Using Ballistocardiogram and Impedance Plethysmogram for Minimal Contact Measurement of Blood Pressure Based on a Body Weight-Fat Scale
Abstract
1. Introduction
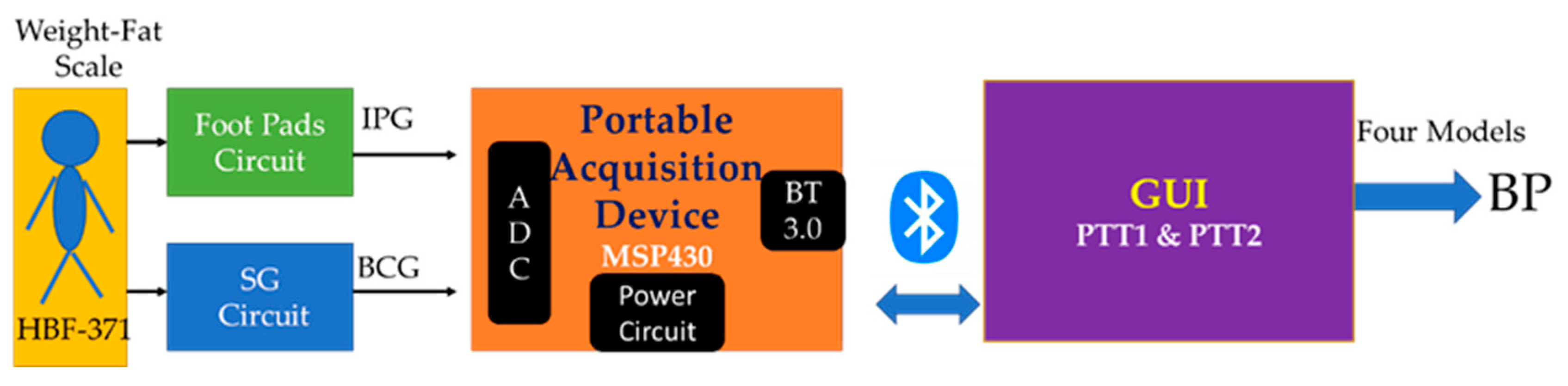
2. Materials and Methods
2.1. Impedance Plethysmography
2.2. Digital Signal Processing
2.3. PTT Models for Blood Pressure Estimation
2.4. Statistic Analysis
2.5. Experiment Protocol
- Subjects stand on the body weight-fat scale to measure ECG, PPG, IPG, and BCG signals for five minutes, and they measure BP once as a baseline.
- Subjects run on a treadmill at a speed of about 6 km/h for at least three minutes, and 8 km/h for the next four minutes. If the SBP is not raised to 20 mmHg higher than the resting SBP, subjects are requested to run longer.
- Subjects stand on the commercial body weight-fat scale again, measuring ECG, PPG, IPG, and BCG signals for six minutes. The BP is measured once a minute when standing on the body weight-fat scale.
- Each measurement session requires about 18 min. Subjects are measured four times. The interval between any two measurement sessions is at least a week.
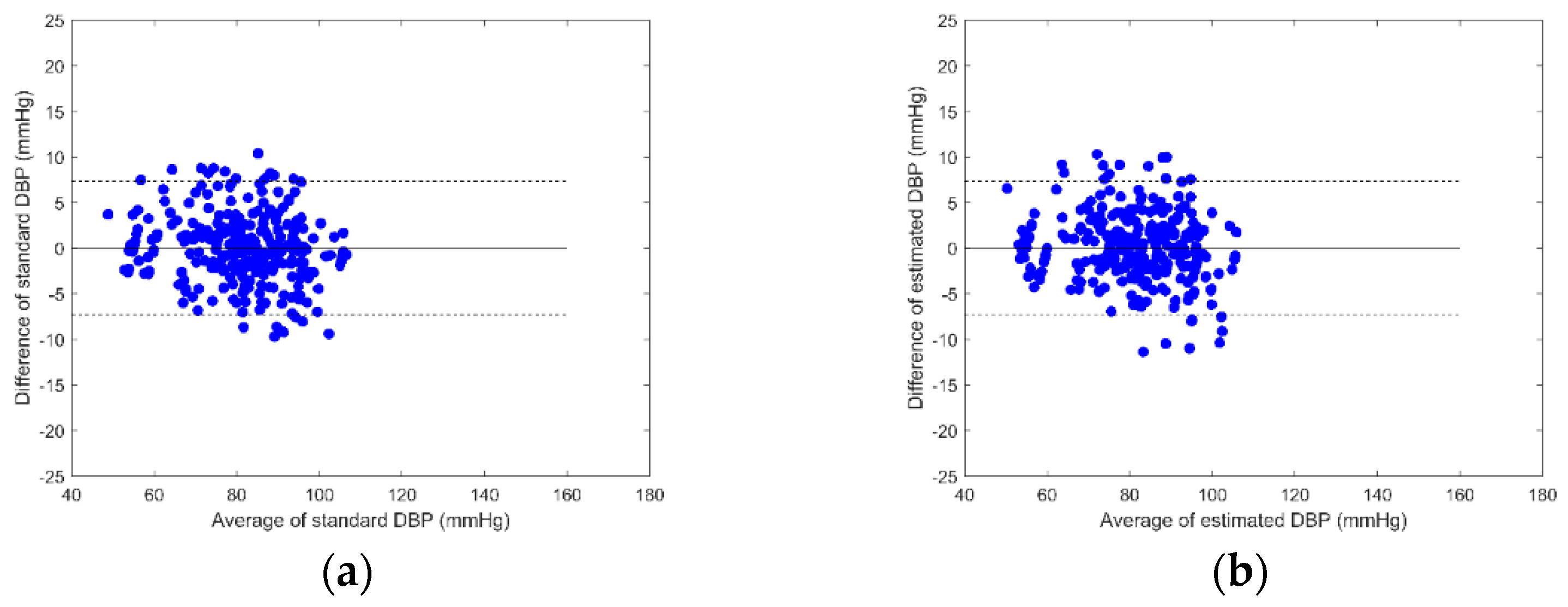
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening. Available online: https://www.who.int/publications/i/item/9789241550505/ (accessed on 1 December 2022).
- Wang, Q.; Su, M.; Zhang, M.; Li, R. Integrating Digital Technologies and Public Health to Fight Covid-19 Pandemic: Key Technologies, Applications, Challenges and Outlook of Digital Healthcare. Int. J. Environ. Res. Public Health 2021, 18, 6053. