1. Introduction
Cryosurgery is a medical procedure that involves the use of extreme cold to destroy abnormal or diseased tissue. It is also known as cryotherapy. The procedure is typically performed using liquid nitrogen or argon gas to freeze and destroy the targeted tissue. Cryosurgery is used to treat a variety of conditions, including skin lesions, prostate cancer, and cervical dysplasia. It is a minimally invasive procedure and recovery time is generally quick [
1].
The physiological mechanisms of cryosurgery involve the freezing and thawing of cells, the constriction of blood vessels, and the oxygen and nutrient deprivation of cells, all of which lead to the destruction of the targeted tissue and the stimulation of the healing process. The destruction of the tissue is a gradual process, as the freezing and thawing cycles cause ice crystals to form and grow within the cells, rupturing the cell membrane [
2]. Additionally, the cells in the treated area become oxygen and nutrient deprived, with further damage to the tissue promoting cell death [
3]. The freezing process also causes an inflammatory response, which contributes to cell death and the healing process [
4].
The crucial parameter, freezing depth—and, therefore, the amount of tissue affected by the cryotherapy—is determined by the duration of freezing and the size of the probe used, without having an objective estimation of the expansion and depth of the “ice ball”. Determining the depth of freezing is necessary to effectively treat the condition without causing excessive damage to surrounding healthy tissue. The depth of freezing can be influenced by a number of factors, including the size and location of the lesion, the type of tissue being treated, and the type of equipment being used. If the freezing is not deep enough, the abnormal cells may not be fully destroyed, and the condition may not be effectively treated. If the freezing is too deep, it can cause significant damage to healthy tissue and potentially result in complications, such as nerve damage, tissue necrosis, or scarring. The depth of freezing is typically monitored by imaging techniques such as ultrasound and MRI [
5,
6,
7]. Other methods include direct temperature measurements utilizing infrared thermography [
8]. Finally, imaging techniques such as optical coherent tomography (OCT) [
9], vibro-acoustography [
10], and electrical impedance tomography [
11] have also been reported.
In this study, we demonstrate a technique based on fiber optic array geometry that exploits the differential optical diffusion and spectral properties of frozen/unfrozen ex vivo porcine tissues, in order to monitor the depth of the frozen region in real time. The fiber optic array sensor was subsequently used for in vivo spectra measurements during the thawing process.
2. Principle of the Measurement Technique
When water-rich living tissues are frozen quickly with liquid nitrogen, their color changes. They turn pale white for a short time after the cryogen is no longer being used. The production of ice crystals and microbubbles contributes to this phenomenon, along with a temporary constriction of arteries and the subsequent lowering of local blood content. Mie or Rayleigh scattering changes the optical characteristics of the frozen tissues, which are dependent on the size of these microstructures [
12]. The differences in the optical properties of frozen and unfrozen tissues can be used to determine the depth of freezing. This is achieved by monitoring the change in intensity of the backscattered light, which is associated with an increase in the number of scatterers in the frozen tissue.
