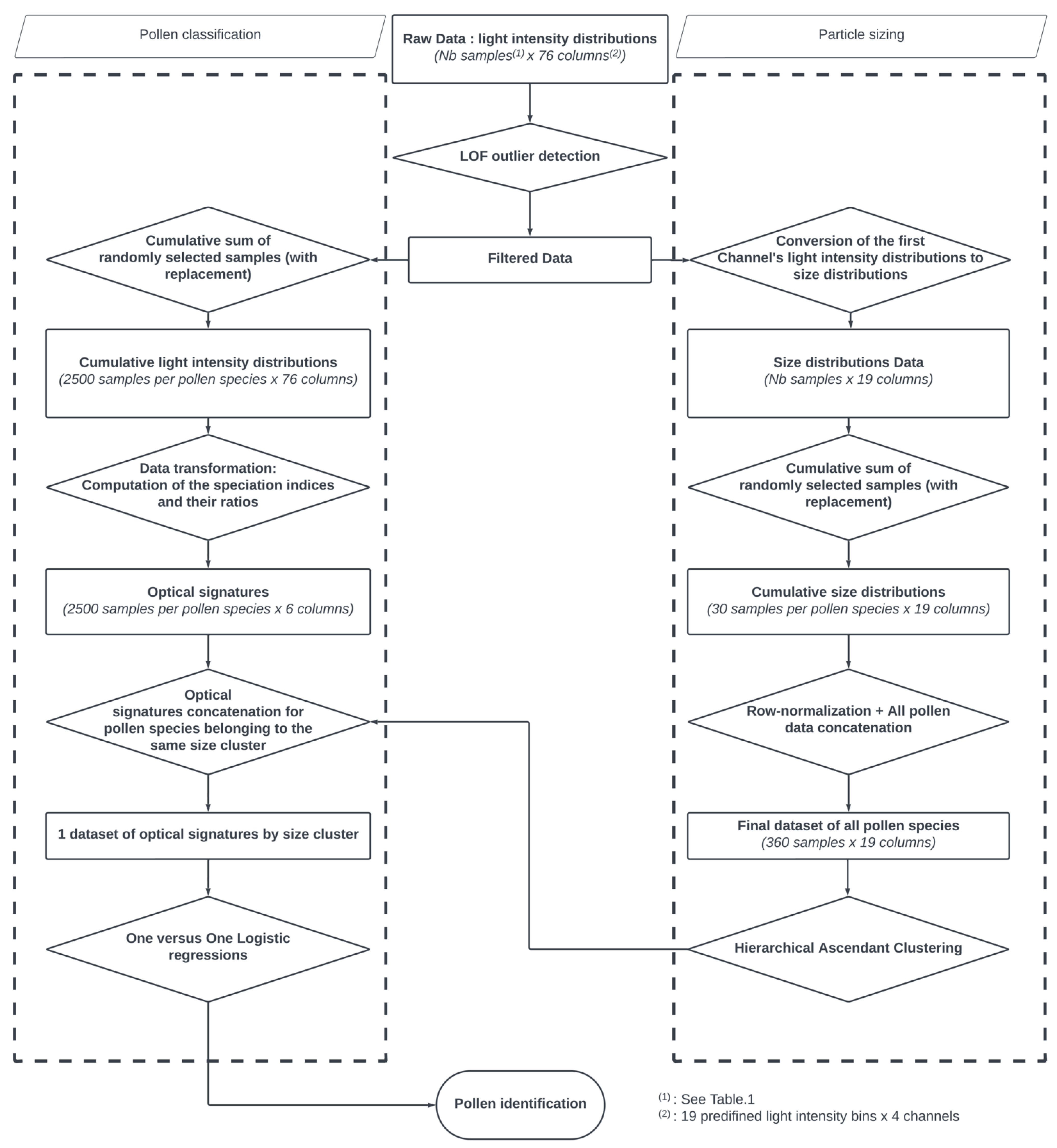
2.4. Data Processing
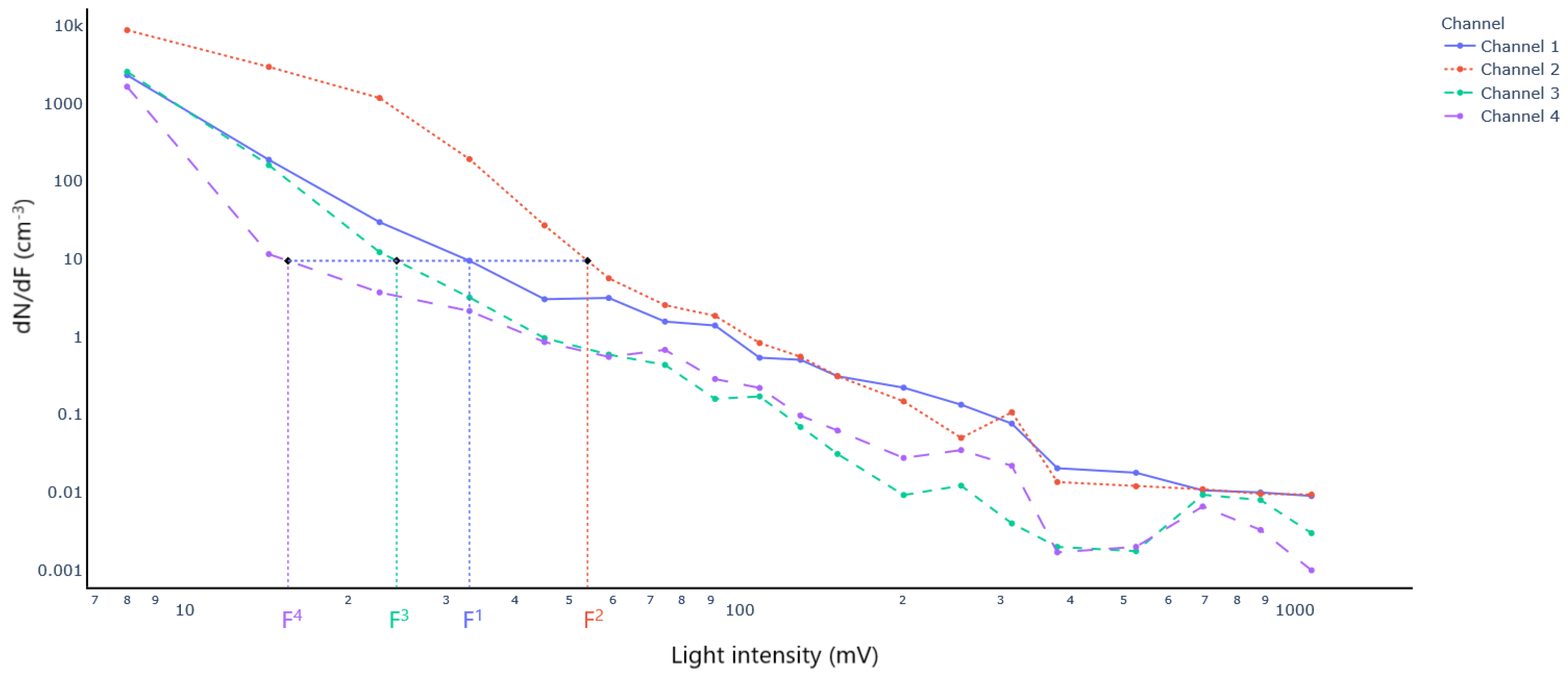
2.4.1. Particle Sizing