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Goetzinger, C.; Rashid, M.A.; Aguayo, G.A.; Huiart, L. Digital Health Strategies to Fight COVID-19 Worldwide: Challenges, Recommendations, and a Call for Papers. J. Med. Internet Res. 2022, 22, e19284. [Google Scholar] [CrossRef]
- Jandoo, T. WHO guidance for digital health: What it means for researchers. Digit. Health 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Lupton, D. Critical Perspectives on Digital Health Technologies. Sociol. Compass 2014, 8, 1344–1359. [Google Scholar] [CrossRef]
- Labrique, A.; Vasudevan, L.; Mehl, G.; Rosskam, E.; Hyder, A.A. Digital Health and Health Systems of the Future. Glob. Health Sci. Pract. 2018, 6, S1–S4. [Google Scholar] [CrossRef]
- Lupton, D. The digitally engaged patient: Self-monitoring and self-care in the digital health era. Soc. Theory Health 2013, 11, 256–270. [Google Scholar] [CrossRef]
- Cook, R.J. Advances in Plant Health Management in the Twentieth Century. Annu. Rev. Phytopathol. 2000, 38, 95–116. [Google Scholar] [CrossRef]
- Mateo, G.F.; Granado-Font, E.; Ferré-Grau, C.; Montaña-Carreras, X. Mobile Phone Apps to Promote Weight Loss and Increase Physical Activity: A Systematic Review and Meta-Analysis. J. Med. Internet Res. 2015, 17, e253. [Google Scholar] [CrossRef]
- Dash, S.; Shakyawar, S.K.; Sharma, M.; Kaushik, S. Big data in healthcare: Management, analysis and future prospects. J. Big Data 2019, 6, 54. [Google Scholar] [CrossRef]
- Apple. ResearchKit/ResearchKit: ResearchKit 1.5. Available online: https://www.apple.com/tw/researchkit/ (accessed on 1 December 2022).