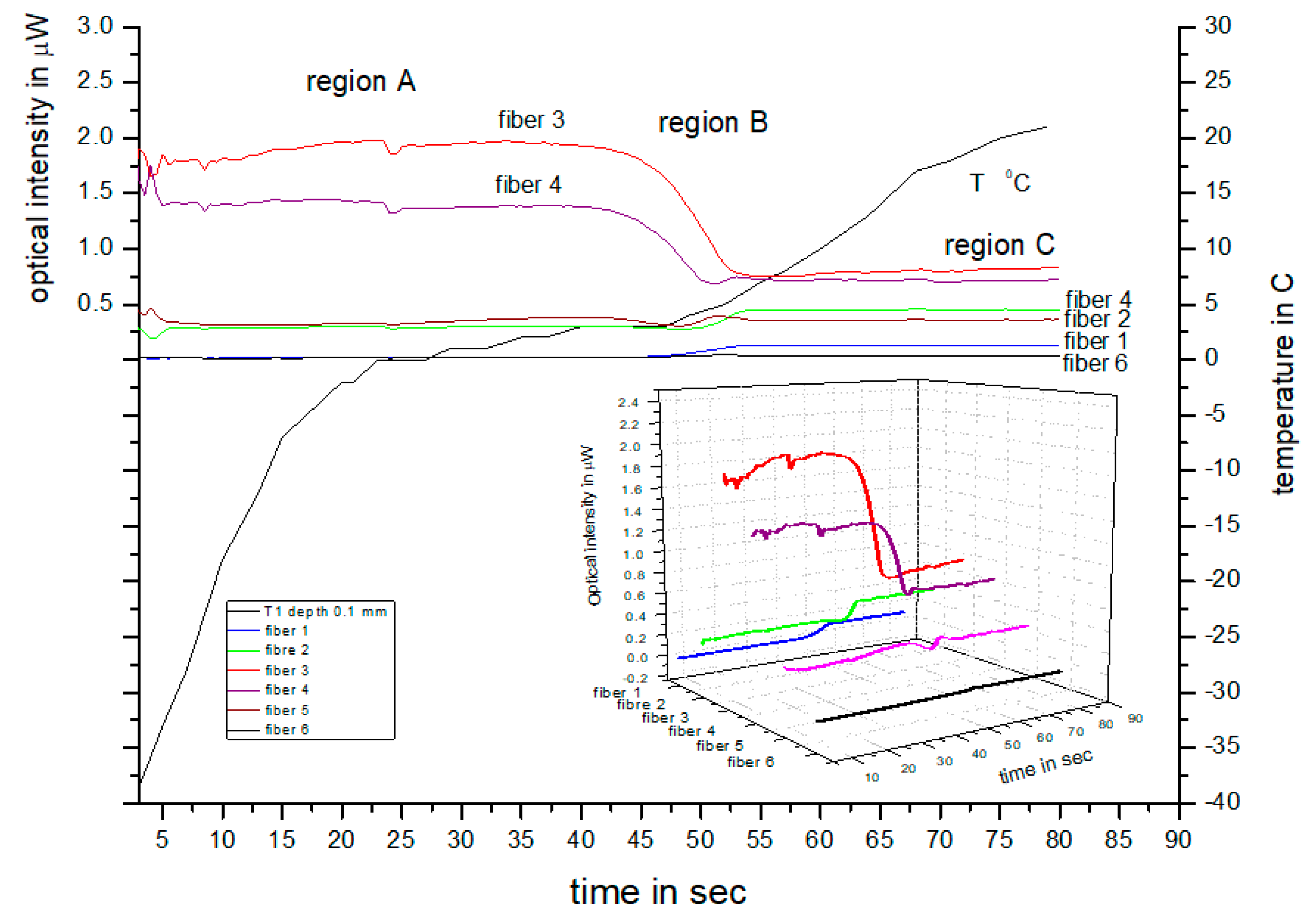
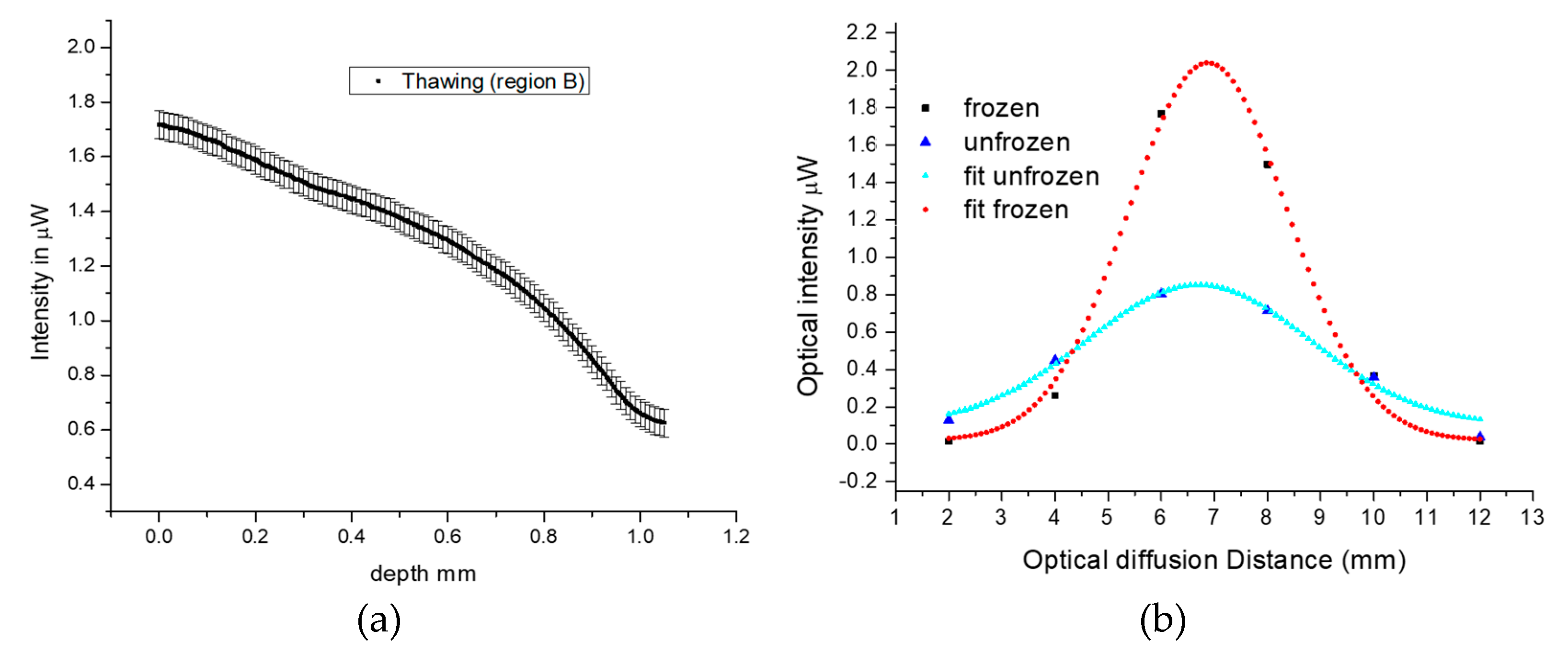
In a previous study [
13], we demonstrated a method that exploits variations in the backscattering monochromatic intensity of frozen and unfrozen ex vivo tissue to obtain information on the depth of freezing during cryotherapy using a fiber optic sensor. This method involves measuring both the transmitted and backscattered light from frozen and unfrozen tissue simultaneously, in order to determine the maximum transmission depth and calibrate it with the backscattered light from the two tissue states. We measured and calibrated the transmitted and backscattered intensities of frozen and unfrozen tissues at different skin depths. We used an independent method involving ultrasound to validate the depth of freezing measured from the backscattered intensity detected by the fiber array during thawing.
In the experiments reported here, we used a similar experimental arrangement to the one presented in our previous work. This comprised a modified multiple fiber array sensor that illuminated and detected the backscattered light from frozen and unfrozen ex vivo porcine tissue, as well as in vivo human skin. We used a similar calibration procedure, involving a monochromatic laser light to illuminate the ex vivo tissue at different depths, in order to calibrate the backscattered and transmitted light during thawing. Following calibration, we used the fiber array sensor to measure the spectra of both the ex vivo and in vivo tissues during thawing. This is described in more detail in the following section.
4. Spectral Study and Comparison of Porcine and Human Spectra
It is possible to assess the depth of freezing during thawing of porcine ex vivo tissue based on our previous results. Due to the difficulty in calibrating the procedure with transmitted light, this method is not suitable for in vivo measurements. However, as described previously, during in vivo cryotherapy, there is a temporary change in the tissue’s color due to the formation of ice crystals and a reduction in blood flow, causing the skin to become temporarily pale and white. To quantify the spectral characteristics during this transition, in vivo skin measurements were performed during cryotherapy in the dermatology clinic. Our aim was to compare the spectral characteristics of thawing porcine ex vivo tissue with those of living human tissue in order to determine, semi-empirically, the frozen depth.
