Nanosatellite Payload for Research on Seed Germination in a 3D Printed Micropot
Abstract
:1. Introduction
Growing Plants in Space
2. Materials and Methods
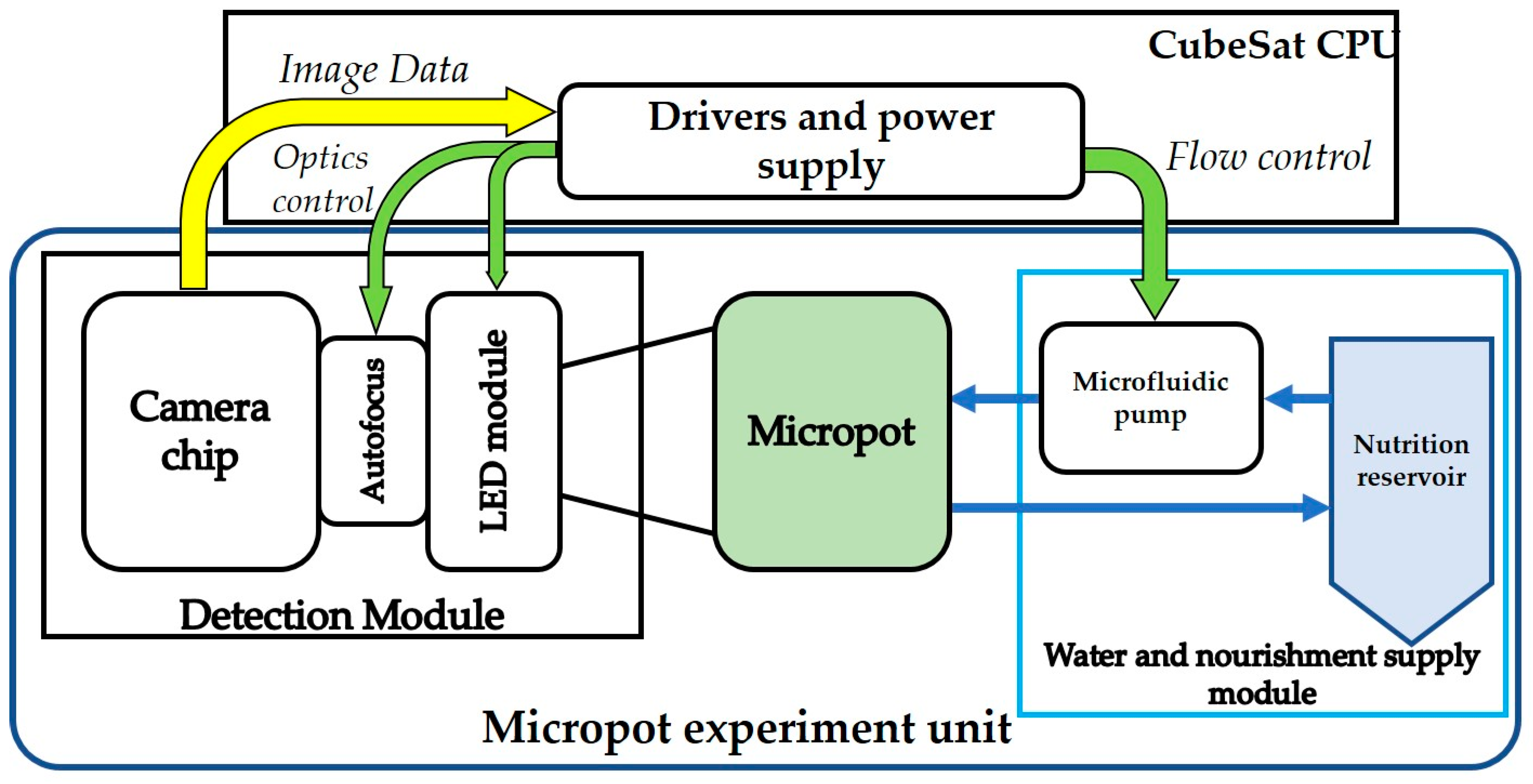
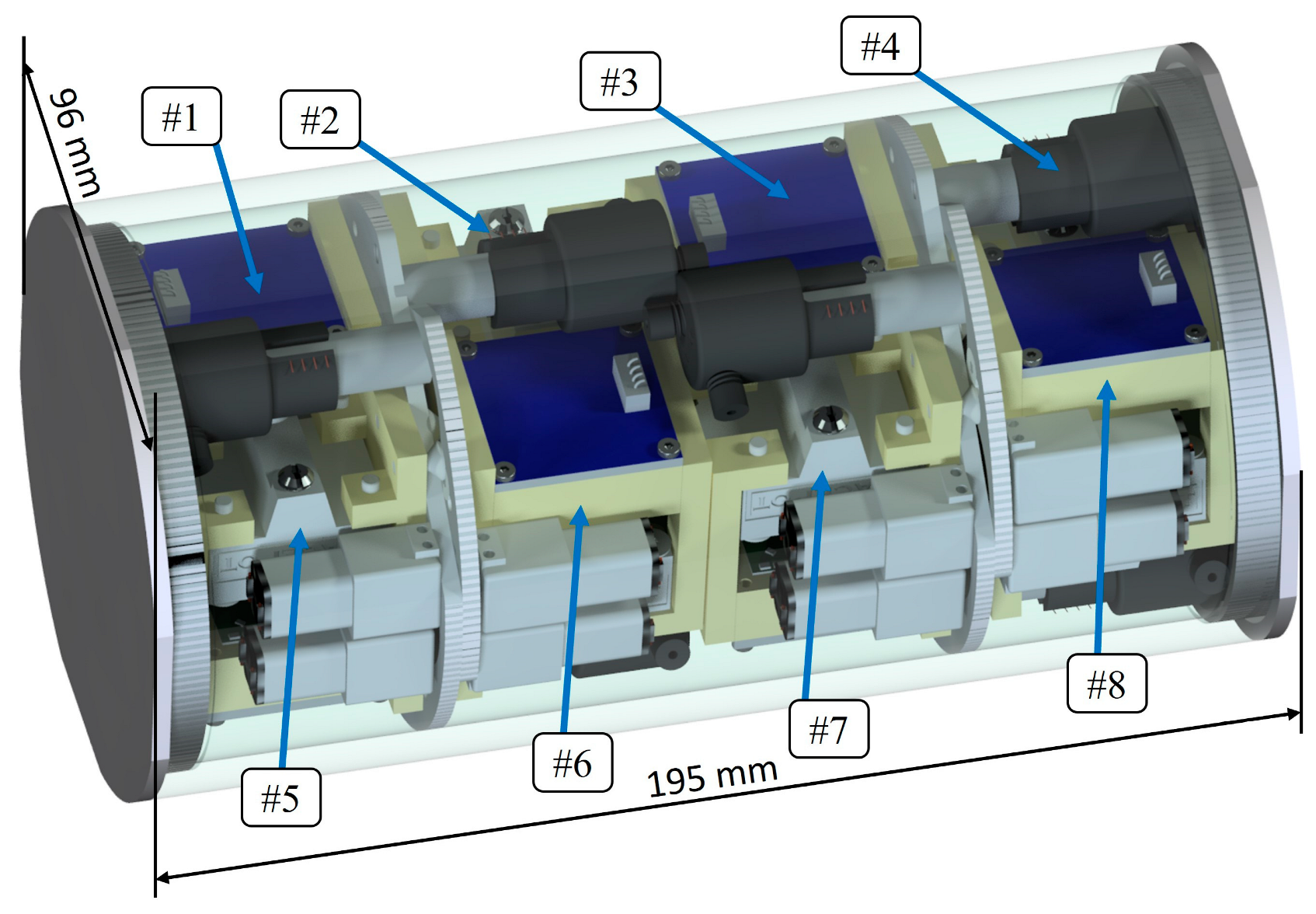
2.1. The Nanosatellite Payload
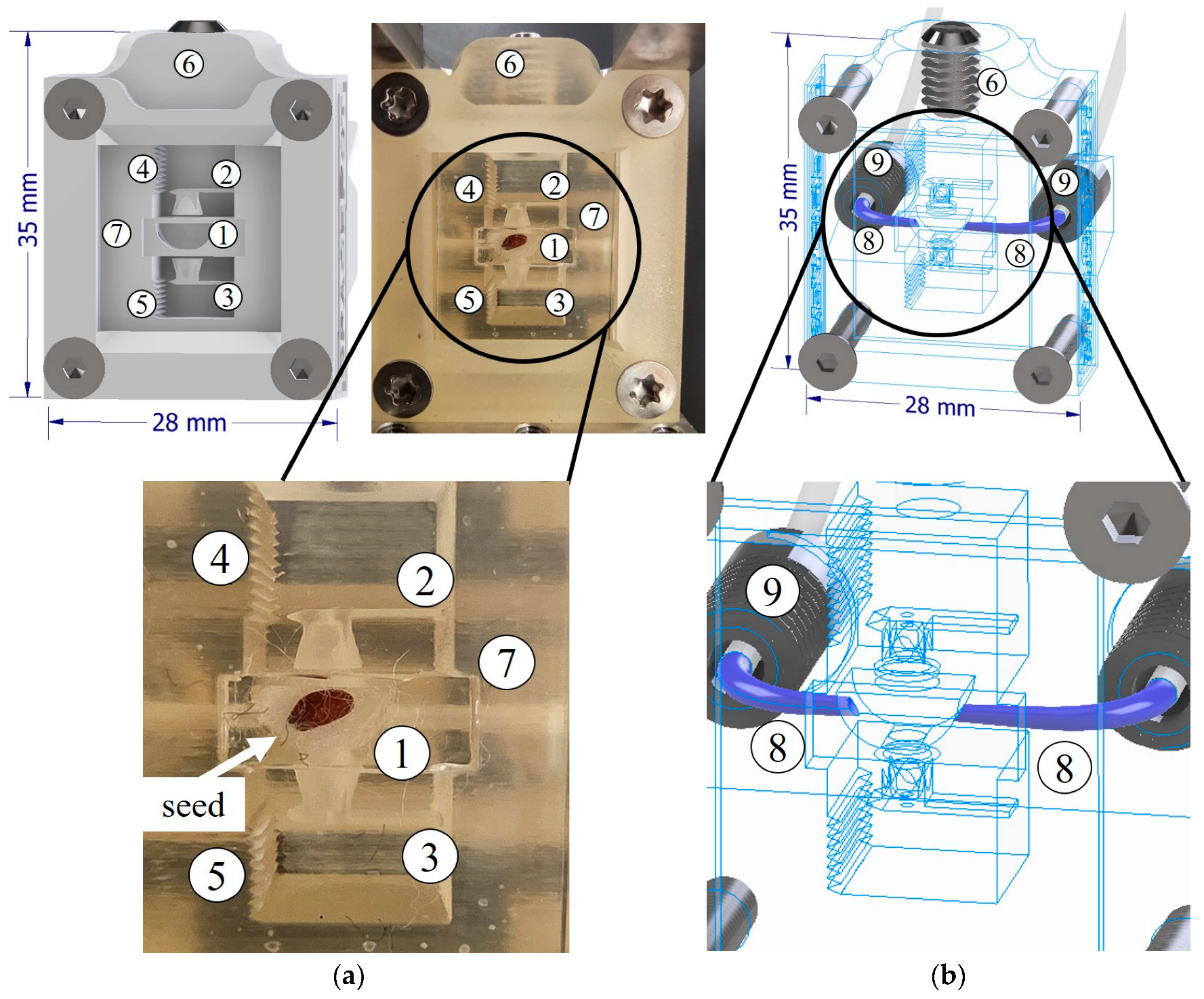
2.2. Micropot Construction and Fabrication