2.4.2. Pollen Classification
3. Results
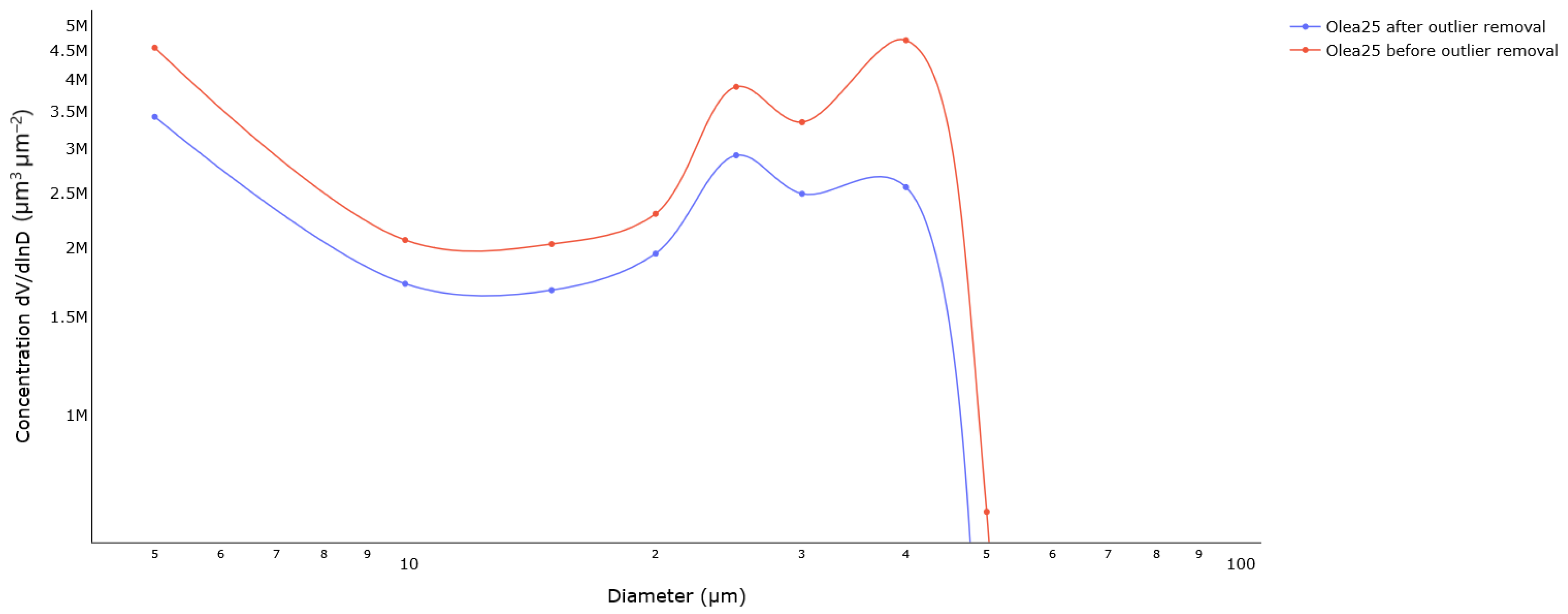
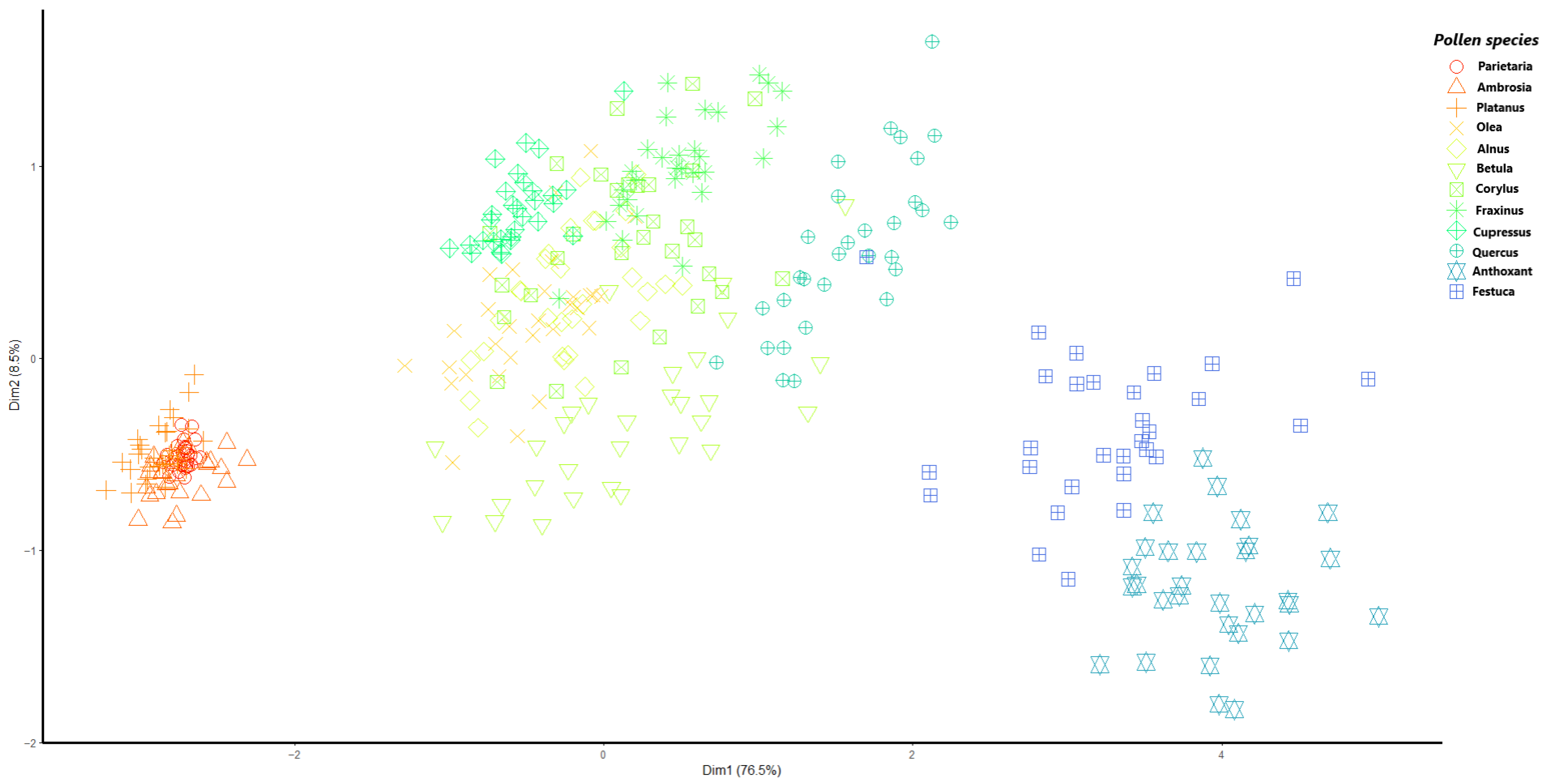