The experimental setup is shown in
Figure 4. To illuminate the tissues, a white light source (WLS) with the peak wavelength centered on 650 nm, consisting of a fiber-pigtailed light-emitting diode (LED) coupling approximately 0.5–0.8 mW of light to the tissue, was used. This peak wavelength was chosen to avoid interference with the intensity variations from oxy-hemoglobin for the in vivo experiments and also so that the results could be compared with the ex vivo results described earlier. The emission bandwidth of the WLS ranged from 350 nm to 900 nm. The backscattered spectrum from the tissues was coupled to the spectrometer by one of the inner fibers 3 or 4, located adjacent to the source fiber, and the data were stored in a computer.
The experimental procedure for measuring porcine ex vivo and in vivo human skin tissue (finger) involved preparing the sensor by lightly wiping the window with IPA to eliminate frozen dew, applying cryogen to the tissues, and placing the sensor in contact with them. Because in vivo human tissue thaws rapidly, measurements had to be obtained immediately after freezing. To simplify data acquisition, the sensor was mounted on a precision sliding stage. The ex vivo porcine tissue was held vertically on a stationary frame, as described in
Section 3.1, without using a hot plate. In vivo cryosurgery was performed on warts growing on fingers by applying cryogen and subsequently positioning the sensor on the finger. Spectral measurements were taken with an Avantes (Avaspec 3648), collecting spectral data every 5–10 s during thawing. To avoid degradation of the ex vivo tissue, several freeze–thaw cycles were performed at different locations on the porcine tissue surface, and the spectral variations with time are shown in
Figure 5a. Similarly, for the in vivo experiments on human fingers, the results were accumulated over several treatments using the aforementioned protocol, and typical results are shown in
Figure 5b. It should be noted, however, that the spectral spike appearing on all spectra at around 425 nm is an artifact of the LED and is of no biological significance. Furthermore, the WLS was chosen due to low emissions above 800 nm, which is the absorption band of oxy-hemoglobin and could contribute to unwanted spectral variations related to heartbeats [
14].
A comparison of the spectral responses between the two cases shows that they are similar, with the backscattering peak appearing at approximately 550–650 nm and overall spectral intensities higher during freezing and lower during thawing. However, the rate at which the overall spectral intensity decreased during thawing was much slower in the ex vivo porcine tissue compared to the in vivo human tissue. This difference was expected since the porcine tissue lacked a blood supply and thawing was mainly influenced by the ambient air temperature, whereas in living tissue, blood flow contributes to temperature equilibration. Additionally, the changes in peak intensity at 650 nm exhibit a similar temporal response to the porcine cases in
Figure 2, with a transition region and two fixed states observed in both cases—one when the tissue is frozen and the other when it is thawed.
The spectra of frozen and unfrozen tissues were plotted in
Figure 6 to compare the ex vivo and in vivo spectral responses. The frozen spectra are shown in black, while the unfrozen ones are shown in red. At first glance, these spectra appear to be similar. However, when the spectra are normalized (min.–max. normalization) and plotted together, as shown in
Figure 6c,d, with the frozen spectra in black and the unfrozen ones in red, the porcine ex vivo tissue shows the same variations. In contrast, the spectrum for the live human frozen/unfrozen tissue in
Figure 6d exhibits spectral variations around 500 nm and 625 nm, which are attributed to the hemoglobin absorption peak [
15].
Based on the above results, it would be interesting to compare the frozen and unfrozen states of the two specimens to see if the calibration method from the study with porcine tissue can be used to measure the frozen depth in live human tissue. This is especially crucial as in vivo depth measurements of frozen-unfrozen tissues are not routinely feasible. To investigate the two specimens, the normalized spectra of their frozen-unfrozen states were compared, and the results are presented in
Figure 7.
Figure 7a shows the frozen porcine ex vivo and human in vivo spectra, which are very similar, indicating that backscattering from the ice in the tissues dominates. In
Figure 7b, the normalized unfrozen spectra of the two specimens are shown, which are generally similar, with some spectral variations around 500–600 nm, associated with the absorption band of hemoglobin [
14].
