Abstract
This systematic review aims to summarise the evidence from studies that examined morphometric alterations of the deep neck muscles using diagnostic imaging (ultrasound imaging, magnetic resonance imaging, and computed tomography) in patients diagnosed with primary headache disorders (PHD). No previous reviews have focused on documenting morphometric changes in this population. We searched five databases (up to 12 November 2022) to identify the studies. The risk of bias (RoB) was assessed using the Quality in Prognostic Studies (QUIPS) tool and the overall quality of the evidence was assessed using The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. A total of 1246 studies were screened and five were finally included; most were at high RoB, and the overall level of confidence in results was very low. Only two studies showed a significant association between morphometric alterations of the deep neck muscles and PHD (p < 0.001); nevertheless, their RoB was high. Contradictory and mixed results were obtained. The overall evidence did not show a clear association between morphometric alterations of the deep neck muscles in patients diagnosed with PHD. However, due to the limited number of studies and low confidence in the evidence, it is necessary to carry out more studies, with higher methodological quality to better answer our question.
1. Introduction
Headache has been defined as localized pain in the head above the orbitomeatal line and/or the nuchal crest. The main tool used to make the diagnosis of headache disorders is The International Classification of Headache Disorders (ICHD). Its last edition, ICHD-3, classifies primary headaches (PH) as follows: migraine, tension-type headache (TTH), trigeminal autonomic cephalalgias (TACs), and other primary headache disorders (PHD) [1]. PH are disorders by themselves, and they are caused by independent pathomechanisms and not by other disorders [1].
Headaches are one of the most common and disabling dysfunctions of the nervous system. About 95% of the world’s population has experienced some type of headache at least once in their lives [2]. The impact of headaches on the population is very high [2]. In Europe [3], headaches have been found to affect 15% of the population [3]. The prevalence of headaches is higher in women between 20 and 50 years old [3]. According to the last Global Burden of Disease Study, headaches were found to be the third most prevalent pain condition in terms of global prevalence, showing an important socio-economic impact [4]. TTH and migraine are among the main neurological causes that can produce sequelae in patients [5]. Despite the importance of these clinical entities, previous publications have shown that migraine patients are undertreated or inadequately treated [6]. The high prevalence of these headaches generates a high socioeconomic cost. The average annual cost of a migraine patient is EUR 1222 and EUR 303 in the case of TTH [7]. In the European Union, the estimated annual cost of migraine is EUR 173 billion, and the cost of migraine is proportionally higher than that of TTH [7].
Previous research has shown that subjects with PH may have musculoskeletal alterations of the cervical spine that may contribute to the persistence and aggravation of symptoms [8,9]. This could be explained by the close connection between the cervical spine and the craniofacial area through the trigeminocervical nucleus [10], which is a region of exchange of nociceptive information between these two areas [11,12,13]. The connection between the cervical spine and the craniofacial region has been especially studied in physiotherapy because of its clinical implications for evaluation and treatment. Studies have reported the presence of trigger points in the neck muscles in patients with migraine [14,15,16] and TTH [16,17,18,19]. In addition, it has been documented that manual therapy and therapeutic exercise focused on the cervical spine can decrease the frequency and intensity of headaches [20,21,22].
The influence of deep neck structures on the signs and symptoms of headaches has been explored in the literature. Some studies have shown that referred pain from the suboccipital muscles can evoke TTH [19]. The relationship between the deep neck muscles and the myodural bridge has also been studied in migraine patients [23]. In addition, a change in the tension of the rectus capitis posterior minor (RCPmi), transmitted to the dura mater through its junction with the myodural bridge, could result in a change of pressure in the subarachnoid space [24] and, therefore, be associated with the initiation of headache [24,25].
Previous systematic reviews have investigated the relationship between the cervical spine and PH. For example, a systematic review with a meta-analysis published in 2019 focused on musculoskeletal alterations in migraine and TTH. Although this review was published recently, the research question does not focus on morphometric alterations of the deep neck muscles as it only considers physical examination. Ultrasound imaging (US) has been commonly used in many studies to determine deep neck muscles size, but in all cases, it has been used in cervicogenic headache (CEH) [26,27,28]. We also found two protocols registered in the “International Prospective Register of Systematic Reviews”(PROSPERO) [29,30]. However, neither of these protocols [29,30] have been published and they do not exactly answer our research question. None of the previous systematic reviews [9] have focused on morphometric alterations of the deep neck muscles in PH and, therefore, an updated systematic review is needed to fill in this gap. We hypothesise that deep neck muscles deficiencies, as evidenced by morphometric alterations, could be a risk/contributing factor for the development/progression of PHD. Therefore, this systematic review aims to: (i) summarise the evidence from studies examining morphometric alterations of the deep neck muscles in patients diagnosed with PHD; (ii) determine whether morphometric alterations of the deep neck muscles can be considered a risk/contributing factor for the development/progression of PH; (iii) assess the methodological quality of studies investigating this issue; (iv) provide a clinical guideline for the practice of health professionals in the treatment of PHD, especially in physiotherapy; and (v) to provide recommendations for future research in the physiotherapeutic treatment of PH.
2. Materials and Methods
2.1. Protocol and Register
The protocol was registered in PROSPERO under the registration number CRD42021252782 [31] and written in accordance with the Preferred Reporting Elements for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The review methods were established prior to the conduct of the review.
2.2. Search Strategy
Keywords related to the main concepts of our topic were used to develop the search strategy: deep neck muscles and PH (Table A1). Searches were conducted in five databases up to 12 November 2022: Medline (Ovid Medline), Pubmed, CINAHL PLUS with Full text (EBSCO host interface), Web of Science, and Scopus. The search dates ranged from 1900 to November 2022. No limits were applied to the date, language, or publication status. All the information about the search strategy is detailed in Appendix A Table A2. Finally, a manual search was carried out in Web of Science (WOS) and Scopus in November 2022, and the bibliography of the selected articles was reviewed.
2.3. Inclusion and Exclusion Criteria
Inclusion criteria: (i) Population: studies with patients of any age or sex with a PHD diagnosis made by a health professional using the ICHD classification; (ii) Intervention/exposure/factor: any study that employed diagnostic imaging methods (US, magnetic resonance imaging (MRI), computed tomography (CT)) [32] with their variable of interest being muscle size (measured by muscle cross-sectional area (CSA) or muscle volume quantification (MVQ)); (iii) Comparison: subjects with no headaches; (iv) Outcome: Our main outcome was the development/progression of PH. However, since we anticipated that prospective cohort studies answering our question would be limited or non-existent, we were also interested in determining whether people with PH in comparison with people without these diagnoses have morphometric impairments of the deep neck muscles evidenced by MRI or US assessment. Measures of association (odds ratios, correlation coefficients) between morphometric impairments of the deep neck muscle and PH (development and/or progression of PH) were sought; and (v) Studies: observational studies such as prospective cohort, cross-sectional studies, retrospective cohort studies, and case control studies were targeted since these designs are suitable to answer our question.
Exclusion criteria: (i) Population: studies whose patients had evidence of any secondary headache, who were receiving physical therapy in the cervical region and diagnosed with medication overuse headache; (ii) Intervention/exposure/factor: studies that used clinical examination, physical examination or a non-validated tool; (iii) Comparison: studies with no comparison group; and (iv) Studies: clinical trials, systematic reviews, meta-analyses, reviews, case reports, letters to the editor, conference articles, book chapters, protocol registries, grey literature, cadaver studies and animal studies.
2.4. Data Collection/Extraction and Risk of Bias
2.4.1. Selection of Studies
All search results were exported to EndNote. All duplicates were removed from EndNote and the results were imported into Covidence (www.covidence.org, accessed on 1 April 2020). The screening process was performed by two independent reviewers based on the inclusion criteria described above and divided into two stages: title/abstract review and full-text review. In cases of disagreement, a third reviewer intervened to discuss and resolve the conflict. The PRISMA 2020 flow chart (Figure 1) was used to organise and keep track of the number of duplicates, selected and eliminated studies.
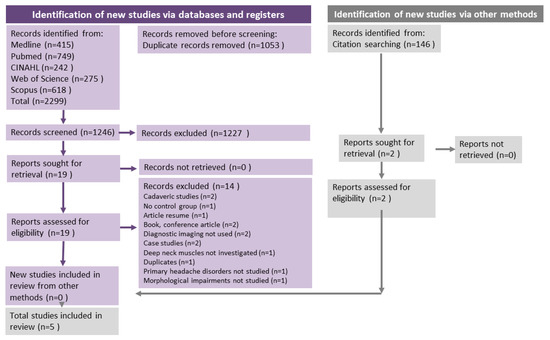
Figure 1.
