Portable TDLAS Sensor for Online Monitoring of CO2 and H2O Using a Miniaturized Multi-Pass Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. TDLAS-WMS Theoretical Principle
2.2. Selection of CO2 and H2O Absorption Lines
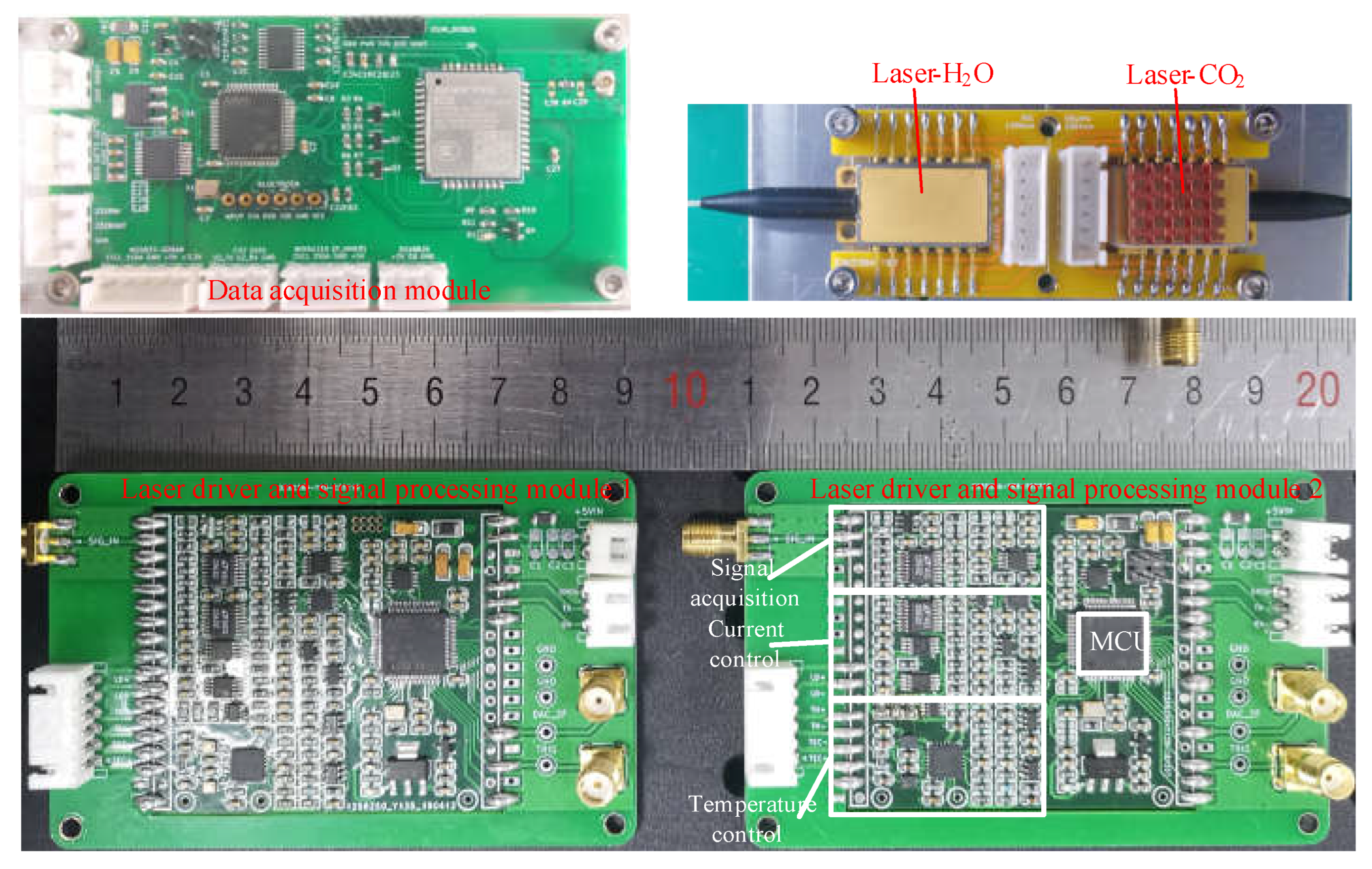
2.3. Experimental Setup
3. Results and Discussion
3.1. Calibration and Measurement Precision
3.2. System Response Time
3.3. Comparison and Field Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joos, F.; Spahni, R. Rates of change in natural and anthropogenic radiative forcing over the past 20,000 years. Proc. Natl. Acad. Sci. USA 2008, 105, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, P.D. Temporal Scaling of Carbon Emission and Accumulation Rates: Modern Anthropogenic Emissions Compared to Estimates of PETM Onset Accumulation. Paleoceanogr. Paleocl. 2019, 34, 329–335. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Huang, G.L.; Guizzardi, D.; Koffi, E.; Muntean, M.; Schieberle, C.; Friedrich, R.; Janssens-Maenhout, G. High resolution temporal profiles in the Emissions Database for Global Atmospheric Research. Sci. Data 2020, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhang, Y.; Ma, Q.L.; Yu, D.J.; Chen, X.J.; Cohen, J.B. Dramatic decline of observed atmospheric CO2 and CH4 during the COVID-19 lockdown over the Yangtze River Delta of China. J. Environ. Sci. 2023, 124, 712–721. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, G.B.H.; Doyle, B.; Fiebrich, C.A.; Schvartzman, D. Low-complexity methods to mitigate the impact of environmental variables on low-cost UAS-based atmospheric carbon dioxide measurements. Atoms. Meas. Technol. 2022, 15, 5599–5618. [Google Scholar] [CrossRef]
- Ng, D.K.T.; Xu, L.; Chen, W.; Wang, H.; Gu, Z.; Chia, X.X.; Fu, Y.H.; Jaafar, N.; Ho, C.P.; Zhang, T.; et al. Miniaturized CO2 Gas Sensor Using 20% ScAlN-Based Pyroelectric Detector. ACS Sens. 2022, 7, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Peng, B.; Zhu, X.Y.; Guo, Y.C. Multi-Gas Detection System Based on Non-Dispersive Infrared (NDIR) Spectral Technology. Sensors 2022, 22, 836. [Google Scholar] [CrossRef]
- Gomez-Pelaez, A.J.; Ramos, R.; Cuevas, E.; Gomez-Trueba, V.; Reyes, E. Atmospheric CO2, CH4, and CO with the CRDS technique at the Izana Global GAW station: Instrumental tests, developments, and first measurement results. Atoms. Meas. Technol. 2019, 12, 2043–2066. [Google Scholar] [CrossRef]
- Hendry, M.J.; Richman, B.; Wassenaar, L.I. Correcting for Methane Interferences on delta H-2 and delta O-18 Measurements in Pore Water Using H2O(liquid)-H2O(vapor) Equilibration Laser Spectroscopy. Anal. Chem. 2011, 83, 5789–5796. [Google Scholar] [CrossRef]