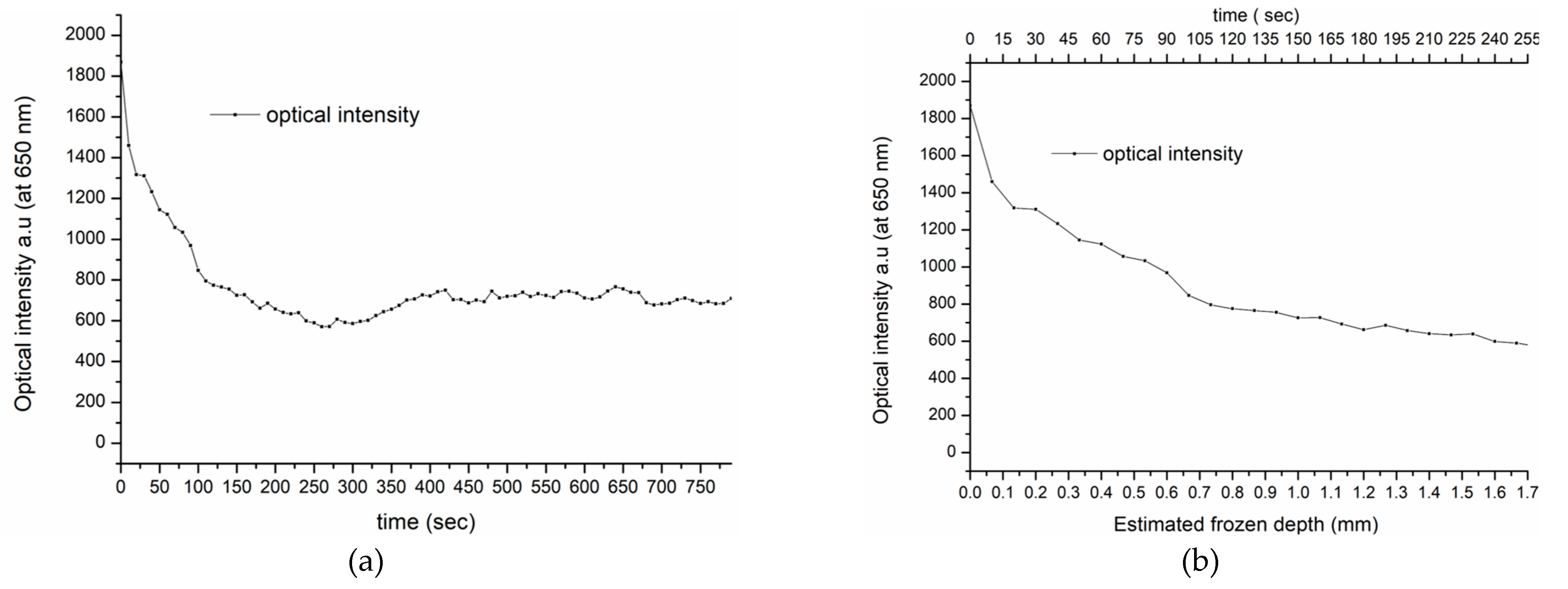
Based on the spectral similarities of the frozen porcine and human tissues, we suggest that the depth of freezing in living human skin is similar to that in the porcine tissue and can be estimated at approximately 1.5 mm. If this assumption is correct, the thawing timeline for in vivo human tissue, taken at 650 nm from the spectra shown in
Figure 5b, can be directly compared with the equivalent porcine specimen, shown in
Figure 3a. Furthermore, at this wavelength, there is minimal interference from the intensity variations due to oxy-hemoglobin, associated with the heartbeat and the backscattering intensity at 650 nm, as shown in
Figure 8a. Therefore, the transition from high to low intensity during thawing is assumed, as was seen in the porcine specimen, to correspond to the maximum frozen depth of 1.5 mm detected by the fiber sensor. This is shown in
Figure 8b, which displays in more detail the intensity transition during thawing.
Consequently,
Figure 8b indicates a similar process to that which determined the frozen depth measurement in the porcine tissue, as shown in
Figure 3a. This finding provides an initial insight into the in vivo thawing process and its potential use in real-life cryotherapy applications.