Gas Sensing Properties of SnO2-Pd Nanoparticles Thick Film by Applying In Situ Synthesis-Loading Method
Abstract
1. Introduction
2. Materials and Methods (Figure 1)
2.1. Preparation and Evaluation of SnO2-Pd NPs
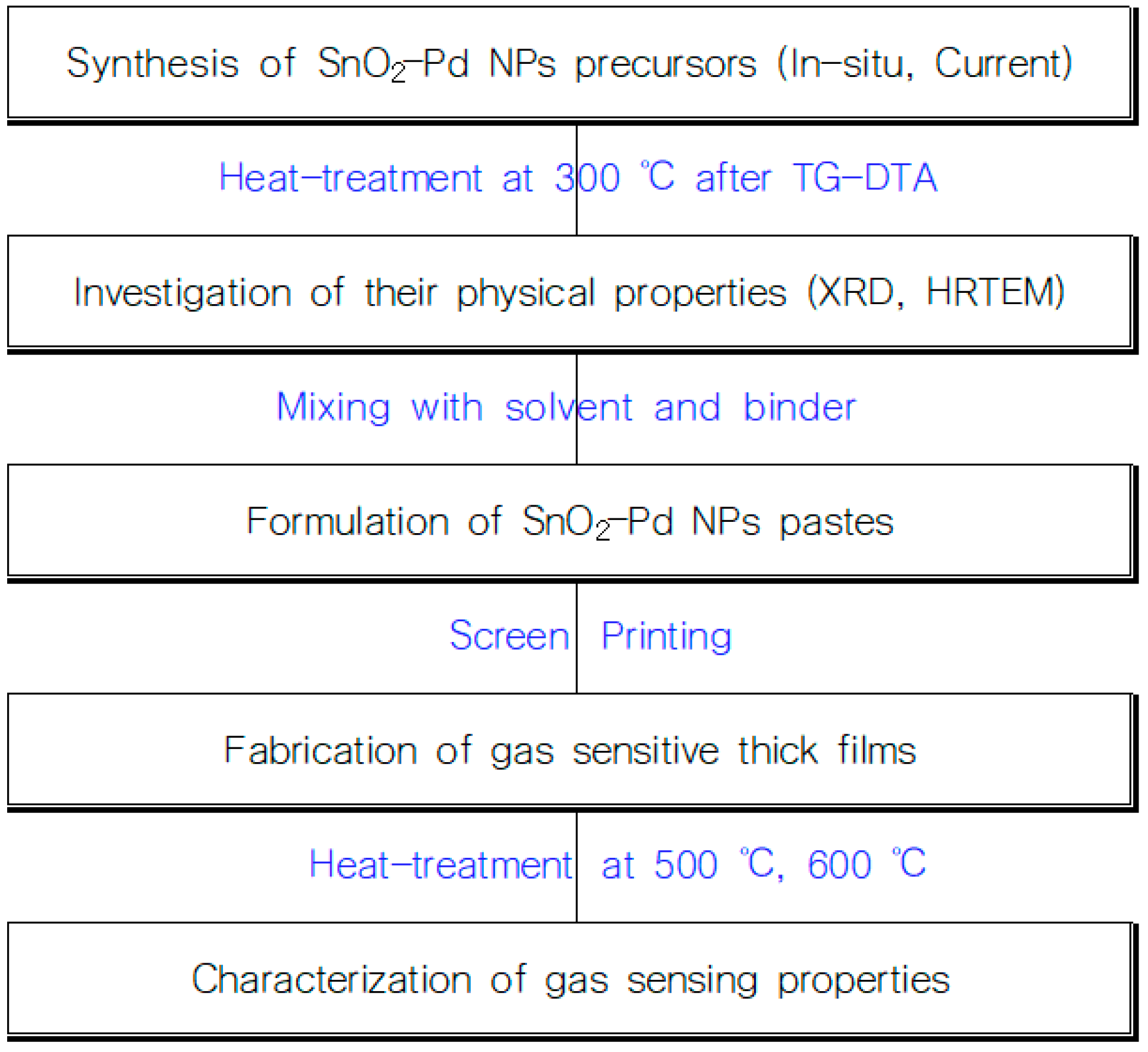
2.2. Preparation and Evaluation of SnO2-Pd NPs Gas Sensitive Thick Films
3. Results
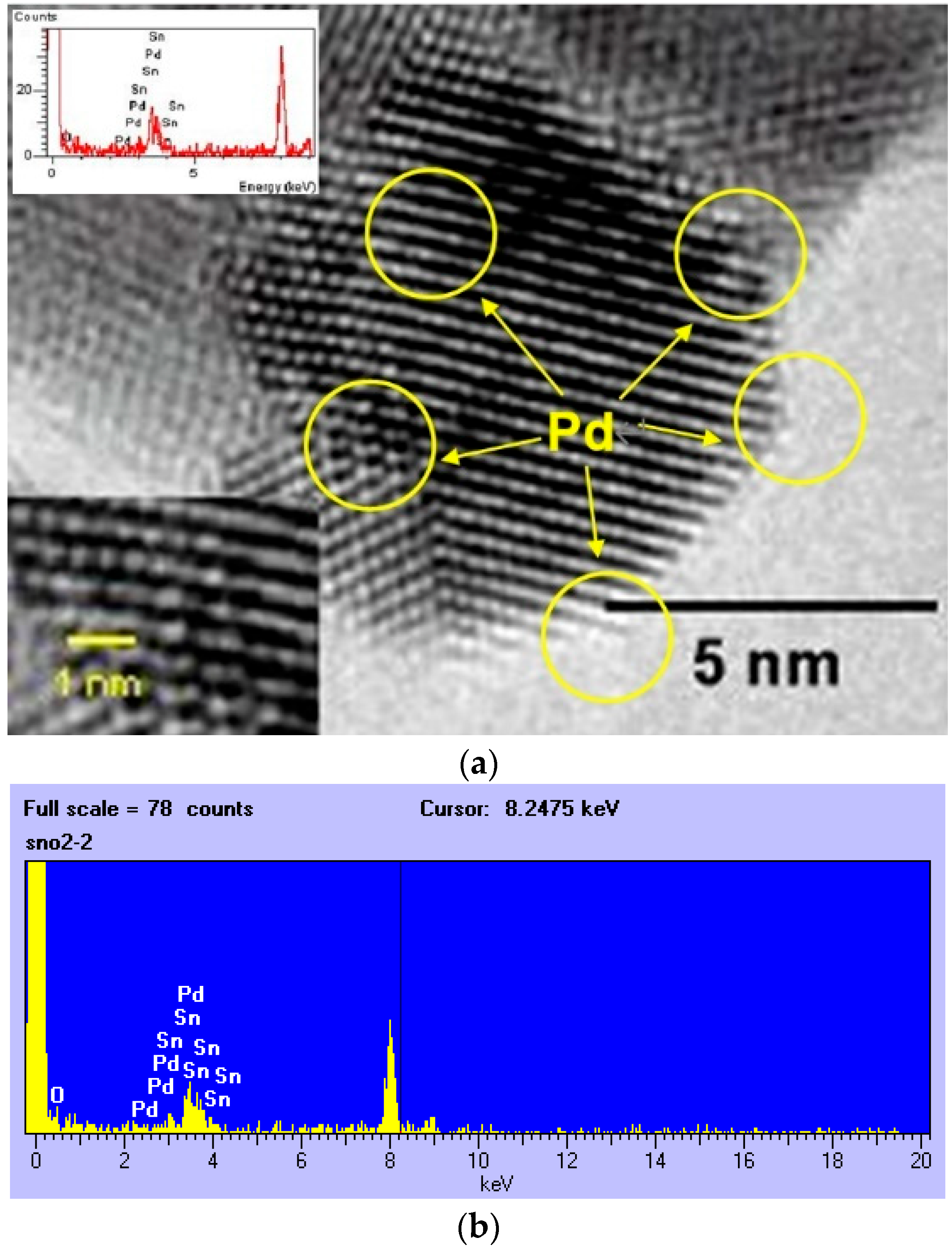
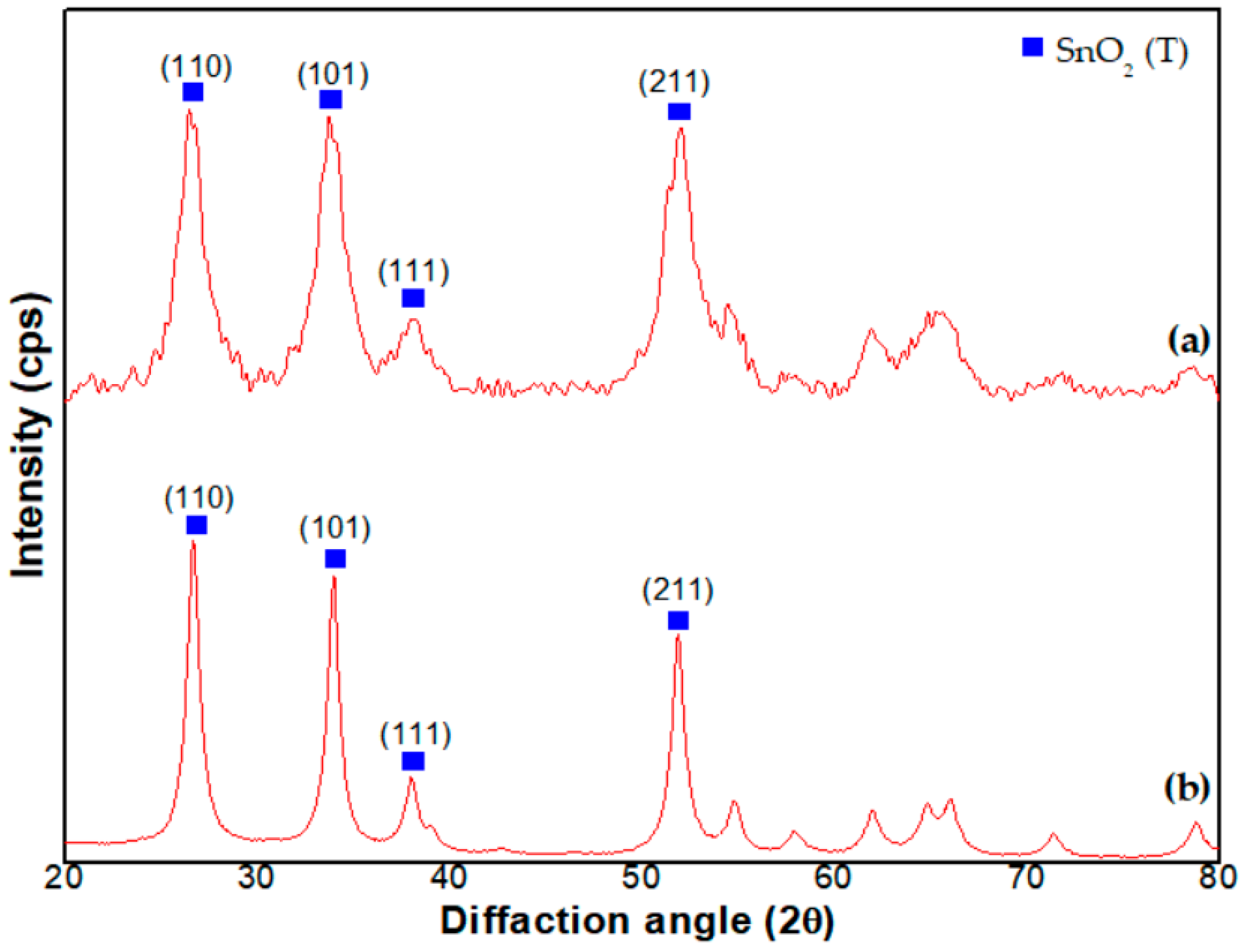
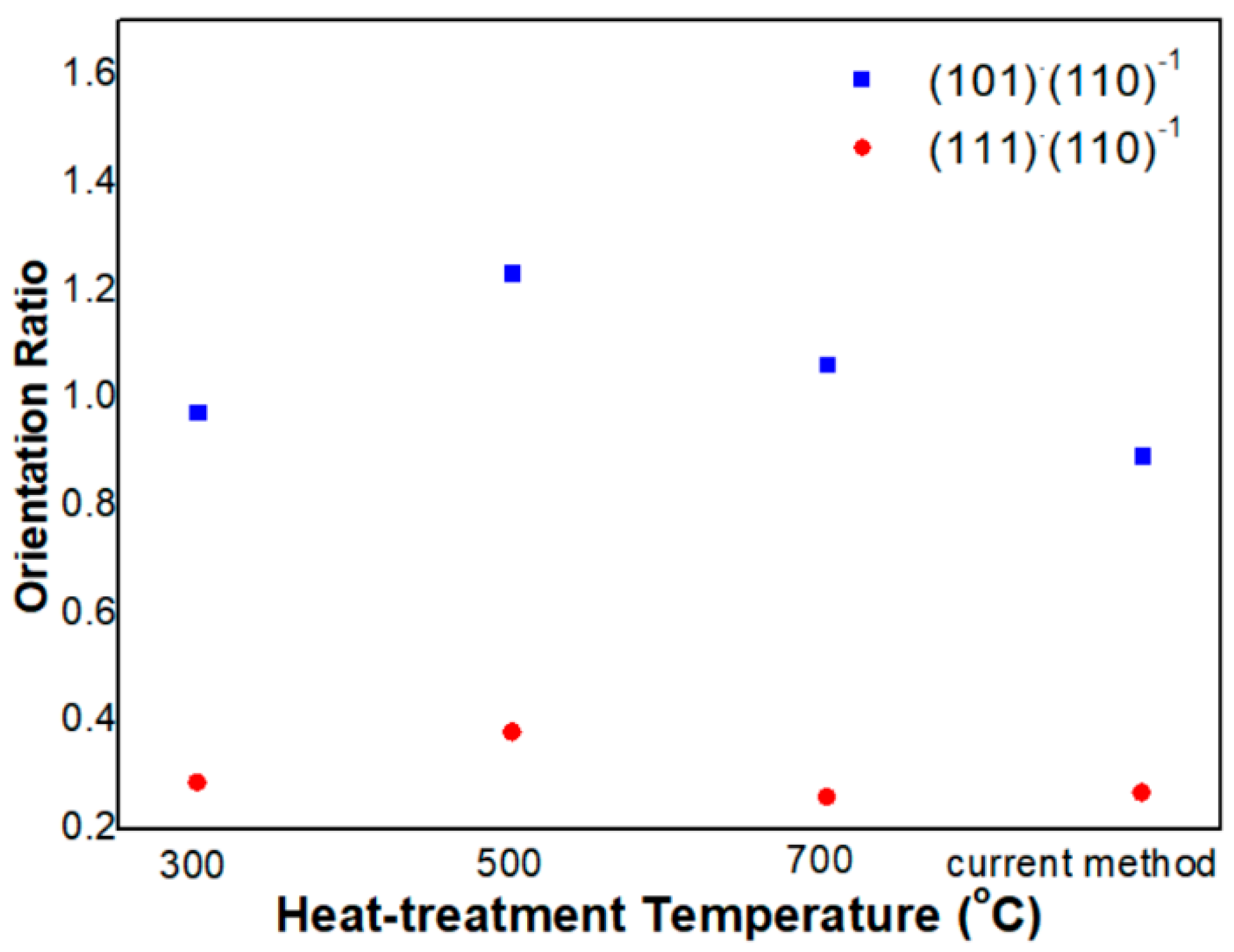
3.1. Physical Properties of SnO2-Pd NPs
3.2. Gas Sensing Properties of SnO2-Pd NPs Gas Sensitive Thick Films
4. Discussion
4.1. Physical Properties of SnO2-Pd NPs
4.2. Gas Sensing Properties of SnO2-Pd NPs Gas Sensitive Thick Films
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Zhan, Q.; Lv, R.; Wu, D.; Zhang, S. Enhancing Formaldehyde Selectivity of SnO2 Gas Sensors with the ZSM-5 Modified Layers. Sensors 2021, 21, 3947. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, A. Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Review in Terms of Central Performances and Outliers. Sensors 2021, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhang, H.; Shi, Y.; Yu, X.; Lan, G. Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material. Polymers 2021, 13, 1360. [Google Scholar] [CrossRef] [PubMed]
- Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Marikutsa, A.; Krivetskiy, V.; Shatalova, T.; Khmelevsky, N.; Gaskov, A. Nanocomposites SnO2/SiO2 for CO Gas Sensors: Microstructure and Reactivity in the Interaction with the Gas Phase. Materials 2019, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K. SnO2-Based Gas Sensor for Detection of Refrigerant Gases. Proceedings 2019, 14, 32. [Google Scholar]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Suematsu, K.; Ma, N.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors. Sensors 2018, 18, 254. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Q.; Zhang, S.; Zong, P.; Yang, F. Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers. Sensors 2017, 17, 2351. [Google Scholar] [CrossRef]
- Tan, W.; Ruan, X.; Yu, Q.; Yu, Z.; Huang, X. Fabrication of a SnO2-Based Acetone Gas Sensor Enhanced by Molecular Imprinting. Sensors 2015, 15, 352–364. [Google Scholar] [CrossRef]
- Batzill, M. Surface Science Studies of Gas Sensing Materials: SnO2. Sensors 2006, 6, 1345–1366. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, X.; Meng, F.; Liu, J. Study of Influencing Factors of Dynamic Measurements Based on SnO2 Gas Sensor. Sensors 2004, 4, 95–104. [Google Scholar] [CrossRef]
- Morrison, S.R. Selectivity in Semiconductor Gas Sensors. Sens. Actuators 1987, 12, 425–440. [Google Scholar] [CrossRef]
- Nasresfahani, S.; Sheikhi, M.H.; Tohidi, M.; Zarifkar, A. Methane Gas Sensing Properties of Pd-Doped SnO2/Reduced Graphene Oxide Synthesized by a Facile Hydrothermal Route. Mater. Res. Bull. 2017, 89, 161–169. [Google Scholar] [CrossRef]
- Luo, C.; Xu, C.; Lv, L.; Li, H.; Huang, X.; Liu, W. Review of Recent Advances in Inorganic Photoresists. RSC Adv. 2020, 10, 8385–8395. [Google Scholar] [CrossRef]
- Blaser, G.; Ruhl, T.; Diehl, C.; Ulrich, M.; Kohl, D. Nanostructured Semiconductor Gas Sensors to Overcome Sensitivity Limitations Due to Percolation Effects. Phys. A 1999, 266, 218–223. [Google Scholar] [CrossRef]
- Schweizer-Berberich, M.; Zheng, J.G.; Weimar, U.; Gopel, W.; Barsan, N.; Pentia, E.; Tomescu, A. The Effect of Pt and Pd Surface Doping on the Response of Nanocrystalline Tin Dioxide Gas Sensors to CO. Sens. Actuators B Chem. 1996, 31, 71–75. [Google Scholar] [CrossRef]