PRISMA Flow Chart. Flow chart of the studies included for this systematic review based on the PRISMA guidelines. The flow chart shows the articles that were found throughout the literature search of the five databases and the number of articles that were reviewed by title, abstract and full text.
2.4.2. Data Extraction
Data extraction (DE) was carried out using a structured excel data collection sheet. This sheet included different fields of interest and drop-down menus to extract all the information of interest. To ensure that all reviewers extracted the information in the same way, a pilot process of data extraction was conducted. One reviewer extracted and organised the data in this DE sheet. A second reviewer checked all the extracted data. If discrepancies occurred, a consensus meeting was held. If consensus was not possible, a third reviewer ensured consensus.
The extracted data were based on characteristics including but not limited to article information (first author’s name, year of publication, language, funding, country, main objective, study design and setting), population information (e.g., age, gender, ethnicity, diagnosis, diagnostic tool, other conditions or characteristics), exposure characteristics (e.g., imaging method used, muscles of interest, validated method), outcomes (type of outcome, tool used, units, meaning of values), summary of results, data analysis, conclusions, limitations/comments, and recommendations. In the case of any missing data of interest, the authors were contacted to obtain the unreported data.
2.4.3. Risk of Bias (RoB)
The RoB assessment of the included studies was performed concurrently with data extraction. This stage was performed by two independent reviewers using The Quality in Prognostic Studies (QUIPS) tool for assessing risk of bias of observational studies. This tool provides six risk of bias domains: (1) study participation, (2) study dropout, (3) measurement of prognostic factors, (4) measurement of outcomes, (5) study confounders, and (6) statistical analysis and reporting. Each of the six domains of potential bias can be rated as high, moderate, or low risk of bias. For the overall quality of the study, we developed decision rules as stated in previous research (Hayden et al. 2019) [33].
- High risk of bias: If the study was rated high in at least one domain.
- Moderate risk of bias: If the study was rated moderate in at least one domain, and the other domains were low.
- Low risk of bias: If the study was rated as low in all six domains.
If consensus could not be reached, an independent third opinion was sought to help resolve any differences.
2.5. Data Synthesis
A narrative (descriptive) synthesis of the results is presented. Evidence tables have been used to compare study details, summarise results, and perform analyses. Data synthesis has been performed according to the type of imaging method used (e.g., US, MRI), the PH diagnosis (e.g., migraine, TTH, TACs, other), and according to the type of outcome (CSA, MVQ).
The results were also summarised according to the risk of bias. A meta-analysis was not carried out due to the great heterogeneity of the headaches analysed, the comparators, the musculature of interest and the outcomes (area/volume measurements). Therefore, we were only able to perform a qualitative narrative synthesis of the results of interest as presented in tables and summarised in figures. We present standardized mean differences (SMD) as measures of effect sizes (ES) of the variables of interest from individual studies to facilitate comparison between studies, as suggested by the Cochrane collaboration.
The quality of the body of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. We used the guidelines provided by Huguet et al. [34] adapted to observational studies. Review Manager (RevMan 5.4.1, The Cochrane Collaboration) software was used to display figures with the distribution of the data obtained. The evidence was classified as high (++++), moderate (+++), low (++) and very low (+), as described by Huguet et al. [34]. For each domain or risk factor, the following was analysed: (1) phase of the research; (2) limitations of the study (3) inconsistency of the results, (4) indirectness (not generalizable), (5) imprecision (insufficient data) and (6) publication bias. The (7) effect size and (8) dose effect were not evaluated as a meta-analysis could not be performed. However, trends in the data were inspected, when possible.
Subgroups analyses were performed when feasible based on the imaging tool and outcome variable obtained to determine the study quality and risk of bias of the included studies.
3. Results
3.1. Selection of Studies
After the electronic search in the five databases, a total of 2299 studies were found, as summarised in the PRISMA flowchart (Figure 1). EndNote and Covidence identified 1053 duplicates and these were removed before screening. A total of 1246 articles were screened and after the first screening of titles/abstract, n = 1227 studies were eliminated. A total of 19 studies were read in full text. The reasons for exclusion are available in Table A3 and outlined in the PRISMA flowchart (Figure 1). Five studies remained and were selected and analysed for this review: Fernández de las Peñas et al. [35], Hvedstrup et al. [23], Wanderley et al. [36], Oksanen et al. [37] and Xiao Ying-Yuan et al. [25].
A summary of the general characteristics of the five studies included in this review is shown in Table 1 and Table A4. The studies included in this systematic review were published between 2007 and 2020 and all were observational cross-sectional studies.

Table 1.
General characteristics of the included studies. Authors: Fernández de las Peñas et al. [35], Wanderley et al. [36], Hvedstrup et al. [23], Oksanen et al. [37], and Xiao-Ying et al. [25].
The five studies included a total sample of 458 participants. The mean ages of the patients varied from 17 years [37] to 43.5 years [25].
Two of the studies only considered women [35,36], and in the remaining three studies, the sex was mixed (in total, 318 women and 140 men were included).
3.2. Primary Headaches Disorders
The studies included different diagnoses of PH. Two studies focused on a single type of PH: TTH [23,35], and migraine [23]. Two studies included both TTH and migraine [36,37]. In the remaining article [25], although the author was contacted by email, we could only confirm that they studied PH and that they used the ICHD criteria for their diagnosis; however, the specific diagnosis was not provided.
All the studies used the criteria of the International Headache Society (IHS) as a diagnostic tool. The classification used in four of them was the 2nd edition of ICHD. In the study by Oksanen et al. [37], the IHS criteria (1988) were used as the diagnosis of the patients was made before the publication of the ICHD-2. Information on headache frequency and years lived with headaches is provided in Table 1.
3.3. Diagnostic Imaging
Two imaging methods were used in the selected studies: MRI and US. Four studies used MRI [23,25,35,37] and only one [36] used US. The radiological interpretation was blinded in four of the studies, but we do not have this information for the article by Yuan et al. [25].
3.4. Muscles Tested
The muscle most frequently examined in the included studies was the RCPmi, as three of the studies [23,25,35] analysed its morphometric alterations. Only one study [36] examined the longus colli (LC), and the rectus capitis posterior major (RCPma) was also examined by one study [35]. Finally one study analysed the rotators, multifidus colli and semispinalis colli [37]. This last study [37] grouped the morphometric measures of the three muscles into a single area. Both Fernández de las Peñas et al. [35] and Oksanen et al. [37] examined the size of the superficial cervical musculature as well. They analysed the semispinalis capitis, splenius capitis [35,37], sternocleidomastoid, scalenus, splenius colli, levator scapulae and trapezius [37].
3.5. Outcomes of Interest
Two different outcomes were used in the selected studies: CSA and MVQ. Four articles used CSA as an outcome [25,36,37].
A meta-analysis was not possible due to the great heterogeneity of the headaches analysed, the comparators, the musculature of interest and the outcome variables (area/volume measurements). Therefore, we only were able to perform a qualitative analysis and a narrative synthesis of the outcomes, which are presented in Figure 2, Figure 3 and Figure 4.
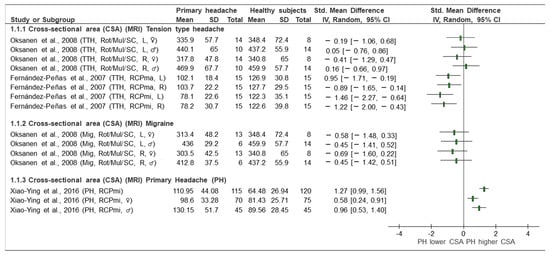
Figure 2.
Forest plot of cross-sectional area based on magnetic resonance imaging (MRI). Graph explains the direction of change in muscle size in patients with primary headache towards a lower or higher CSA. TTH: tension type headache, Mig: Migraine, PH: primary headache, CSA: cross-sectional area, MRI: magnetic resonance imaging, Rot: rotators, Mul: multifidus, SC: semispinalis cervicis, RCPmin: rectus capitis posterior minor, RCPmaj: rectus capitis posterior major, L: left, R: right, ♂: male, ♀: female, Lower CSA: smaller area, Higher CSA: larger area. Authors: Fernández de las Peñas et al. [35], Oksanen et al. [37], and Xiao-Ying et al. [25].