- Prawiro, E.A.P.J.; Chou, N.-K.; Lee, M.-W.; Lin, Y.-H. A Wearable System That Detects Posture and Heart Rate: Designing an Integrated Device With Multiparameter Measurements for Better Health Care. IEEE Consum. Electron. Mag. 2019, 8, 78–83. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Yu, L.; Liu, J.; Zwetsloot, I.M.; Cabrera, J.; Tsui, K.-L. A Personalized Health Monitoring System for Community-Dwelling Elderly People in Hong Kong: Design, Implementation, and Evaluation Study. J. Med. Internet Res. 2018, 22, e19223. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wang, J.-J.; Su, C.-H.; Tan, T.-H. Development of a Patch-Type Electrocardiographic Monitor for Real Time Heartbeat Detection and Heart Rate Variability Analysis. J. Med. Biol. Eng. 2018, 38, 411–423. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, C.-B.; Chen, Y.; Chen, W.; Huang, T.-S.; Hsu, C.-Y. An EMG Patch for the Real-Time Monitoring of Muscle-Fatigue Conditions During Exercise. Sensors 2019, 19, 3108. [Google Scholar] [CrossRef]
- Liu, S.-H.; Liu, H.-C.; Chen, W.; Tan, T.-H. Evaluating Quality of Photoplethymographic Signal on Wearable Forehead Pulse Oximeter With Supervised Classification Approaches. IEEE Access 2022, 8, 185121–185135. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wang, J.-J.; Chen, W.; Pan, K.-L.; Su, C.-H. An Examination System to Detect Deep Vein Thrombosis of a Lower Limb Using Light Reflection Rheography. Sensors 2021, 21, 2446. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chen, W.; Su, C.-H.; Pan, K.-L. Convolutional neural Network-based detection of deep vein thrombosis in a low limb with light reflection rheography. Measurement 2022, 189, 110457. [Google Scholar] [CrossRef]
- Bramwell, J.C.; Hill, A.V. The velocity of pulse wave in man. Proc. R. Soc. London. Ser. B Contain. Pap. Biol. Character 1922, 93, 298–306. [Google Scholar] [CrossRef]
- Newman, D.L.; Greenwald, S.E. Validity of the Moens-Korteweg Equation. In The Arterial System; Springer: Berlin, Germany, 1978; pp. 109–115. [Google Scholar] [CrossRef]
- Sharwood-Smith, G.; Bruce, J.; Drummond, G. Assessment of pulse transit time to indicate cardiovascular changes during obstetric spinal anaesthesia. Br. J. Anaesth. 2006, 96, 100–105. [Google Scholar] [CrossRef]
- Sharma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.K.; Hsiai, T.K.; Chiao, J.-C.; Cao, H. Cuff-Less and Continuous Blood Pressure Monitoring: A Methodological Review. Technologies 2017, 5, 21. [Google Scholar] [CrossRef]
- Huynh, T.H.; Jafari, R.; Chung, W.-Y. Noninvasive Cuffless Blood Pressure Estimation Using Pulse Transit Time and Impedance Plethysmography. IEEE Trans. Biomed. Eng. 2014, 66, 967–976. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.-Y. Multimodal Wrist Biosensor for Wearable Cuff-less Blood Pressure Monitoring System. Sci. Rep. 2019, 9, 7947. [Google Scholar] [CrossRef]