- Wang, J.J.; Tian, X.; Dong, Y.; Zhu, G.D.; Chen, J.J.; Tan, T.; Liu, K.; Chen, W.D.; Gao, X.M. Enhancing off-axis integrated cavity output spectroscopy (OA-ICOS) with radio frequency white noise for gas sensing. Opt. Express 2019, 27, 30517–30529. [Google Scholar] [CrossRef]
- Wang, K.Y.; Shao, L.G.; Chen, J.J.; Wang, G.S.; Liu, K.; Tan, T.; Mei, J.X.; Chen, W.D.; Gao, X.M. A Dual-Laser Sensor Based on Off-Axis Integrated Cavity Output Spectroscopy and Time-Division Multiplexing Method. Sensors 2020, 20, 6192. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Zheng, C.T.; Li, J.H.; Ma, N.N.; Liu, Z.D.; Zhang, Y.; Wang, Y.D.; Tittel, F.K. Near-infrared methane sensor system using off-axis integrated cavity output spectroscopy with novel dual-input dual-output coupling scheme for mode noise suppression. Sens. Actuators B 2020, 308, 127674. [Google Scholar] [CrossRef]
- Zazzeri, G.; Lowry, D.; Fisher, R.E.; France, J.L.; Lanoiselle, M.; Nisbet, E.G. Plume mapping and isotopic characterisation of anthropogenic methane sources. Atmos. Environ. 2015, 110, 151–162. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, M.; Guo, J.J.; Du, B.L.; Zheng, R.E. A Portable Tunable Diode Laser Absorption Spectroscopy System for Dissolved CO2 Detection Using a High-Efficiency Headspace Equilibrator. Sensors 2021, 21, 1723. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Dong, L.; Wu, H.; Ma, W.; Xiao, L.; Jia, S.; Chen, W.; Tittel, F.K. Three-Dimensional Printed Miniature Fiber-Coupled Multipass Cells with Dense Spot Patterns for ppb-Level Methane Detection Using a Near-IR Diode Laser. Anal. Chem. 2020, 92, 13034–13041. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.T.; Sima, C.; Xiao, Y.F.; Wang, X.F.; Ai, Y.; Li, T.L.; Lu, P.; Liu, D.M. Modified laser scanning technique in wavelength modulation spectroscopy for advanced TDLAS gas sensing. Opt. Lasers Eng. 2022, 151, 106906. [Google Scholar] [CrossRef]
- Wang, Q.J.; Sun, P.S.; Zhang, Z.R.; Zhang, L.W.; Pang, T.; Wu, B.; Xia, H.; Guo, Q.; Sigrist, M.W.; Culshaw, B. Multi-component gas measurement aliasing spectral demodulation method for interference separation in laser absorption spectroscopy. Sens. Actuators B 2022, 369, 132292. [Google Scholar] [CrossRef]
- Du, B.L.; Li, M.; Guo, J.J.; Zhang, Z.H.; Ye, W.Q.; Zheng, R.E. The Experimental Research on In-Situ Detection for Dissolved CO2 in Seawater Based on Tunable Diode Laser Absorption Spectroscopy. Spectrosc. Spectral Anal. 2022, 42, 1264–1269. [Google Scholar] [CrossRef]