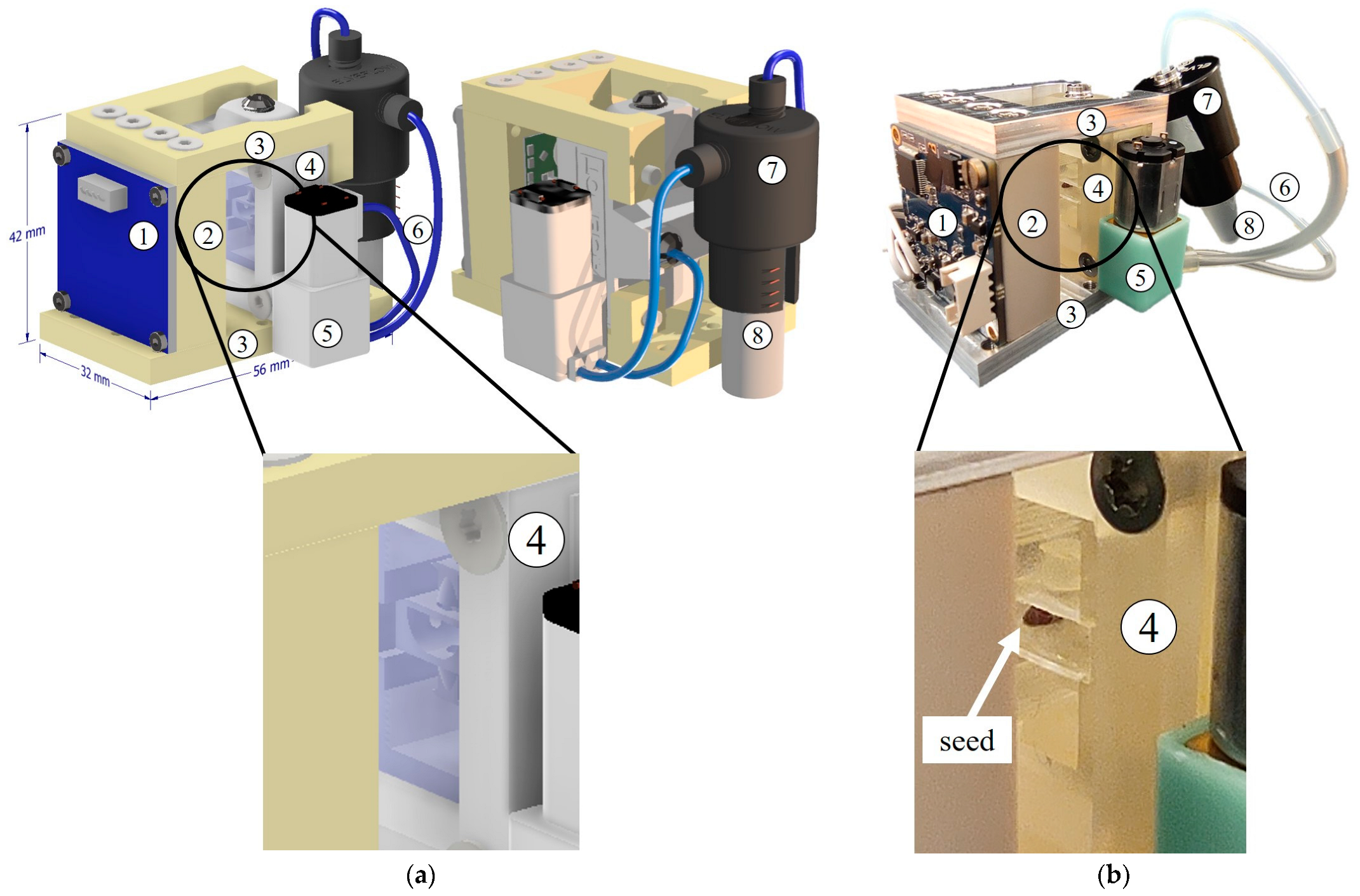
2.3. Monitoring and Nourishment Modules
2.3.1. Optical Monitoring Module
2.3.2. Nourishment Module
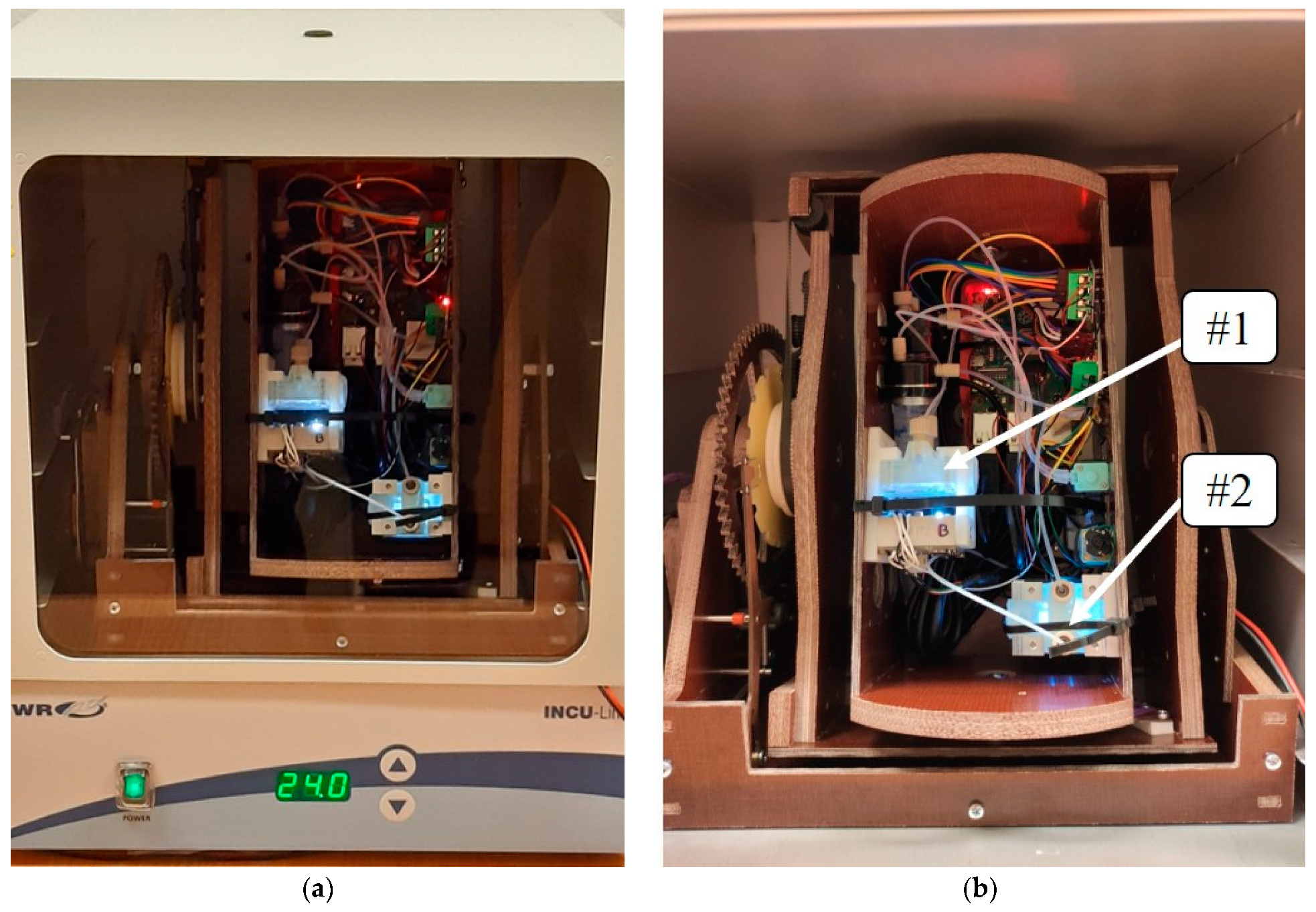
2.4. Microgravity Simulation
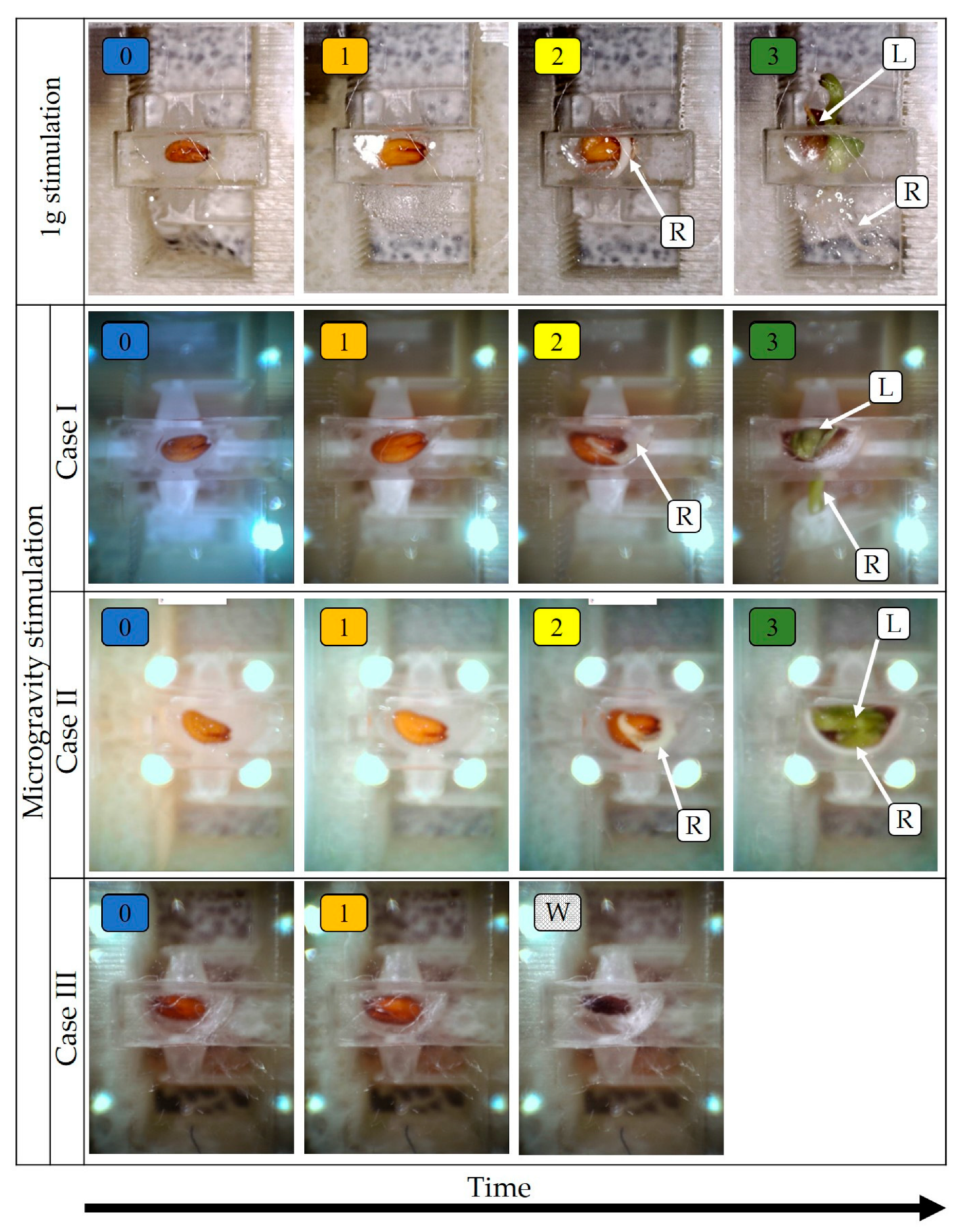
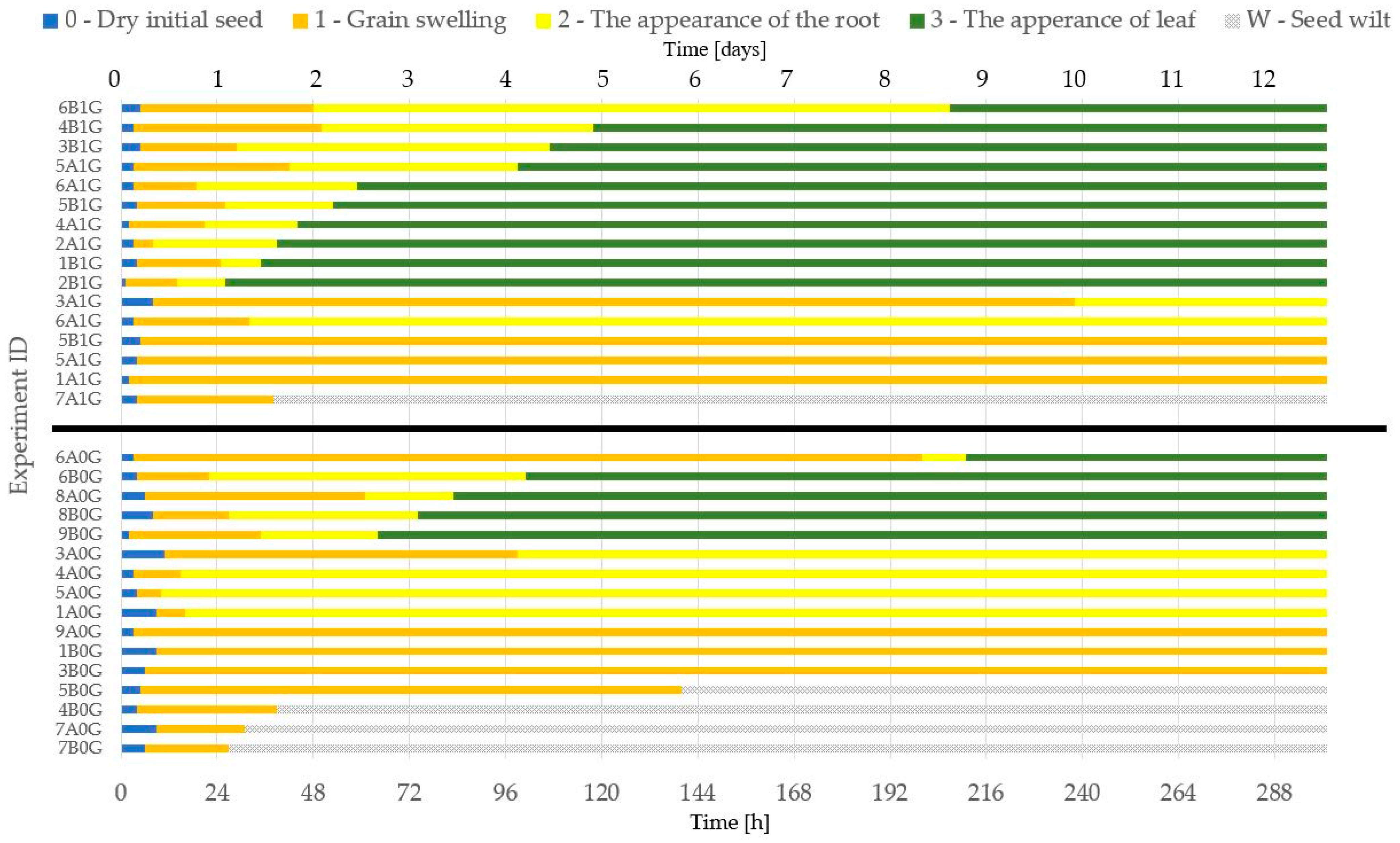
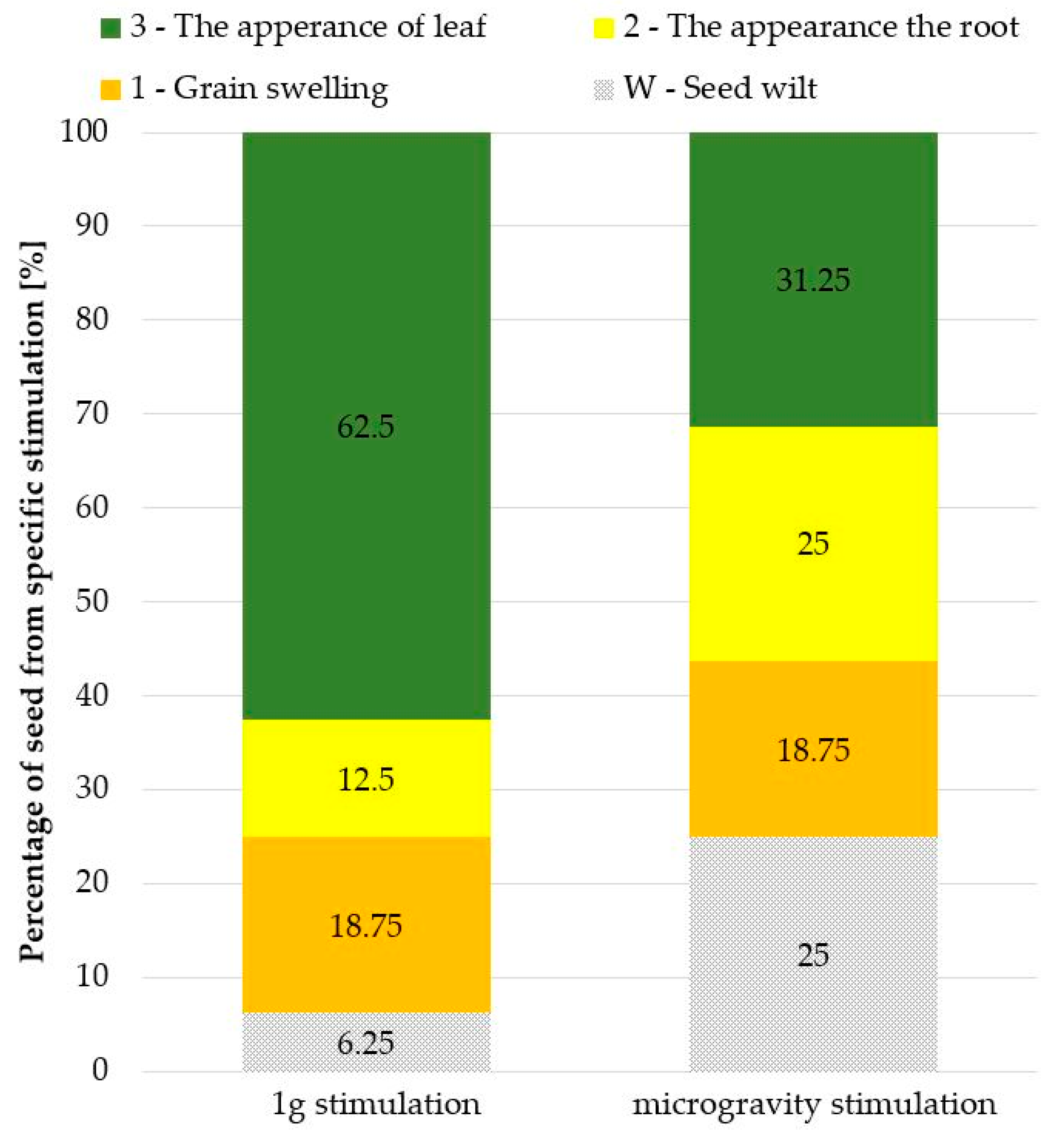