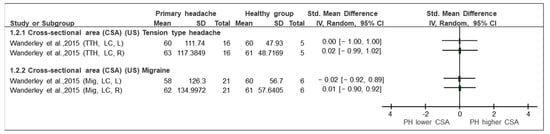
Figure 3.
Forest plot of cross-sectional area based on ultrasound imaging. Graph explains the direction of change in muscle size in patients with primary headache towards a lower or higher CSA. TTH: tension type headache, Mig: Migraine, CSA: cross-sectional area, US: ultrasound imaging, LC: longus colli, L: left, R: right, Lower CSA: smaller area, Higher CSA: larger area. Authors: Wanderley et al. [36].
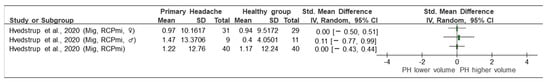
Figure 4.
Forest plot of muscle volume quantification by magnetic resonance imaging (MRI). Graph shows the direction of change in muscle size in patients with primary headache towards a lower or higher MVQ. Mig: migraine, RCPmi: rectus capitis posterior minor, PH: primary headache, ♀: female, ♂: male, lower volume: smaller area, higher volume: larger area. Authors: Hvedstrup et al. [23].
3.6. Morphometric Alterations
This section is divided according to the analysis presented by the studies and as reflected in the forest plots (Figure 2, Figure 3 and Figure 4).
- TTH vs. control (CSA, MRI) (Figure 2): Two studies [35,37] investigated TTH and analysed the CSA of the RCPmi, RCPma, rotators, multifidus, and semispinalis cervicis compared with an asymptomatic control group. As shown in the forest plot (Figure 2), the study by Oksanen et al. [37] did not observe statistically significant differences in any of the variables when comparing TTH and the control subjects. This study assessed the CSA in rotator, multifidus, and semispinalis cervicis, both in females in the right side. The SMD ranged from 0.16 to −0.41. In contrast, Fernández de las Peñas et al. [35] observed statistically significant differences between groups. The magnitude of effects (SMD) in the different comparisons was greater. The SMDs ranged from −0.89 to −1.46. These differences were considered clinically relevant [35].
- Migraine vs. control (CSA, MRI) (Figure 2): Only one [37] of the five included studies evaluated the CSA of the rotator, multifidus and semispinalis cervicis, comparing subjects with migraine and asymptomatic subjects. The results, displayed in the forest plot (Figure 2), show changes in the different groups of interest. The area of the three named muscles was assessed with a unique measure separated by sexes. The values were grouped for males and females. The SMDs ranged from 0.69 to −0.45. Changes were noted, but they were not of a sufficient magnitude to be considered relevant. The CSA in the extensor muscles was greater (p < 00.1) in men than in women [37].
- Primary headache (general) vs. control (CSA, MRI) (Figure 2): Only the study by Yuan et al. [25] evaluated the CSA as a variable in a primary (general) headache group in the RCPmi of the head compared with an asymptomatic group. Looking at the analysis of the different comparison groups in the study by Yuan et al. [25] (headache vs. control), in general, considering women and men together, a SMD [95% CI] = 1.27 [0.99, 1.56] was obtained, which is considered a large effect. In addition, differences between groups were observed when women and men were analysed separately. In this comparison group, it was also observed that the RCPmi had a larger area in men than in women (p < 0.001).
- TTH vs. control (CSA, US) (Figure 3): One of the included studies [36] evaluated the CSA in the longus capitis in patients with tension headaches compared with the control group. The forest plot (Figure 3) presents the qualitative comparison, showing that no changes were found in the comparison between groups. The assessment of neck length on both the right side (SMD [95% CI] = 0.00 [−1.00, 1.00]) and left side (SMD [95% CI] = 0.02 [−0.99,1.02]) did not reflect any significant (statistical or clinical) change between the groups.
- Migraine vs. control (CSA, US) (Figure 3): Only the study by Wanderley et al. [36] compared the CSA in the LC in patients with migraine with healthy subjects. No significant (statistical or clinical) difference in the CSA of the LC between groups was observed on the right (SMD [95% CI] = 0.01 [−0.10, 0.92]) or on the left side (SMD [95% CI] = −0.02 [−0.92, 0.89]).
- Migraine vs. control (MCQ, MRI) (Figure 4): Of the five included studies, only the study by Hvedstrup et al. [23] evaluated the volume of the RCPmi in subjects with migraine compared with the control group. No differences (either statistical or clinical) in the volume of the RCPmi between groups was identified in this study. (SMD [95% CI] = 0.00 [−0.43, 0.44]) (Figure 4). However, this study showed a statistically significant (p < 0.001) higher volume in the male group than in the female group.
3.7. Risk of Bias (QUIPS)
As previously mentioned, the risk of bias of each study was assessed by a set of domains of the QUIPS tool. Each domain analysed is summarised in Table 2. Four studies (80%) were assessed as having a high overall risk of bias, and the remaining article (20%) was considered to have a moderate risk of bias. Most studies (80%) had a moderate risk assessment in the outcome measurement domain, and 80% also had a high-risk of bias in the confounding domain. All of the studies (100%) were judged to be at low risk of bias in the study dropout domain (Figure 5). As all studies were of cross-sectional design, it was anticipated that attrition biases were not an issue.

Table 2.
Risk of Bias domains. This table shows the different domains of the risk of bias (RoB) assessment performed with the QUIPS tool. It represents the six domains assessed: study participation, study attrition, prognostic factor measurement, outcome measurement, potential confounders, and statistical analysis and reporting of statistical data.
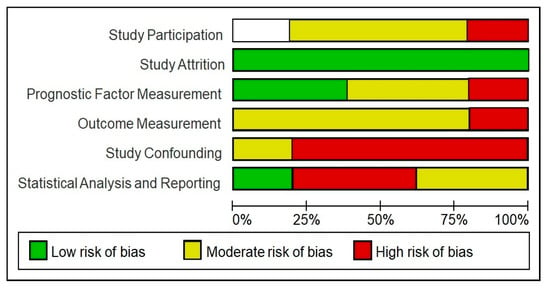
Figure 5.
Risk of bias of included studies using the QUIPS tool. This graph shows the different items assessed in the QUIPS tool and the percentage of studies with a high, moderate or low risk of bias.
3.8. Quality of Studies (GRADE)
The certainty of the studies was assessed using the GRADE [33,34] system, as shown in Table 3. The adaptation of Huguet et al. [34] for articles assessing prognostic factors was used. The overall quality of evidence in the studies was very low in the different comparisons due to heterogeneity, high risk of bias and imprecision. The inconsistency parameter was not assessed in all cases since, if the comparison only included one study, this section was considered as “not applicable”. Publication bias was not an element that devalued quality in this systematic review since the search for all included studies was performed carefully, as shown in Table 3.

Table 3.
GRADE. CI: confidence interval, SD: study design, RoB: Risk of bias, OS: observational studies and ⊕◯◯: very low.
4. Discussion
4.1. Main Results
This systematic review shows controversial findings regarding whether morphometric alterations of the deep neck muscles are present in subjects with PH. There was not a clear pattern or direction of results. It is uncertain whether the size of the deep neck muscles is reduced or not in individuals with PH based on the included studies. We can say that patients with PH may present morphometric alterations of the deep cervical musculature. However, the direction of this alteration is not clear.
For example, Fernández de las Peñas et al. [35] obtained statistically significant results and a large effect, showing that RCPmi and RCPma have a smaller CSA compared with the control group. Therefore, this study reported the presence of atrophy of these muscles in patients with PH, specifically chronic TTH. It should be noted that the RoB in this study is high, and the sample size is small. In contrast, the study by Yuan et al. [25], which also obtained statistically significant results and a large effect, concluded that the RCPmi has a larger CSA, and thus, that there is hypertrophy of these muscles in patients with primary (chronic) headaches. This study was also evaluated at a high RoB. Although morphometric changes of the cervical musculature were observed in both studies, it was not possible to determine whether this factor was a primary or secondary phenomenon to the PH due to the simplistic analyses conducted in these studies.
The study by Oksanen et al. [37] shows lower CSA of the rotators, multifidus cervicis, and semispinalis cervicis in subjects with PH. However, the study carried out by Hvedstrup et al. [23], in agreement with Yuan et al. [25], observed greater volumes of the RCPmi (without being statistically significant, and presenting a small effect size), which contradicts the results found by Oksanen et al. [37]. The mean age of the sample in this study by Hvedstrup et al. [23] was 17 years, comparatively lower than in the rest of the studies, which may affect both the chronicity of the headache and the years of evolution. The heterogeneity of the studies could explain in part the differences in their results.