3.1. Particle Sizing
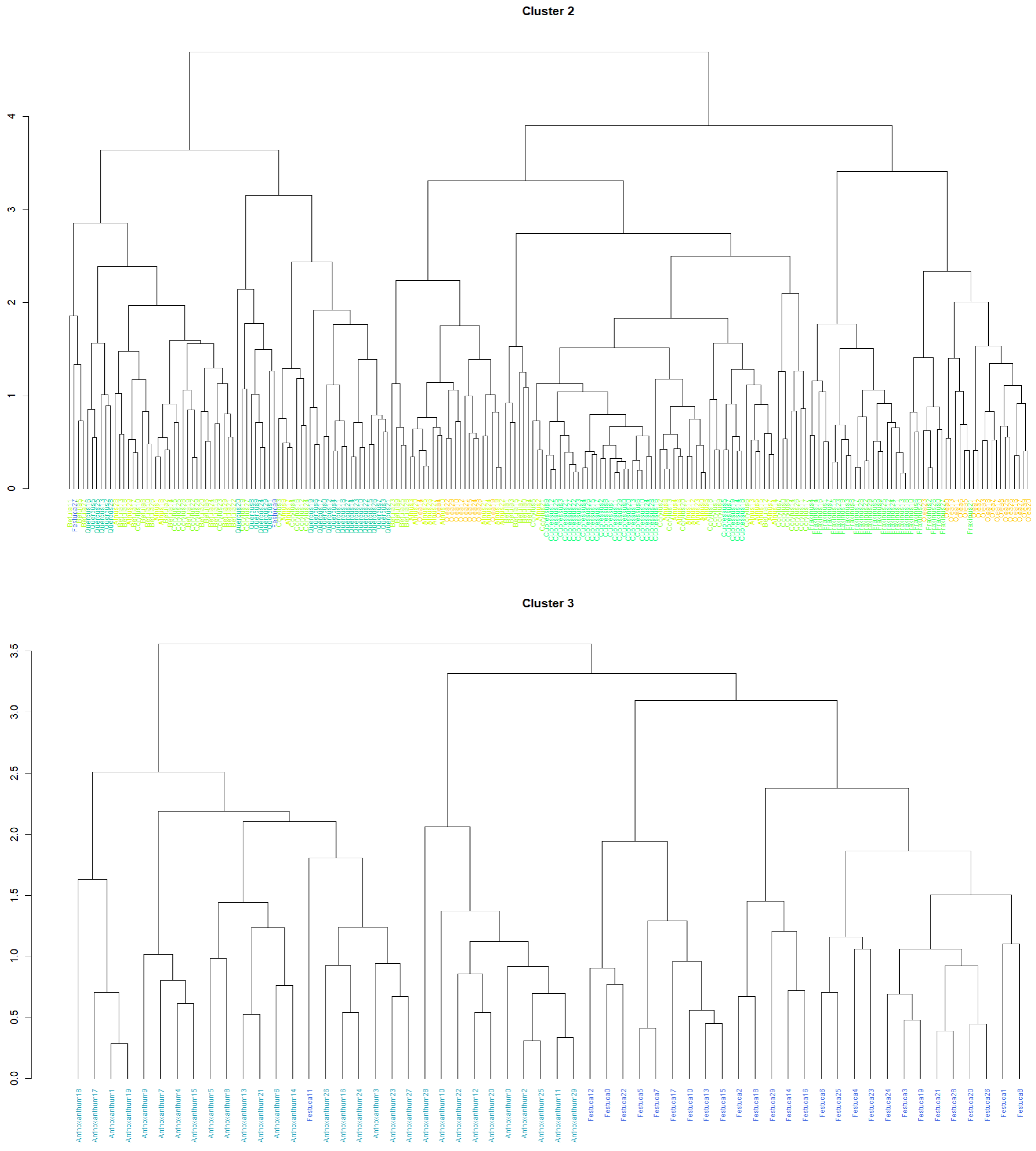
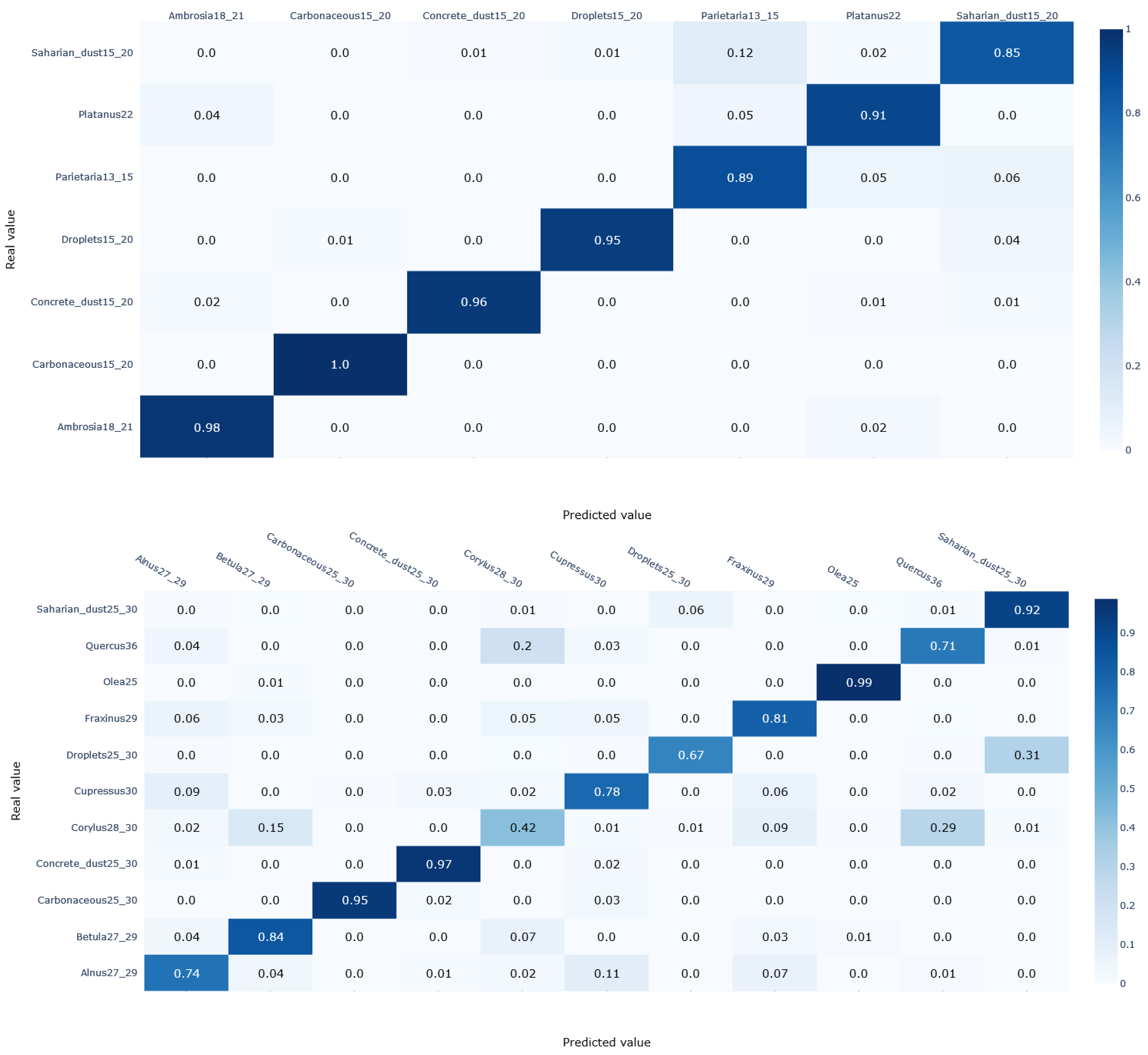