- Dieguez, A.; Vila, A.; Cabot, A.; Romano-Rodriguez, A.; Morante, J.R.; Kappler, J.; Barsan, N.; Weimar, U.; Gopel, W. Influence on the Gas Sensor Performances of the Metal Chemical States Introduced by Impregnation of Calcinated SnO2 Sol–Gel Nanocrystals. Sens. Actuators B Chem. 2000, 68, 94–99. [Google Scholar] [CrossRef]
- Santos, R.B.D.; Rivelino, R.; Mota, F.D.B.; Kakanakova-Georgieva, A.; Gueorguiev, G.K. Feasibility of novel (H3C)nX(SiH3)3−n compounds(X = B, Al, Ga, In): Structure, stability, reactivity, and Raman characterization from ab initio calculations. Dalton Trans. 2015, 44, 3356–3366. [Google Scholar] [CrossRef]
- Bakoglidis, K.D.; Palisaitis, J.; Santos, R.B.D.; Rivelino, R.; Persson, P.O.Å.; Gueorguiev, G.K.; Hultman, L. Self-Healing in Carbon Nitride Evidenced as Material Inflation and Superlubric Behavior. ACS Appl. Mater. Interfaces 2018, 10, 16238–162430. [Google Scholar] [CrossRef]
- Cirera, A.; Romano-Rodríguez, A.; Morante, J.R.; Weimar, U.; Schweizer-Berberich, M.; Gopel, W. Morphological Analysis of Nanocrystalline SnO2 for Gas Sensor Applications. Sens. Actuators B Chem. 1996, 31, 1–8. [Google Scholar]
- Cirera, A.; Diéguez, A.; Diaz, R.; Cornet, A.; Morante, J.R. New Method to Obtain Stable Small-Sized SnO2 Powders for Gas Sensors. Sens. Actuators B Chem. 1999, 58, 360–364. [Google Scholar] [CrossRef]