The literature has highlighted the crucial role that the deep neck muscles play in the cervical spine due to its stabilisation function [38]. In particular, the suboccipital musculature is characterised by a high concentration of muscle spindles, up to five times more than the splenius capitis or three times more than the semispinalis capitis [39]. In addition, these muscles which have a high concentration of muscle spindles play an important role in correct motor control of the neck [40]. Therefore, it is expected that these muscles could be affected in subjects with head- and neck-related disorders. However, based on recent evidence, there is no direct relationship between cervical motor control (in this case, of the suboccipital muscles) and PH [41]. This aligns with the results of our systematic review, where a direct relationship between morphometric alterations of the deep neck muscles and PH was also not observed.
4.2. Previous Studies and Systematic Reviews
We could say that the hypothesis raised in our study is an emerging question since, as described above, we have not found previous systematic reviews analysing the same topic of interest. The systematic review published in 2019 by Liang et al. [9] focuses on functional, rather than structural, alterations of the cervical musculature in patients with migraine and TTH. Although the review by Liang et al. [9] did not determine a direct relationship between both variables, greater functional alterations, such as greater forward head posture or less cervical range of motion were identified in patients with TTH when compared with subjects with migraine. Other systematic reviews, such as the one published by Ignacio Elizagaray et al. [41], have focused on forward head posture in patients with PHD. This publication [41], consistent with Liang et al. [9], concludes that there may be a greater FHP in patients with chronic PH in comparison with those with episodic PH or healthy controls.
Previous research has been interested in morphometric alterations of different muscles related to PH. For example, the publication by Pereira de Castro Lopes et al. [42] reported that patients with migraine (and associated signs and symptoms in the temporomandibular joint) presented hypertrophy of the lateral pterygoid muscle.
On the other hand, US has been used to determine muscle thickness in the obliquus capitis inferior (OCI) [27], LC [26] and longus capitis, obliquus capitis superior (OCS) and RCPma muscles [28], and in most cases, determined a lower muscle thickness in patients than in healthy subjects. In all three studies, however, US was used in patients with CEH, which is not applicable to our research.
However, as mentioned before, none of the previous reviews have focused on documenting morphometric changes in this population. Thus, our review provides novel evidence regarding this literature.
4.3. Heterogeneity of Parameters
As we have already explained, several factors contributed to the great heterogeneity found in the included studies. For example, the selected studies had different characteristics in relation to types of PH, sexes, methods of morphometric measures, methods of diagnostic imaging used, MRI equipment, patient positioning, cervical spine levels for taking measurements, and software used, among other factors. This great variability in the studies prevented us summarising the evidence efficiently or performing a meta-analysis. In addition, we were unable to explore sources of heterogeneity with subgroup analyses (e.g., based on the RoB, types of headaches, outcomes) or meta-regression since the number of studies was limited.
4.4. Risk of Bias/Certainty of the Evidence
We undertook a careful RoB assessment process, and several key elements from the analysed studies reduced our confidence in the results. First, the RoB due to potential confounders was high in many of the studies. Several publications did not control for confounding variables such as height, weight, body mass index, physical activity, cervical spine symptoms (pain, cervical disability index, myofascial trigger points), headache frequency or years lived with headache. In other cases, despite collecting data on some of the variables, they were not included in the statistical analyses. Regarding the quality of evidence, the confidence level of these studies was low due to the heterogeneity of the comparisons, the small sample sizes, as well as the methodological approaches of the studies.
One of the main limitations of the results obtained in this systematic review was the type of study designs used in the included studies. All the publications included in this review were cross-sectional studies. This made it impossible to follow up the participants to establish an association between the alterations in the deep cervical muscles and the onset of headache. Therefore, it is not possible to conclude a causal association between cervical alterations and the development of headache.
As suggested by other publications, there could be an overlap between the symptoms of chronic migraine and CEH [43], and thus, patients with chronic migraine are commonly diagnosed as having CEH. This could affect the accuracy of the diagnoses used in the included studies [44].
Age also has been considered a factor which is related to muscle mass. Starting in middle-aged adults, there is a loss of muscle mass, and thus, studies should be limited to a more specific age range [45] or consider age as a covariate in their statistical analyses. In addition, physical activity has been reported to influence muscle mass [46,47], and thus, analyses of studies should also take this factor into account.
4.5. Limitations and Strengths of This Review
We followed strict standards and up-to-date methods to conduct this systematic review. Comprehensive search strategies as well as manual search and tracking of references were conducted to identify potential studies for inclusion. The newly developed PRISMA guidelines were used to report the results. Furthermore, all the authors were well trained to perform data extraction and quality assessment in a reliable and consistent manner. In addition, the full text of the selected articles was read by two independent reviewers to avoid selection biases.
Due to the insufficient number of studies in the comparisons, publication bias was not assessed in this systematic review. In any case, the search strategy was performed in an exhaustive and expert-supervised manner to avoid publication biases.
In addition, all the included studies based their findings on very small sample sizes, and it is possible that these results are not applicable to the whole population since their variability was very high.
4.6. Implications for Clinical Practice and Research
Due to the limited evidence (low number of studies) and their high RoB as well as their inconsistent results, we can say that there is no clear pattern of morphometric alterations of the deep neck muscles in patients with PH and practical recommendations are uncertain. The results of this systematic review highlight the need for well-planned future studies with better methodological quality and longitudinal design to determine a possible causality of this factor on PH and, therefore, to establish guidelines in the treatment of PH. The need for more research in this area is by itself an important and useful finding for clinical and research practice.
As explained in other sections, previous systematic reviews show the existence of functional alterations in the deep neck muscles in primary headaches [9]. Other studies have also observed these functional changes in cervicogenic headache [48]. In both cases, the evaluation by a physical therapist is recommended. On the other hand, a systematic review conducted in 2022 concluded that craniocervical exercises (involving the deep cervical musculature [49]) improve disability and quality of life in patients with primary headache, especially in patients with TTH [50]. In our systematic review we observed morphometric changes in patients with primary headache in comparison with healthy subjects. Although the evidence from the analysed studies is still limited and firm conclusions cannot be stated, our results point out the need for these muscles to be evaluated by specialized health professionals to determine their role in the symptomatology of individual patients.
We believe that the use of US should be implemented as a method of measuring morphology as it is cost effective, rapid [51] and a more accessible tool for physical therapists and other health professionals for rehabilitation and diagnosis [52]. Previous studies have validated the use of US to measure the CSA of the deep cervical muscles and have found US to be as effective as MRI [53]. The development of US in recent years has allowed better visualisation of the deep neck muscles [53]. We therefore believe that there should be future studies using this diagnostic imaging tool as the method of choice.
Although the evidence from the analyzed studies is still limited and firm conclusions cannot be stated, our results pointed out the need to evaluate these muscles by a specialized health professional, to determine their role in the symptomatology of individual patients.
5. Conclusions
Based on the included studies, it can be said that there are morphometric alterations of the deep cervical musculature in patients with primary headache compared with individual without primary headaches. However, the magnitude or direction of these alterations are not clear. Due to the variability of results, the heterogeneity of the comparisons, and the design of the studies, we cannot conclude what influence that morphometric alterations of the deep cervical muscles may have on the pathogenesis of primary headaches.
Future studies are needed to increase the body of evidence. These preliminary data show the urgent need for quality research on the direction of morphometric changes of the deep neck muscles in primary headaches to guide decision making.
Author Contributions
Conceptualization, J.A.M.-J. and C.C.R.d.l.H.; methodology, S.A.-O. and J.A.M.-J.; software, S.A.-O., C.C.R.d.l.H. and C.J.M.; validation, S.A.-O., C.C.R.d.l.H. and C.J.M.; formal analysis, S.A.-O. and C.C.R.d.l.H.; investigation, J.A.M.-J. and S.A.-O.; writing—original draft preparation, C.C.R.d.l.H. and S.A.-O.; writing—review and editing, S.A.-O., J.A.M.-J. and C.C.R.d.l.H.; visualization, C.C.R.d.l.H. and C.J.M.; supervision, S.A.-O. and J.A.M.-J.; project administration, S.A.-O. and J.A.M.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Search terms. This table shows the different terms used in the database searches.
Table A1.