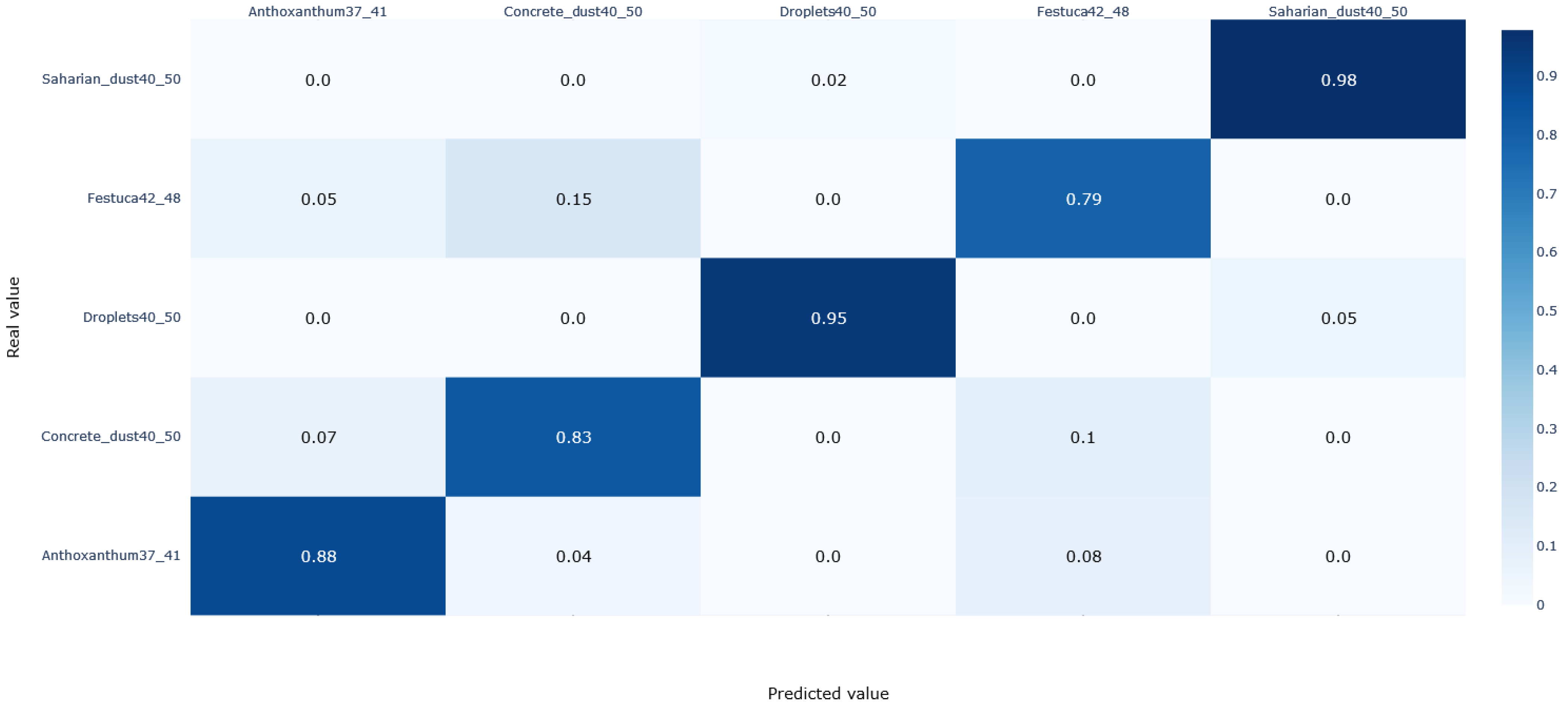
3.2. Pollen Classification
4. Discussion
5. Conclusions
- 1-
- A consistent clustering of the pollen species based on their size properties.
- 2-
- An accurate separation between pollen and non-pollen particles.