- Yousefian, P.; Shin, S.; Mousavi, A.S.; Kim, C.-S.; Finegan, B.; McMurtry, M.S.; Mukkamala, R.; Jang, D.-G.; Kwon, U.; Kim, Y.H.; et al. Physiological Association between Limb Ballistocardiogram and Arterial Blood Pressure Waveforms: A Mathematical Model-Based Analysis. Sci. Rep. 2019, 9, 5146. [Google Scholar] [CrossRef]
- Inan, O.T.; Migeotte, P.-F.; Park, K.-S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Informatics 2015, 19, 1414–1427. [Google Scholar] [CrossRef]
- Starr, I.; Schroeder, H.A. Ballistocardiogram. II. Normal Standards, abnormalities commonly found in diseases of the heart and circulation, and their significance. J. Clin. Investig. 1940, 19, 437–450. [Google Scholar] [CrossRef]
- Etemadi, M.; Inan, O.T.; Giovangrandi, L.; Kovacs, G.T.A. Rapid Assessment of Cardiac Contractility on a Home Bathroom Scale. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 864–869. [Google Scholar] [CrossRef]
- Shin, S.; Yousefian, P.; Mousavi, A.S.; Kim, C.-S.; Mukkamala, R.; Jang, D.-G.; Ko, B.-H.; Lee, J.; Kwon, U.-K.; Kim, Y.H.; et al. A Unified Approach to Wearable Ballistocardiogram Gating and Wave Localization. IEEE Trans. Biomed. Eng. 2020, 68, 1115–1122. [Google Scholar] [CrossRef]
- Martin, S.L.-O.; Carek, A.M.; Kim, C.-S.; Ashouri, H.; Inan, O.T.; Hahn, J.-O.; Mukkamala, R. Weighing Scale-Based Pulse Transit Time is a Superior Marker of Blood Pressure than Conventional Pulse Arrival Time. Sci. Rep. 2016, 6, 39273. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zhang, B.-H.; Chen, W.; Su, C.-H.; Chin, C.-L. Cuffless and Touchless Measurement of Blood Pressure from Ballistocardiogram Based on a Body Weight Scale. Nutrients 2022, 14, 2552. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Genton, L.; Hans, D.; Kyle, U.G.; Pichard, C. Dual-Energy X-ray absorptiometry and body composition: Differences between devices and comparison with reference methods. Nutrition 2002, 18, 66–70. [Google Scholar] [CrossRef]
- Xie, X.; Kolthoff, N.; Bärenholt, O.; Nielsen, S. Validation of a leg-to-leg bioimpedance analysis system in assessing body composition in postmenopausal women. Int. J. Obes. 1999, 23, 1079–1084. [Google Scholar] [CrossRef]
- Jebb, S.A.; Cole, T.J.; Doman, D.; Murgatroyd, P.R.; Prentice, A.M. Evaluation of the novel tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br. J. Nutr. 2000, 83, 115–122. [Google Scholar] [CrossRef]
- Gudivaka, R.; Schoeller, D.A.; Kushner, R.F.; Bolt, M.J.G. Single- and multifrequency models for bioelectrical impedance analysis of body water compartments. J. Appl. Physiol. 1999, 87, 1087–1096. [Google Scholar] [CrossRef]
- Sherwood(Chair), A.; Allen, M.T.; Fahrenberg, J.; Kelsey, R.M.; Lovallo, W.R.; Doornen, L.J. Methodological Guidelines for Impedance Cardiography. Psychophysiology 1990, 27, 1–23. [Google Scholar] [CrossRef]
- Liu, S.-H.; Cheng, D.-C.; Su, C.-H. A Cuffless Blood Pressure Measurement Based on the Impedance Plethysmography Technique. Sensors 2017, 17, 1176. [Google Scholar] [CrossRef]