- Ibarguen, C.A.; Mosquera, A.; Parra, R.; Castro, M.S.; Rodríguez-Páez, J.E. Synthesis of SnO2 Nanoparticles through the Controlled Precipitation Route. Mater. Chem. Phys. 2007, 101, 433–440. [Google Scholar] [CrossRef]
- Hong, S.-J.; Han, J.-I. Effect of Low Temperature Composite Catalyst Loading (LTC2L) on Sensing Properties of Nano Gas Sensor. Sens. Actuators A Phys. 2004, 112, 80–86. [Google Scholar] [CrossRef]
- Hong, S.-J.; Han, J.-I. Low-Temperature Catalyst Adding for Tin–Oxide Nanostructure Gas Sensors. IEEE Sens. J. 2005, 5, 12–19. [Google Scholar] [CrossRef]
- Fenerty, J.; Humphries, P.G.; Pearce, J. The Reconstructive Decomposition of Tin(II) Formate in Oxidising and Inert Atmospheres. Thermochim. Acta 1983, 61, 319–327. [Google Scholar] [CrossRef]
- Pramanik, N.C.; Das, S.; Biswas, P.K. The Effect of Sn (IV) on Transformation of Co-Precipitated Hydrated In (III) and Sn (IV) Hydroxides to Indium Tin Oxide (ITO) Powder. Mater. Lett. 2002, 56, 671–679. [Google Scholar] [CrossRef]
- De, G.; Licciulli, A.; Massaro, C.; Quirini, A.; Rella, R.; Siciliano, P.; Vasanelli, L. Sol–Gel Derived Pure and Palladium Activated Tin Oxide Films for Gas-Sensing Applications. Sens. Actuators B Chem. 1999, 55, 134–139. [Google Scholar] [CrossRef]