- 3-
- A correct recognition of the pollen species with 9 out of 12 of the species covered in our study having a prediction score above 78%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina, R.T.; Rodríguez, A.M.; Palaciso, I.S.; López, F.G. Pollen production in anemophilous trees. Grana 1996, 35, 38–46. [Google Scholar] [CrossRef]
- Piotrowska, K. Pollen production in selected species of anemophilous plants. Acta Agrobot. 2012, 61, 41–52. [Google Scholar] [CrossRef]
- Taylor, P.E.; Flagan, R.C.; Valenta, R.; Glovsky, M. Release of allergens as respirable aerosols: A link between grass pollen and asthma. J. Allergy Clin. Immunol. 2002, 109, 51–56. [Google Scholar] [CrossRef]
- De Weger, L.; Bergmann, K.-C.; Rantio-Lehtimäki, A.; Dahl, Å.; Buters, J.; Déchamp, C.; Belmonte, J.; Thibaudon, M.; Cecchi, L.; Besancenot, J.-P.; et al. Impact of pollen. In Allergenic Pollen: A review of the Production, Release, Distribution and Health Impacts; Sofiev, M., Bergman, K.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Gilles, S.; Blume, C.; Wimmer, M.; Damialis, A.; Meulenbroek, L.; Gökkaya, M.; Bergougnan, C.; Eisenbart, S.; Sundell, N.; Lindh, M.; et al. Pollen exposure weakens innate defense against respiratory viruses. Allergy 2019, 75, 576–587. [Google Scholar] [CrossRef]
- Traidl-Hoffmann, C.; Kasche, A.; Menzel, A.; Jakob, T.; Thiel, M.; Ring, J.; Behrendt, H. Impact of Pollen on Human Health: More Than Allergen Carriers? Int. Arch. Allergy Immunol. 2003, 131, 1–13. [Google Scholar] [CrossRef]
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F.; Blaiss, M. The WAO White Book on Allergy; World Allergy Organization: Milwaukee, WI, USA, 2013.
- Meltzer, E.O.; Gross, G.N.; Katial, R.; Storms, W.W. Allergic rhinitis substantially impacts patient quality of life: Findings from the Nasal Allergy Survey Assessing Limitations. J. Fam. Pract. 2012, 61, S5–S10. [Google Scholar]
- Šaulienė, I.; Šukienė, L.; Kainov, D.; Greičiuvienė, J. The impact of pollen load on quality of life: A questionnaire-based study in Lithuania. Aerobiologia 2015, 32, 157–170. [Google Scholar] [CrossRef]
- Malone, D.C.; Lawson, K.A.; Smith, D.H.; Arrighi, H.; Battista, C. A cost of illness study of allergic rhinitis in the United States. J. Allergy Clin. Immunol. 1997, 99, 22–27. [Google Scholar] [CrossRef]
- Crystal-Peters, J.; Crown, W.H.; Goetzel, R.Z.; Schutt, D.C. The cost of productivity losses associated with allergic rhinitis. Am. J. Manag. Care 2000, 6, 373–378. [Google Scholar]
- Blaiss, M.S. Allergic rhinoconjunctivitis: Burden of disease. Allergy Asthma Proc. 2007, 28, 393–397. [Google Scholar] [CrossRef]
- Zuberbier, T.; Lötvall, J.; Simoens, S.; Subramanian, S.V.; Church, M.K. Economic burden of inadequate management of allergic diseases in the European Union: A GA2LEN review. Allergy 2014, 69, 1275–1279. [Google Scholar] [CrossRef]
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solomon, F.; Storkey, J.; Vautard, R.; et al. Climate Change and Future Pollen Allergy in Europe. Environ. Health Perspect. 2017, 125, 385–391. [Google Scholar] [CrossRef]
- Sedghy, F.; Varasteh, A.R.; Sankian, M.; Moghadam, M. Interaction between Air Pollutants and Pollen Grains: The Role on the Rising Trend in Allergy. Rep. Biochem. Mol. Biol. 2018, 6, 219–224. [Google Scholar]
- Stas, M.; Aerts, R.; Hendrickx, M.; Delcloo, A.; Dendoncker, N.; Dujardin, S.; Linard, C.; Nawrot, T.; Van Nieuwenhuyse, A.; Aerts, J.-M.; et al. Exposure to green space and pollen allergy symptom severity: A case-crossover study in Belgium. Sci. Total. Environ. 2021, 781, 146682. [Google Scholar] [CrossRef]
- Beggs, P.J. Environmental Allergens: From Asthma to Hay Fever and Beyond. Curr. Clim. Chang. Rep. 2015, 1, 176–184. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; De Martino, A.; Viegi, G.; Lanza, M.; Molino, A.; Sanduzzi, A.; Vatrella, A.; Annesi-Maesano, I.; D’Amato, M. Effects on asthma and respiratory allergy of Climate change and air pollution. Multidiscip. Respir. Med. 2015, 10, 39. [Google Scholar] [CrossRef]
- Wayne, P.; Foster, S.; Connolly, J.; Bazzaz, F.; Epstein, P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann. Allergy Asthma Immunol. 2002, 88, 279–282. [Google Scholar] [CrossRef]