- Shao, L.G.; Chen, J.J.; Wang, K.Y.; Mei, J.X.; Tan, T.; Wang, G.S.; Liu, K.; Gao, X.M. Highly precise measurement of atmospheric N2O and CO using improved White cell and RF current perturbation. Sens. Actuators B 2022, 352, 130995. [Google Scholar] [CrossRef]
- Shao, L.G.; Mei, J.X.; Chen, J.J.; Tan, T.; Wang, G.S.; Liu, K.; Gao, X.M. Simultaneous Sensitive Determination of delta C-13, delta O-18, and delta O-17 in Human Breath CO2 Based on ICL Direct Absorption Spectroscopy. Sensors 2022, 22, 1527. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Sun, P.S.; Pang, T.; Xia, H.; Cui, X.J.; Li, Z.; Han, L.; Wu, B.; Wang, Y.; Sigrist, M.W.; et al. Reconstruction of combustion temperature and gas concentration distributions using line-of-sight tunable diode laser absorption spectroscopy. Opt. Eng. 2016, 55, 076107. [Google Scholar] [CrossRef]
- Avetisov, V.; Bjoroey, O.; Wang, J.Y.; Geiser, P.; Paulsen, K.G. Hydrogen Sensor Based on Tunable Diode Laser Absorption Spectroscopy. Sensors 2019, 19, 5313. [Google Scholar] [CrossRef]
- Tang, Q.X.; Zhang, Y.J.; Chen, D.; Zhang, K.; He, Y.; You, K.; Liu, G.H.; Lu, Y.B.; Fan, B.Q.; Yu, D.Q. Laser absorption spectroscopy data processing method based on co-frequency and dual-wavelength and its application. Opt. Express 2018, 26, 4459–4469. [Google Scholar] [CrossRef]
- Gu, M.; Chen, J.; Mei, J.; Tan, T.; Wang, G.; Liu, K.; Liu, G.; Gao, X. Open-path anti-pollution multi-pass cell-based TDLAS sensor for the online measurement of atmospheric H2O and CO2 fluxes. Opt. Express 2022, 30, 43961–43972. [Google Scholar] [CrossRef]
- Pogany, A.; Wagner, S.; Werhahn, O.; Ebert, V. Development and Metrological Characterization of a Tunable Diode Laser Absorption Spectroscopy (TDLAS) Spectrometer for Simultaneous Absolute Measurement of Carbon Dioxide and Water Vapor. Appl. Spectrosc. 2015, 69, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.X.; Guo, J.J.; Sun, J.Y.; Li, J.; Zhao, C.F.; Liu, Z.S. Research on atmospheric CO2 remote sensing with open-path tunable diode laser absorption spectroscopy and comparison methods. Opt. Eng. 2017, 56, 066113. [Google Scholar] [CrossRef]
- Luo, L.Z.; Li, T.; Deng, J.G.; Zhao, R.Z.; Wang, J.K. An Improved WMS-2f/1f Spectral Fitting Method Using Orthogonal Test in Initial Parameters Selection. Sensors 2022, 22, 7430. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; He, Q.X.; Li, J.K.; Feng, Q.B. Oxygen detection system based on TDLAS-WMS and a compact multipass gas cell. Microw. Opt. Technol. Lett. 2022. early view. [Google Scholar] [CrossRef]