Search terms. This table shows the different terms used in the database searches.
| MeSH | DeCS | Free Terms | |
|---|---|---|---|
| Paraspinal muscles | Neck muscles | Deep neck muscles | |
| Neck muscles | Deep cervical muscles | ||
| Musculi suboccipitales | |||
| Suboccipital muscles | |||
| Rectus capitis | |||
| Rectus capitis posterior | |||
| minor | |||
| Rectus capitis posterior | |||
| Deep neck | major | ||
| muscles | Rectus capitis lateralis | ||
| Rectus capitis anterior | |||
| Obliquus capitis | |||
| Obliquus capitis superior | |||
| Obliquus capitis inferior | |||
| Multifidus cervicis | |||
| Semispinalis cervicis | |||
| Longus cervicis | |||
| Longus colli | |||
| Longus capitis | |||
| Headache disorders, primary | Migraine disorders | Sick headache | |
| Tension type headache | Headache | Primary headache | |
| Migraine disorders | Headache disorders | Primary cephalalgias | |
| Cluster headache | Tension headache | ||
| Primary | Trigeminal autonomic cephalalgia | Stress headache | |
| Headache | Histamine cephalalgia | ||
| disorders | Megrim | ||
| Migrainous | |||
| Cephalalgia | |||
| Migraine | |||
| Cephalalgy |

Table A2.
Search strategy. This table describes the different combinations of terms used in each of the databases are grouped below: Pubmed, Medline, CINAHL, Web of Science and SCOPUS.
Table A2.
Search strategy. This table describes the different combinations of terms used in each of the databases are grouped below: Pubmed, Medline, CINAHL, Web of Science and SCOPUS.
| Pubmed (n = 749, Filters: no) |
| (paraspinal muscles or neck muscles or “deep neck muscles” or “deep cervical muscles” or musculi suboccipitales or suboccipital muscles or rectus capitis or “rectus capitis posterior minor” or “rectus capitis posterior major” or “rectus capitis lateralis” or “rectus capitis anterior” or obliquus capitis or obliquus capitis superior or obliquus capitis inferior or multifidus colli or multifidus cervicis or semispinalis or “rectus capitis anterior” or obliquus capitis or obliquus capitis superior or obliquus capitis inferior or multifidus colli or multifidus cervicis or semispinalis colli or “semispinalis cervicis” or longus colli or “longus cervicis” or longus capitis) AND (headache disorders, primary or tension type headache or migraine disorders or “cluster headache” or trigeminal autonomic cephalalgias or headache or “headache disorders” or “sick headache” or “primary headache” or “primary cephalalgias” or “tension headache” or “stress headache” or “histamine cephalalgia” or megrim or migrainous or cephalalgia or migraine or cephalalgy) |
| Medline (Ovid) (n = 415 Filters: no) |
| exp *Neck Muscles/OR neck muscl*.mp. OR exp *Paraspinal Muscles/OR paraspinal muscl*.mp. OR (deep neck muscl* or deep cervical muscl* or musculi suboccipitales or suboccipital muscl* or rectus capitis or rectus capitis posterior major or rectus capitis posterior minor or rectus capitis lateralis or rectus capitis anterior or obliquus capitis or obliquus capitis superior or obliquus capitis inferior or multifidus colli or multifidus cervicis or semispinalis colli or semispinalis cervicis or longus cervicis or longus colli or longus capitis).mp. AND exp *headache disorders, primary/or exp *migraine disorders/or exp *tension- type OR headache/or exp *trigeminal autonomic cephalalgias/OR headache disorders, primary.mp. OR exp *Tension-Type Headache/OR tension-type headache.mp. OR exp *migraine disorders/or exp *alice in wonderland syndrome/or exp *migraine with aura/or exp *migraine without aura/or exp *ophthalmoplegic migraine/migraine.mp. OR exp *trigeminal autonomic cephalalalgias/or exp *cluster headache/or exp *paroxysmal hemicrania/or exp *sunct syndrome/OR cluster headache.mp. OR exp *Headache/OR headache.mp. OR exp *headache disorders/OR exp *headache disorders, primary/OR headache disorders.mp. OR ((sick or primary or tension or stress) adj3 headache).mp. OR (sick headache* or primaryheadache* or primary cephalalgia* or tension headache* or stress headache* or histamine cephalagia* or megrim or migrainous or cephalalgia* or cephalalgy).mp. |
| CINHAL (n = 242 Filters: academic publications) |
| The same terms were used as in Medline. |
| Web of Science (WOS) (n = 275 Filters: no) |
| (TS = headache disorders, primary OR TS = tension type headache OR TS = migraine disorders OR TS = cluster headache OR TS = trigeminal autonomic cephalalalgias OR TS = headache OR TS = headache disorders OR TS = sick headache OR TS = primary headache OR TS = primary cephalalalgias OR TS = tension headache OR TS = stress headache OR TS = histamine cephalalalgia OR TS = megrim OR TS = migrainous OR TS = cephalalalgia OR TS = cephalalalgy) AND (KP = paraspinal muscles OR KP = neck muscles OR KP = deep neck muscles OR KP = deep cervical muscles OR KP = musculi suboccipitales OR KP = suboccipital muscles OR KP = rectus capitis OR KP = rectus capitis posterior minor OR KP = rectus capitis posterior major OR KP = rectus capitis lateralis OR KP = rectus capitis anterior OR KP = obliquus capitis OR KP = obliquus capitis superior OR KP = obliquus capitis inferior OR KP = multifidus colli OR KP = multifidus cervicis OR KP = semispinalis colli OR KP = semispinalis cervicis OR KP = longus cervicis OR KP = longus colli OR KP = longus capitis) OR (AK = paraspinal muscles OR AK = neck muscles OR AK = deep neck muscles OR AK = deep cervical muscles OR AK = musculi suboccipitales OR AK = suboccipital muscles OR AK = rectus capitis OR AK = rectus capitis posterior minor OR AK = rectus capitis posterior major OR AK = rectus capitis lateralis OR AK = rectus capitis anterior OR AK = obliquus capitis OR AK = obliquus capitis superior OR AK = obliquus capitis inferior OR AK = multifidus colli OR AK = multifidus cervicis OR AK = semispinalis colli OR AK = semispinalis cervicis OR AK = longus cervicis OR AK = longus colli OR AK = longus capitis) |
| SCOPUS (n = 618 Filters: TITLE-ABS-KEY, LIMIT-TO (DOCTYPE, “ar”)) |
| TITLE-ABS-KEY( (“paraspinal muscles” OR “neck muscles” OR “deep neck muscles” OR “deep cervical muscles” OR “musculi suboccipitales” OR “suboccipital muscles” OR “rectus capitis” OR “rectus capitis posterior minor” OR “rectus capitis posterior major” OR “rectus capitis lateralis” OR “rectus capitis anterior” OR “obliquus capitis” OR “obliquus capitis superior” OR “obliquus capitis inferior” OR “multifidus colli” OR “multifidus colli OR “rectus capitis anterior” OR “obliquus capitis” OR “obliquus capitis superior” OR “obliquus capitis inferior” OR “multifidus colli” OR “multifiduscervicis” OR “semispinaliscervicis” OR “semispinalis colli” OR “longus cervicis” OR “longus colli” OR “longus capitis” OR “longus capitis”)) AND (TITLE-ABS- KEY(“headache disorders, primary” OR “tension type headache “OR “migraine disorders” OR “cluster headache” OR “trigeminal autonomic cephalalgias” OR headache OR “headache disorders” OR “sick headache” OR “primary headache” OR “primary cephalalgias” OR “tension headache” OR “stress headache” OR “histamine cephalalalgia” OR megrim OR migrainous OR cephalalalgia OR cephalalgy)) AND (LIMIT-TO (DOCTYPE, “ar”)) |

Table A3.
Reason for exclusion. This table shows the reasons for exclusion of the studies discarded in the full-text screening phase.
Table A3.