- Ziska, L.H.; Makra, L.; Harry, S.K.; Bruffaerts, N.; Hendrickx, M.; Coates, F.; Saarto, A.; Thibaudon, M.; Oliver, G.; Damialis, A.; et al. Temperature-related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: A retrospective data analysis. Lancet Planet. Health 2019, 3, e124–e131. [Google Scholar] [CrossRef]
- Bruffaerts, N.; De Smedt, T.; Delcloo, A.; Simons, K.; Hoebeke, L.; Verstraeten, C.; Van Nieuwenhuyse, A.; Packeu, A.; Hendrickx, M. Comparative long-term trend analysis of daily weather conditions with daily pollen concentrations in Brussels, Belgium. Int. J. Biometeorol. 2017, 62, 483–491. [Google Scholar] [CrossRef]
- Hirst, J.M. An Automatic Volumetric Spore Trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Grinnell, S.W.; Perkins, W.A.; Vaughan, L.M. Sampling Apparatus And Method. U.S. Patent 2,973,642, 7 March 1961. [Google Scholar]
- Magill, P.L.; Lumpkins, E.D.; Arveson, J.S. A System for Appraising Airborne Populations of Pollens and Spores. Am. Ind. Hyg. Assoc. J. 1968, 29, 293–298. [Google Scholar] [CrossRef]
- Buters, J.T.M.; Antunes, C.; Galveias, A.; Bergmann, K.C.; Thibaudon, M.; Galán, C.; Schmidt-Weber, C.; Oteros, J. Pollen and spore monitoring in the world. Clin. Transl. Allergy 2018, 8, 9. [Google Scholar] [CrossRef]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.E.; Clot, B.; Brandao, R. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar] [CrossRef]
- Adamov, S.; Lemonis, N.; Clot, B.; Crouzy, B.; Gehrig, R.; Graber, M.-J.; Sallin, C.; Tummon, F. On the measurement uncertainty of Hirst-type volumetric pollen and spore samplers. Aerobiologia 2021, 1–15. [Google Scholar] [CrossRef]
- Werchan, B.; Werchan, M.; Mücke, H.-G.; Gauger, U.; Simoleit, A.; Zuberbier, T.; Bergmann, K.-C. Spatial distribution of allergenic pollen through a large metropolitan area. Environ. Monit. Assess. 2017, 189, 169. [Google Scholar] [CrossRef]
- Katz, D.S.W.; Batterman, S.A. Urban-scale variation in pollen concentrations: A single station is insufficient to characterize daily exposure. Aerobiologia 2020, 36, 417–431. [Google Scholar] [CrossRef]
- Zapata-Marin, S.; Schmidt, A.M.; Weichenthal, S.; Katz, D.S.; Takaro, T.; Brook, J.; Lavigne, E. Within city spatiotemporal variation of pollen concentration in the city of Toronto, Canada. Environ. Res. 2021, 206, 112566. [Google Scholar] [CrossRef]
- Oteros, J.; Pusch, G.; Weichenmeier, I.; Heimann, U.; Möller, R.; Röseler, S.; Traidl-Hoffmann, C.; Schmidt-Weber, C.; Buters, J.T. Automatic and Online Pollen Monitoring. Int. Arch. Allergy Immunol. 2015, 167, 158–166. [Google Scholar] [CrossRef]
- Oteros, J.; Weber, A.; Kutzora, S.; Rojo, J.; Heinze, S.; Herr, C.; Gebauer, R.; Schmidt-Weber, C.B.; Buters, J.T. An operational robotic pollen monitoring network based on automatic image recognition. Environ. Res. 2020, 191, 110031. [Google Scholar] [CrossRef]
- Healy, D.A.; O’Connor, D.J.; Burke, A.M.; Sodeau, J.R. A laboratory assessment of the Waveband Integrated Bioaerosol Sensor (WIBS-4) using individual samples of pollen and fungal spore material. Atmos. Environ. 2012, 60, 534–543. [Google Scholar] [CrossRef]
- Sauvageat, E.; Zeder, Y.; Auderset, K.; Calpini, B.; Clot, B.; Crouzy, B.; Konzelmann, T.; Lieberherr, G.; Tummon, F.; Vasilatou, K. Real-time pollen monitoring using digital holography. Atmos. Meas. Tech. 2020, 13, 1539–1550. [Google Scholar] [CrossRef]
- Šaulienė, I.; Šukienė, L.; Daunys, G.; Valiulis, G.; Vaitkevičius, L.; Matavulj, P.; Brdar, S.; Panic, M.; Sikoparija, B.; Clot, B.; et al. Automatic pollen recognition with the Rapid-E particle counter: The first-level procedure, experience and next steps. Atmos. Meas. Tech. 2019, 12, 3435–3452. [Google Scholar] [CrossRef]
- Kawashima, S.; Clot, B.; Fujita, T.; Takahashi, Y.; Nakamura, K. An algorithm and a device for counting airborne pollen automatically using laser optics. Atmospheric Environ. 2007, 41, 7987–7993. [Google Scholar] [CrossRef]
- Renard, J.-B.; El Azari, H.; Richard, J.; Lauthier, J.; Surcin, J. Towards an Automatic Pollen Detection System in Ambient Air Using Scattering Functions in the Visible Domain. Sensors 2022, 22, 4984. [Google Scholar] [CrossRef]
- Lurton, T.; Renard, J.-B.; Vignelles, D.; Jeannot, M.; Akiki, R.; Mineau, J.-L.; Tonnelier, T. Light scattering at small angles by atmospheric irregular particles: Modelling and laboratory measurements. Atmos. Meas. Tech. 2014, 7, 931–939. [Google Scholar] [CrossRef]