- Maruyama, T.; Morishita, T. Tin Dioxide Thin Films Prepared by Photochemical Vapour Deposition from Tin (II) Acetate. Thin Solid Films 1994, 251, 19–22. [Google Scholar] [CrossRef]
- Cirera, A.; Cornet, A.; Morante, J.R.; Olaizola, S.M.; Castano, E.; Gracia, J. Comparative structural study between sputtered and liquid pyrolysis nanocrystaline SnO2. Mater. Sci. Eng. B 2000, 69–70, 406–410. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.I.; Hong, S.-J. Gas Sensing Properties of SnO2-Pd Nanoparticles Thick Film by Applying In Situ Synthesis-Loading Method. Sensors 2023, 23, 2404. https://doi.org/10.3390/s23052404
Han JI, Hong S-J. Gas Sensing Properties of SnO2-Pd Nanoparticles Thick Film by Applying In Situ Synthesis-Loading Method. Sensors. 2023; 23(5):2404. https://doi.org/10.3390/s23052404
Chicago/Turabian StyleHan, Jeong In, and Sung-Jei Hong. 2023. "Gas Sensing Properties of SnO2-Pd Nanoparticles Thick Film by Applying In Situ Synthesis-Loading Method" Sensors 23, no. 5: 2404. https://doi.org/10.3390/s23052404
APA StyleHan, J. I., & Hong, S.-J. (2023). Gas Sensing Properties of SnO2-Pd Nanoparticles Thick Film by Applying In Situ Synthesis-Loading Method. Sensors, 23(5), 2404. https://doi.org/10.3390/s23052404