Reason for exclusion. This table shows the reasons for exclusion of the studies discarded in the full-text screening phase.
| First Author, Year, Title | Reason for Exclusion |
|---|---|
| Fernández de las Peñas, 2008, Association of cross-sectional area of the rectus capitis posterior minor muscle with active trigger points in chronic tension-type headache: a pilot study | Did not have control group |
| Edmeads, 1978, Headaches and head pains associated with diseases of the cervical spine | Conference paper |
| Luedtke, 2017, Does the rectus capitis posterior minor muscle contribute to the pathogenesis of chronic headache? | Abstract of a paper we have already included |
| Xu Q, 2017, Anatomical Parameters of the Rectus Capitis Posterior Minor Muscle Based on a New Magnetic Resonance Scan Method | Cadaveric study |
| Kalmanson OA, 2017, Anatomic considerations in headaches associated with cervical sagittal imbalance: A cadaveric biomechanical study. | Cadaveric study |
| Vemon H, 1999, Musculoskeletal abnormalities in chronic headache | Did not use diagnostic imaging methods |
| Peterson C, 2005, An observational study of musculoskeletal symptoms in migraine patients between attacks | Did not use diagnostic imaging methods |
| Taptas JN, 1965, [Cervical disorders and headache]. Munch Med Wochenschr | Book |
| Winter SM, 2019, What is causing this patient’s headache and stiff neck? JAAPA: Journal of the American Academy of Physician Assistants (Lippincott Williams & Wilkins) | Case study article |
| Marmion DE, 1954, The role of the muscles in the aetiology of headache | Case study article |
| Jull G, 2007, Cervical musculoskeletal impairment in frequent intermittent headache. Part 1: Subjects with single headaches | Did not study deep neck muscles |
| Hallgren RC, 1994, Atrophy of suboccipital muscles in patients with chronic pain: a pilot study. | Did not study primary headache disorders |
| 2014, Tension headaches: Cramped neck muscles are often the cause | Did not study morphological changes in muscles |
| 2014, Tension headaches: Cramped neck muscles are often the cause | Duplicate |

Table A4.
Summary of the main characteristics of the five observational studies included in this systematic review.
Table A4.
Summary of the main characteristics of the five observational studies included in this systematic review.
| Study | Sample | Outcome | Statistical Analysis | Results/Conclusions |
|---|---|---|---|---|
| First author: C. Fernández de las Peñas, Year: 2007 Title: Magnetic resonance imaging study of the morphometry of cervical extensor muscles in chronic tension-type headache. Main objective: To analyse the differences in the morphometry of cranio-cervical extensor muscles (i.e., rectus capitis posterior minor, rectus capitis posterior major, semispinalis capitis and splenius capitis muscles) between CTTH patients and healthy controls. Secondary objective: Assess the relationship between muscle size and several clinical variables concerning the intensity and the temporal profile of headache. Study design: Cross-sectional Study country: Spain | Whole sample age: Control group mean: 40, SD: 10 years/Tension type headache group mean: 43, SD: 12 years Whole sample gender: Female Whole sample size: n = 30 Headache diagnosis: Tension type headache (TTH) Headache diagnosis tool: IHDC-2, Neurological examination Headache frequency: Chronic | Main outcome name: relative cross-sectional area (rCSA) Main outcome tool: magnetic resonance (MRI) Main outcome units: mm2 Deep muscles: rectus capitis posterior major, rectus capitis posterior minor Superficial muscles: semispinalis capitis, splenius capitis | Data analysis: ANOVA Data analysis description: Differences in side-to-side rCSA for all muscles were analysed with three-way (patient and controls, left and right sides, LRCQ activity levels) analysis of variance (ANOVA). Bonferroni post hoc analysis was performed to identify specific differences between the variables. The unpaired Student’s t-test was used to calculate differences in rCSA based on BMI levels and in rCSA between patients and controls. The Pearson’s correlation test (r) was used to explore the relationship between rCSA for the cervical muscles and age. Spearman’s rho (rs) test was used to analyse the association between the rCSA of the explored muscles and the clinical variables relating to headache in CTTH patients. 95% confidence level. p-value < 0.05 statistically significant. | Study results: No significant differences were found between right and left Rcsa. Patients with CTTH showed reduced rCSA in RCPmin and RCPmaj. The greater the headache intensity, duration or frequency, the smaller the Rcsa. TTH vs. control CSA RCPmi: R: p = 0.002 *, SMD = −1.22 [−2.00, −0.43] L: p = 0.001 *, SMD = −1.46 [−2.27, −0.64] CSA RCPma: R: p = 0.01 *, SMD = −0.89 [−1.65, −0.14] L: p = 0.01 *, SMD = −0.95 [ −1.71, −0.19] Authors’ conclusions: RCPmin and RCPmaj muscles showed reduced rCSA in CTTH patients. Headache intensity, frequency and duration were greater in those CTTH patients with more reduced rCSA in both RCPmin and RCPmaj muscles. Muscle size was not influenced by the factors of age, activity levels or BMI in either group. |
| First author: Débora Wanderley, Year: 2015 Title: Analysis of dimensions, activation and median frequency of cervical flexor muscles in young women with migraine or tension-type headache Main objective: Assess the association between the presence of migraine or tension-type headache and changes in the longus colli muscle dimensions and sternocleidomastoid muscle activity, using ultrasonography and surface electromyography Secondary objective: Not reported Study design: Cross-sectional Study country: Brazil | Whole sample age: Mean: 22.67, SD: 22.1–23.23 years Whole sample gender: Female Whole sample size: n = 48 Headache diagnosis: Migraine, Tension_type headache (TTH) Headache diagnosis tool: IHDC-2, Neurological examination Headache frequency: Episodic TTH and migraine | Main outcome name: cross-sectional area (CSA) Main outcome tool: ultrasonography (US) Main outcome units: cm2 Deep muscles: longus colli Superficial muscles: ECOM (electromyography) | Data analysis:t-test, ANOVA, Turkey’s post hoc Data analysis description: Data were presented as mean (confidence interval) and percentage. All variables exhibited normal distribution in the Kolmogorov–Smirnov test. To verify the association between variables, paired t-test, one-way ANOVA (for intergroup comparisons separately), repeated measures two-way ANOVA (for intra and intergroup comparisons performed simultaneously) and Tukey’s post hoc test were performed. Statistical significance was set at the 95% confidence level (p < 0.05). | Study results: Ultrasonographic analysis of the left and right longus colli muscle during rest and contraction revealed no intergroup difference in cross-sectional area or lateral, anteroposterior, and shape ratio dimensions. The hypothesis was not confirmed. CSA LC TTH vs. Control R: SMD = 0.02 [−0.99, 1.02] L: SMD = 0.00 [−1.00, 1.00] Mig vs. control R: SMD = 0.01 [0.90, 0.92] L: SMD = −0.02 [−0.92, 0.89] CSA rest: R: p = 0.985 NS L: p = 0.899 NS Authors’ conclusions: No association was observed between the presence of headache and alterations in the dimensions of the longus colli muscle, median frequency, and sternocleidomastoid muscle activation at the end of contraction. |
| First author: Jeppe Hvedstrup, Year: 2020 Title: Volume of the rectus capitis posterior minor muscle in migraine patients: a crosssectional structural MRI study Main objective: Assess the RCPmi in migraine patients compared with controls using muscle volume quantification (MVQ) Secondary objective: Not reported Study design: Cross-sectional Study country: Denmark | Whole sample age: Mean: 35 years Whole sample gender: Mixed Whole sample size: n = 80 Headache diagnosis: Migraine Headache diagnosis tool: IHDC-2, Neurological examination Headache frequency: Episodic | Main outcome name: muscle volume quantification (MVQ) Main outcome tool: magnetic resonance (MRI) Main outcome units: cm3 Deep muscles: rectus capitis posterior minor Superficial muscles: NA | Data analysis: General linear model Data analysis description: Associations were assessed using a general linear model with muscle volume as the dependent variable and the group being compared (i.e., patient/control or with/with out aura) as the covariate. In order to adjust for sex, it was added as a categorical covariate in the model. Paired comparisons were performed using paired t-test. If data were skewed, the paired comparison was performed on logarithmically transformed data. Associations between muscle volume and continuous variables were also examined using a general linear model adjusted for sex. A t-test was used to compare volume in females and males. | Study results: RCPmi volume measured with MRI did not differ between episodic migraine patients and controls. RCPmi volume was not associated with the migraine pain side, migraine frequency or years lived with migraine. Mig vs. Control p = 0.549 NS., SMD = 0.00 [−0.43, 0.44] Mig side vs. non attack side p = 0.237 NS Aura vs. no aura p = 0.339 NS Authors’ conclusions: There were no structural alterations in the RCPmi in migraine patients. Further studies are warranted to explore whether the frequent neck pain is associated with functional alterations in the RCPmi. |