- Renard, J.-B.; Dulac, F.; Berthet, G.; Lurton, T.; Vignelles, D.; Jégou, F.; Tonnelier, T.; Jeannot, M.; Couté, B.; Akiki, R.; et al. LOAC: A small aerosol optical counter/sizer for ground-based and balloon measurements of the size distribution and nature of atmospheric particles–Part 1: Principle of measurements and instrument evaluation. Atmos. Meas. Tech. 2016, 9, 1721–1742. [Google Scholar] [CrossRef]
- Breunig, M.M.; Kriegel, H.P.; Ng, R.T.; Sander, J. LOF: Identifying Density-Based Local Outliers. In Proceedings of the 2000 ACM SIGMOD International Conference on Management of Data, Dallas, TX, USA, 15–18 May 2000. [Google Scholar]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Ling, C.X.; Li, C. Data Mining for Direct Marketing: Problems and Solutions. In Proceedings of the 4th International Conference on Knowledge Discovery and Data Mining (KDD’98), New York, NY, USA, 27–31 August 1998; pp. 73–79. [Google Scholar]
- Renard, J.-B.; Geffrin, J.-M.; Valencia, V.T.; Tortel, H.; Ménard, F.; Rannou, P.; Milli, J.; Berthet, G. Number of independent measurements required to obtain reliable mean scattering properties of irregular particles having a small size parameter, using microwave analogy measurements. J. Quant. Spectrosc. Radiat. Transf. 2021, 272, 107718. [Google Scholar] [CrossRef]
- Hopkins, B.; Skellam, J.G. A New Method for determining the Type of Distribution of Plant Individuals. Ann. Bot. 1954, 18, 213–227. [Google Scholar] [CrossRef]
- Wright, K. hopkins: Hopkins Statistic for Clustering. 2022. Available online: https://kwstat.github.io/hopkins (accessed on 20 October 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: https://www.R-project.org/ (accessed on 6 October 2022).
- Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses (R Package Version 1.0.6). 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 26 October 2022).
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning (Information Science and Statistics); Springer: Berlin/Heidelberg, Germany, 2007; p. 183. [Google Scholar]
- Society for the Promotion of Palynological Research in Austria (AutPal). Available online: https;//www.paldat.org/ (accessed on 15 December 2022).



| Common Name | Latin Name | Theoretical Diameter | Number of Samples | Total Number of Grains |
|---|---|---|---|---|
| Alder | Alnus glutinosa | 27 to 29 µm | 216 | 2593 |
| Sweet vernal grass | Anthoxanthum odoratum | 37 to 41 µm | 171 | 6988 |
| Ragweed | Ambrosiaartemisiifolia | 18 to 21 µm | 179 | 1350 |
| Birch | Betula pendula | 27 to 29 µm | 209 | 1789 |
| Hazel | Corylus avellana | 28 to 30 µm | 242 | 1403 |
| Cypress | Cupressus sempervirens | 30 µm | 189 | 3896 |
| Fescue | Festuca pratensis | 42 to 48 µm | 195 | 5196 |
| Ash | Fraxinus excelsior | 29 µm | 198 | 2462 |
| Olive tree | Olea euopaea | 25 µm | 231 | 1244 |
| Wall pellitory | Parietaria officinalis | 13 to 15 µm | 299 | 5150 |
| Plane tree | Platanus acerifolia | 22 µm | 201 | 634 |
| Common oak | Quercus robur | 36 µm | 203 | 3557 |
| Pollen Species | Silhouette Score |
|---|---|
| Alnus glutinosa | 0.6 |
| Anthoxanthum odoratum | 0.41 |
| Ambrosiaartemisiifolia | 0.63 |
| Betula pendula | 0.54 |
| Corylus avellana | 0.59 |
| Cupressus sempervirens | 0.54 |
| Festuca pratensis | 0.65 |
| Fraxinus excelsior | 0.28 |
| Olea euopaea | 0.6 |
| Parietaria officinalis | 0.59 |
| Platanus acerifolia | 0.5 |
| Quercus robur | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Azari, H.; Renard, J.-B.; Lauthier, J.; Dudok de Wit, T. A Laboratory Evaluation of the New Automated Pollen Sensor Beenose: Pollen Discrimination Using Machine Learning Techniques. Sensors 2023, 23, 2964. https://doi.org/10.3390/s23062964
El Azari H, Renard J-B, Lauthier J, Dudok de Wit T. A Laboratory Evaluation of the New Automated Pollen Sensor Beenose: Pollen Discrimination Using Machine Learning Techniques. Sensors. 2023; 23(6):2964. https://doi.org/10.3390/s23062964
Chicago/Turabian StyleEl Azari, Houssam, Jean-Baptiste Renard, Johann Lauthier, and Thierry Dudok de Wit. 2023. "A Laboratory Evaluation of the New Automated Pollen Sensor Beenose: Pollen Discrimination Using Machine Learning Techniques" Sensors 23, no. 6: 2964. https://doi.org/10.3390/s23062964
APA StyleEl Azari, H., Renard, J.-B., Lauthier, J., & Dudok de Wit, T. (2023). A Laboratory Evaluation of the New Automated Pollen Sensor Beenose: Pollen Discrimination Using Machine Learning Techniques. Sensors, 23(6), 2964. https://doi.org/10.3390/s23062964