- Park, D.; Inan, O.T.; Giovangrandi, L. A combined heartbeat detector based on individual BCG and IPG heartbeat detectors. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 3428–3431. [Google Scholar] [CrossRef]
- Inan, O.T.; Park, D.; Giovangrandi, L.; Kovacs, G.T.A. Noninvasive Measurement of Physiological Signals on a Modified Home Bathroom Scale. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 59, pp. 2137–2143. [Google Scholar] [CrossRef]
- Ryan, K.; Dockray, S.; Linehan, C. A systematic review of tailored eHealth interventions for weight loss. Digit. Health 2019, 5, 1–23. [Google Scholar] [CrossRef]
- Rollo, M.E.; Burrows, T.; Vincze, L.J.; Harvey, J.; Collins, C.E.; Hutchesson, M.J. Cost evaluation of providing evidence-based dietetic services for weight management in adults: In-person versus eHealth delivery. Nutr. Diet. 2018, 75, 35–43. [Google Scholar] [CrossRef]
- Omboni, S.; Panzeri, E.; Campolo, L. E-Health in Hypertension Management: An Insight into the Current and Future Role of Blood Pressure Telemonitoring. Curr. Hypertens. Rep. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Saz-Lara, A.; Sequí-Dominguez, I.; Gómez-Guijarro, M.D.; Ruiz-Grao, M.C.; Martinez-Vizcaino, V.; Álvarez-Bueno, C. Comparative effect of eHealth interventions on hypertension management-related outcomes: A network meta-analysis. Int. J. Nurs. Stud. 2021, 124, 104085. [Google Scholar] [CrossRef]
- Harsha, D.W.; Bray, G.A. Weight loss and blood pressure control (Pro). Hypertension 2008, 51, 1420–1425. [Google Scholar] [CrossRef]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of Weight Reduction on Blood Pressure: A meta-analysis of randomized controlled trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wang, J.-J.; Tan, T.-H. A Portable and Wireless Multi-Channel Acquisition System for Physiological Signal Measurements. Sensors 2019, 19, 5314. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Liu, S.-H.; Liu, L.-J.; Pan, K.-L.; Chen, W.; Tan, T.-H. Using the Characteristics of Pulse Waveform to Enhance the Accuracy of Blood Pressure Measurement by a Multi-Dimension Regression Model. Appl. Sci. 2019, 9, 2922. [Google Scholar] [CrossRef]
- Mukkamala, R.; Yavarimanesh, M.; Natarajan, K.; Hahn, J.-O.; Kyriakoulis, K.G.; Avolio, A.P.; Stergiou, G.S. Evaluation of the Accuracy of Cuffless Blood Pressure Measurement Devices: Challenges and Proposals. Hypertension 2021, 78, 1161–1167. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.-T. Pulse transit time technique for cuffless unobtrusive blood pressure measurement: From theory to algorithm. Biomed. Eng. Lett. 2019, 9, 281–304. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef]
- Kenney, M.J.; Seals, D.R. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 1993, 22, 653–664. [Google Scholar] [CrossRef]
- IEEE Standard Association. IEEE Standard for Wearable Cuffless Blood Pressure Measuring Devices. IEEE Std. 2019, 1–38, 1708–2014. [Google Scholar] [CrossRef]