| First author: Airi Oksanen, Year: 2008 Title: Neck muscles cross-sectional area in adolescents with and without headache—MRI study Main objective: To examine the CSA of neck flexion and extension muscles between groups of adolescents with migraine or tension-type headache and healthy controls Secondary objective: Not reported Study design: Cross-sectional Study country: Finland | Whole sample age: Mean: 17, SD: 0.5 years Whole sample gender: Mixed Whole sample size: n = 65 Headache diagnosis: Migraine, Tension type headache Headache diagnosis tool: IHDC-1, Pediatrician Headache frequency: Migraine (Not reported), Episodic TTH | Main outcome name: cross-sectional area (CSA) Main outcome tool: magnetic resonance (MRI) Main outcome units: mm2 Deep muscles: semispinalis colli, multifidus colli, rotators Superficial muscles: ECOM, scalenus, semispinalis capitis, levator scapulae, splenius colli, splenius cervicis, trapezius | Data analysis: General linear mixed model Data analysis description: Statistical analysis was carried out using general linear mixed models with gender and group as fixed effects and side as repeated factor. All pairwise comparisons (between groups) were calculated within gender and side. These were adjusted using Bonferroni method. ANOVA was used to compare characteristic measures between groups. In cases of non-normal distribution, the Kruskal–Wallis test was used to compare groups. The Chi square or Fisher’s exact test was used in case of categorical measures to compare groups. The intraclass correlation coefficient, ICC (3, 1) was calculated to evaluate the trial-to-trial repeatability. p-values less than 0.05 were considered as significant | Study results: The CSA of the neck extension muscles was significantly larger (<0.001) in boys than in girls. In boys, the CSA of both deep neck extension muscles in the migraine group were smaller than in the healthy control group, the differences being not significant. No significant differences were observed in the other CSA values in boys between different study groups. In girls, there were no significant differences in the size of neck extension muscles between the study groups; however, most of the CSA values were smaller in the headache group ♂ Mig vs. control CSA: NS L: SMD = −0.45 [−1.41, 0.52] R: SMD = −0.45 [−1.42, 0.51] TTH vs. control CSA: NS L: SMD = −0.19 [−1.6, 0.68] R: SMD = −0.41 [−1.29, 0.47] Authors’ conclusions: There are changes in the CSA of neck flexion and extension muscles in adolescent patients with migraine and tensiontype headache. |
| First author: Xiao-Ying Yuan, Year: 2016 Title: Correlation between chronic headaches and the rectus capitis posterior minor muscle: A comparative analysis of cross-sectional trail Main objective: Investigate the differences in the RCPmi between normal adults and patients who suffer from chronic headaches and assess the potential effects of the myodural bridge (MDB), the aim of which was to find a new explanation of the chronic headache using MRI Secondary objective: Not reported Study design: Cross-sectional Study country: China | Whole sample age: Control group mean: 42.50, SD: 15.20 years/Headache group mean: 43.63, SD: 15.69 years Whole sample gender: Mixed Whole sample size: n = 235 Headache diagnosis: Primary headache Headache diagnosis tool: Not reported Headache frequency: Chronic | Main outcome name: Cross-sectional area (CSA) Main outcome tool: magnetic resonance (MRI) Main outcome units: mm2 Deep muscles: rectus capitis posterior minor Superficial muscles: NA | Data analysis:t-test Data analysis description: The 95% confidence interval and mean standard deviation (SD) of the RCPmi were recorded, and the mean values between two groups were examined for statistical significance using an independent-sample t-test. A two-sided p-value of less than 0.05 indicated statistically significant differences. The p-values reported in this paper are not adjusted for multiplicity. | Study results: Based on the statistics, the headache group showed greater hypertrophy than the control group in both males (p < 0.001) and females (p ¼ 0.001). Headache vs. Control CSA: p < 0.001 *, SMD =1.27 [0.99, 1.56] ♂CSA: p < 0.001 *, SMD = 0.96 [0.53, 1.40] ♀CSA: p < 0.001 *, SMD = 0.58 [0.29, 0.91]. Authors’ conclusions:. It is believed that hypertrophy of the RCPmi may lead to chronic headaches. |
ICHD: International Classification of Headache Disorders; rCSA: relative cross-sectional area; MRI: magnetic resonance imaging; RCPmi: rectus capitis posterior minor; RCPma: rectus capitis posterior major; LC: longus colli; R: right; L: left; SD: standard deviation; SMD: standardized mean difference; TTH: tension type headache; CSA: cross sectional area; MVQ: muscle volume quantification; IHS: international headache society; Mig: migraine; *: Statistically Significant; NS: Not Statistically Significant; ♂: men; ♀: women.
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.; Hagen, K.; Jensen, R.; Katsarava, Z.; Lipton, R.; Scher, A.; Steiner, T.; Zwart, J.A. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007, 27, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stovner, L.J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; Ruiz de la Torre, E.; Tassorelli, C.; Barré, J.; et al. The impact of headache in Europe: Principal results of the Eurolight project. J. Headache Pain 2014, 15, 31. [Google Scholar] [CrossRef]
- Fuensalida-Novo, S.; Palacios-Ceña, M.; Fernández-Muñoz, J.J.; Castaldo, M.; Wang, K.; Catena, A.; Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C. The burden of headache is associated to pain interference, depression and headache duration in chronic tension type headache: A 1-year longitudinal study. J. Headache Pain 2017, 18, 119. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Katsarava, Z.; Mania, M.; Lampl, C.; Herberhold, J.; Steiner, T.J. Poor medical care for people with migraine in Europe—Evidence from the Eurolight study. J. Headache Pain 2018, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barré, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; et al. The cost of headache disorders in Europe: The Eurolight project. Eur. J. Neurol. 2012, 19, 703–711. [Google Scholar] [CrossRef]
- Luedtke, K.; Starke, W.; May, A. Musculoskeletal dysfunction in migraine patients. Cephalalgia 2018, 38, 865–875. [Google Scholar] [CrossRef]
- Liang, Z.; Galea, O.; Thomas, L.; Jull, G.; Treleaven, J. Cervical musculoskeletal impairments in migraine and tension type headache: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2019, 42, 67–83. [Google Scholar] [CrossRef]
- Armijo Olivo, S.; Magee, D.J.; Parfitt, M.; Major, P.; Thie, N.M. The association between the cervical spine, the stomatognathic system, and craniofacial pain: A critical review. J. Orofac. Pain 2006, 20, 271–287. [Google Scholar]
- Piovesan, E.J.; Kowacs, P.A.; Tatsui, C.E.; Lange, M.C.; Ribas, L.C.; Werneck, L.C. Referred pain after painful stimulation of the greater occipital nerve in humans: Evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia 2001, 21, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, E.J.; Kowacs, P.A.; Oshinsky, M.L. Convergence of cervical and trigeminal sensory afferents. Curr. Pain Headache Rep. 2003, 7, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.W.; Sun, K.Q.; Vernon, H.; Sessle, B.J. Craniofacial inputs to upper cervical dorsal horn: Implications for somatosensory information processing. Brain Res. 2005, 1044, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, G.N.; Florencio, L.L.; Dach, F.; Chaves, T.C.; Palacios-Ceña, M.; Fernández-de-Las-Peñas, C.; Bevilaqua-Grossi, D.; Speciali, J.G. Myofascial Trigger Points and Migraine-related Disability in Women With Episodic and Chronic Migraine. Clin. J. Pain 2017, 33, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Cuadrado, M.L.; Pareja, J.A. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia 2006, 26, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Do, T.P.; Heldarskard, G.F.; Kolding, L.T.; Hvedstrup, J.; Schytz, H.W. Myofascial trigger points in migraine and tension-type headache. J. Headache Pain 2018, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Ceña, M.; Castaldo, M.; Wang, K.; Catena, A.; Torelli, P.; Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine 2017, 96, e6548. [Google Scholar] [CrossRef]