- Feng, S.L.; Qiu, X.B.; Guo, G.Q.; Zhang, E.H.; He, Q.S.; He, X.H.; Ma, W.G.; Fittschen, C.; Li, C.L. Palm-Sized Laser Spectrometer with High Robustness and Sensitivity for Trace Gas Detection Using a Novel Double-Layer Toroidal Cell. Anal. Chem. 2021, 93, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Tuzson, B.; Mangold, M.; Looser, H.; Manninen, A.; Emmenegger, L. Compact multipass optical cell for laser spectroscopy. Opt. Lett. 2013, 38, 257–259. [Google Scholar] [CrossRef] [PubMed]
- He, Q.X.; Zheng, C.T.; Liu, H.F.; Li, B.; Wang, Y.D.; Tittel, F.K. A near-infrared acetylene detection system based on a 1.534 mu m tunable diode laser and a miniature gas chamber. Infrared. Phys. Techn. 2016, 75, 93–99. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Pang, T.; Yang, Y.; Xia, H.; Cui, X.J.; Sun, P.S.; Wu, B.; Wang, Y.; Sigrist, M.W.; Dong, F.Z. Development of a tunable diode laser absorption sensor for online monitoring of industrial gas total emissions based on optical scintillation cross-correlation technique. Opt. Express 2016, 24, A943-55. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Mei, J.X.; Tian, X.; Liu, K.; Tan, T.; Chen, W.D.; Gao, X.M. Laser frequency locking and intensity normalization in wavelength modulation spectroscopy for sensitive gas sensing. Opt. Express 2019, 27, 4878–4885. [Google Scholar] [CrossRef] [PubMed]
- Herriott, D.; Kompfner, R.; Kogelnik, H. Off-Axis Paths in Spherical Mirror Interferometers. Appl. Opt. 1964, 3, 523–526. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, M.; Chen, J.; Zhang, Y.; Tan, T.; Wang, G.; Liu, K.; Gao, X.; Mei, J. Portable TDLAS Sensor for Online Monitoring of CO2 and H2O Using a Miniaturized Multi-Pass Cell. Sensors 2023, 23, 2072. https://doi.org/10.3390/s23042072
Gu M, Chen J, Zhang Y, Tan T, Wang G, Liu K, Gao X, Mei J. Portable TDLAS Sensor for Online Monitoring of CO2 and H2O Using a Miniaturized Multi-Pass Cell. Sensors. 2023; 23(4):2072. https://doi.org/10.3390/s23042072
Chicago/Turabian StyleGu, Mingsi, Jiajin Chen, Yiping Zhang, Tu Tan, Guishi Wang, Kun Liu, Xiaoming Gao, and Jiaoxu Mei. 2023. "Portable TDLAS Sensor for Online Monitoring of CO2 and H2O Using a Miniaturized Multi-Pass Cell" Sensors 23, no. 4: 2072. https://doi.org/10.3390/s23042072
APA StyleGu, M., Chen, J., Zhang, Y., Tan, T., Wang, G., Liu, K., Gao, X., & Mei, J. (2023). Portable TDLAS Sensor for Online Monitoring of CO2 and H2O Using a Miniaturized Multi-Pass Cell. Sensors, 23(4), 2072. https://doi.org/10.3390/s23042072