- Nyboer, J.; Kreider, M.M.; Hannapel, L. Electrical Impedance Plethysmography. Circulation 1950, 2, 811–821. [Google Scholar] [CrossRef]
- Liu, S.H. Motion Artifact Reduction in Electrocardiogram Using Adaptive Filter. J. Med. Biol. Eng. 2011, 31, 67–72. [Google Scholar] [CrossRef]
- Schlink, B.R.; Nordin, A.D.; Ferris, D.P. Comparison of Signal Processing Methods for Reducing Motion Artifacts in High-Density Electromyography During Human Locomotion. IEEE Open J. Eng. Med. Biol. 2020, 1, 156–165. [Google Scholar] [CrossRef]





| Subject (N) | SBP Max.~Min. mmHg | DBP Max.~Min. mmHg | Subject (N) | SBP Max.~Min. mmHg | DBP Max.~Min. mmHg |
|---|---|---|---|---|---|
| 01 (N = 15) | 161~129 | 107~91 | 11 (N = 19) | 172~124 | 107~85 |
| 02 (N = 17) | 148~118 | 95~69 | 12 (N = 15) | 146~105 | 88~59 |
| 03 (N = 19) | 148~101 | 88~67 | 13 (N = 14) | 140~112 | 88~76 |
| 04 (N = 17) | 133~96 | 74~53 | 14 (N = 13) | 128~98 | 88~76 |
| 05 (N = 14) | 129~96 | 60~47 | 15 (N = 13) | 137~105 | 90~80 |
| 06 (N = 13) | 154~118 | 100~80 | 16 (N = 13) | 129~82 | 94~68 |
| 07 (N = 15) | 150~126 | 99~85 | 17 (N = 12) | 147~116 | 78~70 |
| 08 (N = 14) | 173~134 | 98~84 | 18 (N = 13) | 163~120 | 106~87 |
| 09 (N = 12) | 143~113 | 79~59 | 19 (N = 15) | 133~96 | 85~74 |
| 10 (N = 15) | 133~96 | 85~74 | 20 (N = 13) | 126~84 | 87~69 |
| Subjects | Reference Method | Proposed Method | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |||||
| ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | |
| 01 | 4.7 | 0.845 | 3.2 | 0.482 | 10.7 | 0.190 | 4.5 | 0.000 |
| 02 | 9.8 | 0.023 | 8.5 | 0.010 | 9.9 | 0.001 | 8.4 | 0.027 |
| 03 | 9.3 | 0.532 | 4.9 | 0.435 | 12.4 | 0.177 | 6.1 | 0.129 |
| 04 | 11.2 | 0.272 | 7.4 | 0.216 | 19.4 | 0.482 | 6.7 | 0.355 |
| 05 | 9.6 | 0.064 | 3.3 | 0.262 | 7.8 | 0.376 | 3.7 | 0.083 |
| 06 | 8.9 | 0.284 | 7.4 | 0.000 | 10.6 | 0.000 | 7.4 | 0.012 |
| 07 | 7.0 | 0.305 | 3.9 | 0.263 | 7.9 | 0.099 | 4.3 | 0.091 |
| 08 | 8.1 | 0.550 | 4.7 | 0.074 | 12.0 | 0.002 | 4.9 | 0.001 |
| 09 | 9.0 | 0.010 | 6.3 | 0.001 | 8.6 | 0.099 | 5.8 | 0.141 |
| 10 | 8.0 | 0.558 | 5.1 | 0.034 | 11.2 | 0.130 | 5.1 | 0.022 |
| 11 | 9.3 | 0.595 | 5.1 | 0.041 | 14.5 | 0.018 | 5.0 | 0.104 |
| 12 | 4.4 | 0.875 | 4.6 | 0.343 | 10.7 | 0.258 | 4.4 | 0.389 |
| 13 | 4.1 | 0.778 | 2.9 | 0.033 | 8.6 | 0.011 | 2.9 | 0.052 |
| 14 | 7.4 | 0.448 | 3.4 | 0.008 | 6.6 | 0.561 | 3.3 | 0.079 |
| 15 | 6.1 | 0.450 | 1.7 | 0.641 | 7.3 | 0.209 | 2.8 | 0.038 |
| 16 | 5.7 | 0.864 | 6.3 | 0.439 | 13.9 | 0.202 | 8.3 | 0.016 |
| 17 | 4.0 | 0.828 | 2.8 | 0.000 | 7.5 | 0.383 | 2.8 | 0.000 |
| 18 | 6.0 | 0.790 | 5.6 | 0.628 | 13.1 | 0.000 | 9.1 | 0.007 |
| 19 | 6.6 | 0.580 | 2.6 | 0.000 | 9.9 | 0.049 | 2.6 | 0.015 |
| 20 | 7.8 | 0.545 | 5.6 | 0.166 | 11.6 | 0.012 | 6.5 | 0.060 |
| Mean ± SD | 7.3 2.1 | 0.510 0.272 | 4.8 1.8 | 0.204 0.215 | 10.2 2.2 | 0.163 0.168 | 5.2 2.0 | 0.081 0.106 |
| Subjects | Reference Method | Proposed Method | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |||||
| ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | |
| 01 | 6.0 | 0.751 | 3.5 | 0.385 | 10.7 | 0.200 | 4.5 | 0.001 |
| 02 | 9.7 | 0.044 | 8.4 | 0.031 | 9.6 | 0.057 | 8.0 | 0.127 |
| 03 | 9.1 | 0.552 | 4.9 | 0.435 | 13.0 | 0.089 | 6.5 | 0.014 |
| 04 | 9.8 | 0.441 | 6.3 | 0.433 | 5.7 | 0.807 | 3.0 | 0.871 |
| 05 | 9.1 | 0.146 | 3.4 | 0.236 | 7.2 | 0.463 | 3.5 | 0.161 |
| 06 | 8.4 | 0.363 | 7.4 | 0.009 | 10.5 | 0.009 | 7.4 | 0.004 |
| 07 | 6.9 | 0.310 | 3.9 | 0.246 | 7.8 | 0.132 | 4.4 | 0.030 |
| 08 | 7.8 | 0.578 | 4.7 | 0.085 | 12.1 | 0.000 | 4.9 | 0.001 |
| 09 | 8.0 | 0.229 | 5.4 | 0.263 | 8.5 | 0.117 | 5.9 | 0.114 |
| 10 | 6.9 | 0.669 | 5.0 | 0.060 | 11.1 | 0.138 | 5.2 | 0.015 |
| 11 | 9.4 | 0.586 | 5.2 | 0.016 | 14.0 | 0.084 | 5.0 | 0.073 |
| 12 | 5.6 | 0.800 | 4.6 | 0.318 | 11.5 | 0.137 | 3.8 | 0.531 |
| 13 | 4.3 | 0.755 | 3.0 | 0.011 | 8.4 | 0.053 | 2.9 | 0.038 |
| 14 | 7.3 | 0.452 | 3.4 | 0.010 | 7.1 | 0.489 | 3.2 | 0.117 |
| 15 | 6.0 | 0.468 | 1.9 | 0.557 | 8.1 | 0.029 | 2.8 | 0.033 |
| 16 | 4.5 | 0.915 | 5.9 | 0.511 | 13.0 | 0.301 | 8.4 | 0.000 |
| 17 | 4.1 | 0.820 | 2.8 | 0.001 | 7.3 | 0.425 | 2.8 | 0.000 |
| 18 | 5.8 | 0.800 | 6.7 | 0.459 | 11.1 | 0.275 | 8.9 | 0.050 |
| 19 | 6.9 | 0.532 | 2.6 | 0.000 | 9.7 | 0.073 | 2.6 | 0.027 |
| 20 | 7.8 | 0.546 | 5.6 | 0.178 | 11.2 | 0.079 | 5.1 | 0.318 |
| Mean ± SD | 7.2 1.8 | 0.538 0.231 | 4.7 1.7 | 0.212 0.192 | 9.9 2.2 | 0.198 0.201 | 4.9 1.9 | 0.126 0.213 |
| Subjects | Reference Method | Proposed Method | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |||||
| ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | |
| 01 | 5.9 | 0.771 | 3.6 | 0.418 | 7.4 | 0.643 | 3.8 | 0.352 |
| 02 | 7.9 | 0.402 | 7.1 | 0.355 | 7.9 | 0.406 | 6.9 | 0.398 |
| 03 | 6.5 | 0.787 | 4.9 | 0.471 | 8.5 | 0.634 | 6.7 | 0.018 |
| 04 | 7.4 | 0.705 | 5.6 | 0.583 | 4.8 | 0.873 | 3.0 | 0.883 |
| 05 | 7.3 | 0.503 | 3.1 | 0.420 | 6.4 | 0.616 | 3.5 | 0.236 |
| 06 | 8.4 | 0.421 | 7.6 | 0.034 | 8.1 | 0.463 | 7.7 | 0.021 |
| 07 | 7.0 | 0.345 | 4.0 | 0.250 | 7.9 | 0.161 | 4.6 | 0.031 |
| 08 | 5.3 | 0.824 | 4.8 | 0.115 | 5.2 | 0.829 | 5.0 | 0.029 |
| 09 | 6.0 | 0.613 | 5.5 | 0.312 | 6.0 | 0.604 | 5.8 | 0.230 |
| 10 | 6.3 | 0.749 | 5.1 | 0.103 | 7.0 | 0.686 | 5.1 | 0.107 |
| 11 | 9.5 | 0.601 | 5.1 | 0.101 | 13.9 | 0.149 | 5.1 | 0.117 |
| 12 | 5.8 | 0.800 | 4.5 | 0.401 | 9.5 | 0.461 | 3.7 | 0.610 |
| 13 | 4.4 | 0.764 | 3.0 | 0.038 | 7.3 | 0.344 | 3.0 | 0.051 |
| 14 | 7.5 | 0.483 | 3.5 | 0.015 | 7.4 | 0.489 | 3.2 | 0.181 |
| 15 | 5.1 | 0.645 | 2.0 | 0.559 | 5.3 | 0.621 | 2.2 | 0.474 |
| 16 | 4.0 | 0.941 | 5.6 | 0.598 | 6.2 | 0.857 | 3.6 | 0.835 |
| 17 | 4.3 | 0.820 | 3.0 | 0.002 | 6.2 | 0.627 | 3.0 | 0.003 |
| 18 | 5.8 | 0.825 | 4.9 | 0.744 | 10.3 | 0.443 | 9.1 | 0.110 |
| 19 | 6.2 | 0.653 | 2.6 | 0.097 | 5.0 | 0.776 | 2.7 | 0.043 |
| 20 | 3.7 | 0.908 | 2.9 | 0.802 | 5.4 | 0.803 | 2.7 | 0.823 |
| Mean ± SD | 6.2 1.5 | 0.678 0.168 | 4.4 1.5 | 0.321 0.246 | 7.3 2.1 | 0.570 0.205 | 4.5 1.8 | 0.278 0.290 |