- Joseph, K.; Hitchcock, S.A.; Meyer, H.P.; Geyser, M.M.; Becker, P.J. Active myofascial trigger points in head and neck muscles of patients with chronic tension-type headache in two primary health care units in Tshwane. S. Afr. Fam. Pract. 2016, 58, 131–135. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Alonso-Blanco, C.; Cuadrado, M.L.; Gerwin, R.D.; Pareja, J.A. Trigger points in the suboccipital muscles and forward head posture in tension-type headache. Headache 2006, 46, 454–460. [Google Scholar] [CrossRef]
- van Ettekoven, H.; Lucas, C. Efficacy of physiotherapy including a craniocervical training programme for tension-type headache; a randomized clinical trial. Cephalalgia 2006, 26, 983–991. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Wei, N.; Chang, W.; Chen, W.; Sui, H.J. Effectiveness of physical therapy on the suboccipital area of patients with tension-type headache: A meta-analysis of randomized controlled trials. Medicine 2019, 98, e15487. [Google Scholar] [CrossRef] [PubMed]
- Garrigós-Pedrón, M.; La Touche, R.; Navarro-Desentre, P.; Gracia-Naya, M.; Segura-Ortí, E. Effects of a Physical Therapy Protocol in Patients with Chronic Migraine and Temporomandibular Disorders: A Randomized, Single-Blinded, Clinical Trial. J. Oral Facial Pain Headache 2018, 32, 137–150. [Google Scholar] [CrossRef]
- Hvedstrup, J.; Amin, F.M.; Hougaard, A.; Ashina, H.; Christensen, C.E.; Larsson, H.B.W.; Ashina, M.; Schytz, H.W. Volume of the rectus capitis posterior minor muscle in migraine patients: A cross-sectional structural MRI study. J. Headache Pain 2020, 21, 57. [Google Scholar] [CrossRef]
- Zheng, N.; Yuan, X.Y.; Li, Y.F.; Chi, Y.Y.; Gao, H.B.; Zhao, X.; Yu, S.B.; Sui, H.J.; Sharkey, J. Definition of the to be named ligament and vertebrodural ligament and their possible effects on the circulation of CSF. PLoS ONE 2014, 9, e103451. [Google Scholar] [CrossRef]
- Xiao-Ying, Y.; Sheng-Bo, Y.; Cong, L.; Qiang, X.; Nan, Z.; Jian-Fei, Z.; Yan-Yan, C.; Xu-Gang, W.; Xiang-Tao, L.; Hong-Jin, S.; et al. Correlation between chronic headaches and the rectus capitis posterior minor muscle: A comparative analysis of cross-sectional trail. Cephalalgia 2017, 37, 1051–1056. [Google Scholar] [CrossRef]
- Abbaspour Khajeh, O.; Amiri, M.; Javanshir, K.; Karimlo, M. Reliability of longus colli muscle size measurement in healthy subjects and patients with cervicogenic headache using ultrasonography. J. Babol Univ. Med. Sci. 2012, 14, 97–101. [Google Scholar]
- Chen, Y.-Y.; Chai, H.-M.; Wang, C.-L.; Shau, Y.-W.; Wang, S.-F. Asymmetric Thickness of Oblique Capitis Inferior and Cervical Kinesthesia in Patients With Unilateral Cervicogenic Headache. J. Manip. Physiol. Ther. 2018, 41, 680–690. [Google Scholar] [CrossRef]
- Abaspour, O.; Akbari, M.; Rezasoltani, A.; Ahmadi, A. Relationship between thickness of deep neck muscles synergy and painful side in patients with cervicogenic headache. Cranio J. Craniomandib. Pract. 2021, 39, 465–471. [Google Scholar] [CrossRef]
- Elizagaray-García, I.; Garrigós-Pedrón, M.; Beltran-Alacreu, H.; Angulo-Díaz, S.; Gil-Martínez, A. Cervical Musculoskeletal Dysfunction in Patients with Chronic Primary Headache: Systematic Review and Meta-Analysis. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 10 February 2023).
- De Hertogh, W.; Michiels, S.; Castien, R.; Vandaele, L.; Vanbaelen, E.; Amons, A.; De Pauw, J. Cervical Muscle Function in Patients with Migraine, Tensiontype Headache and Cervicogenic Headache: A Systematic Review. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 10 February 2023).
- Caballero, C.; Justribó, C.; Armijo-Olivo, S.; Mesa, J.A. Are Morphological Impairments of the Deep Neck Muscles Related to Primary Headache Disorders? A Systematic Review. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 10 February 2023).
- Moreira, O.C.; de Oliveira, C.E.; Candia-Luján, R.; Romero-Pérez, E.M.; de Paz Fernandez, J.A. Methods of Evaluation of Muscle Mass: A Systematic Review of Randomized Controlled Trials. Nutr. Hosp. 2015, 32, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Hayden, J.A.; Stinson, J.; McGrath, P.J.; Chambers, C.T.; Tougas, M.E.; Wozney, L. Judging the quality of evidence in reviews of prognostic factor research: Adapting the GRADE framework. Syst. Rev. 2013, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Bueno, A.; Ferrando, J.; Elliott, J.M.; Cuadrado, M.L.; Pareja, J.A. Magnetic resonance imaging study of the morphometry of cervical extensor muscles in chronic tension-type headache. Cephalalgia 2007, 27, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, D.; Moura Filho, A.G.; Costa Neto, J.J.S.; Siqueira, G.R.; de Oliveira, D.A. Analysis of dimensions, activation and median frequency of cervical flexor muscles in young women with migraine or tension-type headache. Braz. J. Phys. Ther./Rev. Bras. Fisioter. 2015, 19, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, A.; Erkintalo, M.; Metsahonkala, L.; Anttila, P.; Laimi, K.; Hiekkanen, H.; Salminen, J.J.; Aromaa, M.; Sillanpaa, M. Neck muscles cross-sectional area in adolescents with and without headache—MRI study. Eur. J. Pain 2008, 12, 952–959. [Google Scholar] [CrossRef]
- Kashfi, P.; Karimi, N.; Peolsson, A.; Rahnama, L. The effects of deep neck muscle-specific training versus general exercises on deep neck muscle thickness, pain and disability in patients with chronic non-specific neck pain: Protocol for a randomized clinical trial (RCT). BMC Musculoskelet. Disord. 2019, 20, 540. [Google Scholar] [CrossRef]
- Liu, J.X.; Thornell, L.E.; Pedrosa-Domellöf, F. Muscle spindles in the deep muscles of the human neck: A morphological and immunocytochemical study. J. Histochem. Cytochem. 2003, 51, 175–186. [Google Scholar] [CrossRef]
- Kissane, R.W.P.; Charles, J.P.; Banks, R.W.; Bates, K.T. Skeletal muscle function underpins muscle spindle abundance. Proc. Biol. Sci. 2022, 289, 20220622. [Google Scholar] [CrossRef]
- Elizagaray-Garcia, I.; Beltran-Alacreu, H.; Angulo-Díaz, S.; Garrigós-Pedrón, M.; Gil-Martínez, A. Chronic Primary Headache Subjects Have Greater Forward Head Posture than Asymptomatic and Episodic Primary Headache Sufferers: Systematic Review and Meta-analysis. Pain Med. 2020, 21, 2465–2480. [Google Scholar] [CrossRef]
- Lopes, S.L.; Costa, A.L.; Gamba Tde, O.; Flores, I.L.; Cruz, A.D.; Min, L.L. Lateral pterygoid muscle volume and migraine in patients with temporomandibular disorders. Imaging Sci. Dent. 2015, 45, 1–5. [Google Scholar] [CrossRef]
- Ogince, M.; Hall, T.; Robinson, K.; Blackmore, A.M. The diagnostic validity of the cervical flexion-rotation test in C1/2-related cervicogenic headache. Man Ther. 2007, 12, 256–262. [Google Scholar] [CrossRef]
- Yi, X.; Cook, A.J.; Hamill-Ruth, R.J.; Rowlingson, J.C. Cervicogenic headache in patients with presumed migraine: Missed diagnosis or misdiagnosis? J. Pain 2005, 6, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Goodpaster, B.H. Effects of Exercise and Aging on Skeletal Muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Harber, M.P. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, I.C.; Olivo, S.A.; Magee, D.J. Cervical musculoskeletal impairments in cervicogenic headache: A systematic review and a meta-analysis. Phys. Ther. Rev. 2008, 13, 149–166. [Google Scholar] [CrossRef]
- Jull, G.A.; O’Leary, S.P.; Falla, D.L. Clinical assessment of the deep cervical flexor muscles: The craniocervical flexion test. J. Manip. Physiol. Ther. 2008, 31, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Varangot-Reille, C.; Suso-Martí, L.; Romero-Palau, M.; Suárez-Pastor, P.; Cuenca-Martínez, F. Effects of Different Therapeutic Exercise Modalities on Migraine or Tension-Type Headache: A Systematic Review and Meta-Analysis with a Replicability Analysis. J. Pain 2022, 23, 1099–1122. [Google Scholar] [CrossRef]
- Bemben, M.G. Use of diagnostic ultrasound for assessing muscle size. J. Strength Cond. Res. 2002, 16, 103–108. [Google Scholar]
- O’Riordan, C.; Van De Ven, P.; Nelson, J.; McCreesh, K.; Clifford, A. Reliability of a measurement method for the cross-sectional area of the longus colli using real-time ultrasound imaging. Ultrasound 2016, 24, 154–162. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Gallego-Sendarrubias, G.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Ortega-Santiago, R.; Arias-Buría, J.L. Cross-sectional area of the cervical extensors assessed with panoramic ultrasound imaging: Preliminary data in healthy people. Musculoskelet. Sci. Pract. 2020, 50, 102257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).