| Subjects | Reference Method | Proposed Method | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |||||
| ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | ERMS (mmHg) | r2 | |
| 01 | 4.9 | 0.858 | 2.0 | 0.839 | 7.7 | 0.650 | 3.9 | 0.362 |
| 02 | 7.4 | 0.518 | 7.1 | 0.399 | 7.6 | 0.493 | 6.9 | 0.432 |
| 03 | 7.0 | 0.767 | 5.1 | 0.474 | 5.7 | 0.848 | 5.0 | 0.479 |
| 04 | 6.7 | 0.771 | 4.5 | 0.750 | 5.1 | 0.870 | 3.0 | 0.890 |
| 05 | 7.7 | 0.488 | 2.3 | 0.693 | 6.4 | 0.646 | 3.0 | 0.481 |
| 06 | 8.8 | 0.440 | 7.6 | 0.132 | 8.6 | 0.463 | 7.4 | 0.185 |
| 07 | 6.5 | 0.493 | 4.1 | 0.289 | 8.2 | 0.189 | 4.5 | 0.159 |
| 08 | 5.5 | 0.828 | 5.1 | 0.096 | 4.7 | 0.873 | 5.3 | 0.029 |
| 09 | 5.9 | 0.658 | 3.9 | 0.689 | 6.2 | 0.627 | 6.0 | 0.263 |
| 10 | 6.5 | 0.748 | 5.2 | 0.154 | 7.2 | 0.692 | 5.0 | 0.209 |
| 11 | 9.4 | 0.633 | 4.7 | 0.282 | 14.1 | 0.179 | 4.8 | 0.271 |
| 12 | 3.4 | 0.938 | 4.6 | 0.425 | 9.4 | 0.520 | 3.5 | 0.662 |
| 13 | 4.2 | 0.800 | 2.8 | 0.240 | 7.2 | 0.398 | 3.1 | 0.066 |
| 14 | 7.9 | 0.482 | 3.7 | 0.023 | 5.7 | 0.725 | 3.4 | 0.162 |
| 15 | 5.4 | 0.642 | 1.3 | 0.820 | 5.3 | 0.657 | 2.2 | 0.519 |
| 16 | 3.7 | 0.953 | 5.0 | 0.711 | 6.2 | 0.868 | 2.7 | 0.916 |
| 17 | 4.2 | 0.848 | 3.2 | 0.019 | 5.8 | 0.704 | 3.2 | 0.006 |
| 18 | 5.1 | 0.879 | 4.5 | 0.809 | 11 | 0.438 | 9.5 | 0.143 |
| 19 | 6.4 | 0.662 | 2.7 | 0.130 | 5.8 | 0.725 | 2.3 | 0.327 |
| 20 | 3.6 | 0.914 | 2.9 | 0.803 | 5.7 | 0.802 | 2.6 | 0.860 |
| Mean ± SD | 6.0 1.7 | 0.716 0.162 | 4.1 1.6 | 0.439 0.292 | 7.2 2.2 | 0.618 0.203 | 4.4 1.9 | 0.370 0.275 |
| PTT1ECG (ms) | PTT1BCG (ms) | PTT2ECG (ms) | PTT2BCG (ms) | |
|---|---|---|---|---|
| Mean | 164.6 | 298.9 | 227.1 | 364.1 |
| SD | 22.0 | 80.6 | 28.5 | 89.8 |
| p-value | 0.0 | 0.0 | ||
| DTECG-BCG (ms) | DTPPG-IPG (ms) | |
|---|---|---|
| Mean | 82.1 | 120.7 |
| SD | 20.3 | 40.3 |
| p-value | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Wu, Y.-R.; Chen, W.; Su, C.-H.; Chin, C.-L. Using Ballistocardiogram and Impedance Plethysmogram for Minimal Contact Measurement of Blood Pressure Based on a Body Weight-Fat Scale. Sensors 2023, 23, 2318. https://doi.org/10.3390/s23042318
Liu S-H, Wu Y-R, Chen W, Su C-H, Chin C-L. Using Ballistocardiogram and Impedance Plethysmogram for Minimal Contact Measurement of Blood Pressure Based on a Body Weight-Fat Scale. Sensors. 2023; 23(4):2318. https://doi.org/10.3390/s23042318
Chicago/Turabian StyleLiu, Shing-Hong, Yan-Rong Wu, Wenxi Chen, Chun-Hung Su, and Chiun-Li Chin. 2023. "Using Ballistocardiogram and Impedance Plethysmogram for Minimal Contact Measurement of Blood Pressure Based on a Body Weight-Fat Scale" Sensors 23, no. 4: 2318. https://doi.org/10.3390/s23042318
APA StyleLiu, S.-H., Wu, Y.-R., Chen, W., Su, C.-H., & Chin, C.-L. (2023). Using Ballistocardiogram and Impedance Plethysmogram for Minimal Contact Measurement of Blood Pressure Based on a Body Weight-Fat Scale. Sensors, 23(4), 2318. https://doi.org/10.3390/s23042318