3. Results and Discussion
Microgravity and Reference Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koudelka, O.; Kusching, R.; Wenger, M.; Roamno, P. Nanosatellite missions—The future. PTA Proc. 2017, 5, 63–71. [Google Scholar]
- Camps, A. Nanosatellites and applications to commercial and scientific missions. In Satellites Missions and Technologies for Geosciences; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef]
- Hawkins, E.; Kanapskyte, A.; Santa Maria, S. Developing technologies for biological experiments in deep space. Proceedings 2020, 60, 28. [Google Scholar] [CrossRef]
- Capeeletti, C.; Batiiistini, S.; Malphrus, B. CubeSat Handbook. Academic Press: Cambridge, MA, USA, 2021; ISBN 978-0-12-817884-3. [Google Scholar]
- Ehrenfeud, O.; Ricco, A.J.; Squires, D.; Kitts, C.; Agasid, E.; Bramall, N.; Bryson, K.; Chittenden, J.; Conley, C.; Cook, A.; et al. The O/OREOS missions—Astrobiology in low Erath orbit. Acta Astronaut. 2014, 93, 501–508. [Google Scholar] [CrossRef]
- Ricco, A.; Maria, S.S.; Hanel, R. BioSentinel: A 6U nanosatellite for deep-space biological science. IEEE Aerosp. Electron. Syst. Mag. 2020, 35, 6–18. [Google Scholar] [CrossRef]
- Tieze, S.; Liddell, L.; Maria, S.S.; Bhattacharya, S. BioSentinel: A biological CubeSat for deep space exploration. Astrobiology 2020, 20, 8. [Google Scholar] [CrossRef]
- Zabel, P.; Bamsey, M.; Schubert, D.; Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016, 10, 1–16. [Google Scholar] [CrossRef]
- Bohmer, M.; Schleiff, E. Microgravity research in plants. EMBO Rep. 2019, 20, e48541. [Google Scholar] [CrossRef] [PubMed]
- Kis, J.; Wolverto, C.; Wyatt, S.; Hasenstein, K.; van Loon, J. Comparison of microgravity analogs to spaceflight in studies of plant growth and development. Front. Plant Sci. 2019, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, F.; Nedunhceran, A. Analog and simulated microgravity platforms for life sciences research: Their individual capacities, benefits and limitations. Adv. Space Res. 2022, 69, 2921–2929. [Google Scholar] [CrossRef]
- Walczak, R.; Kawa, B.; Adamski, K. Inkjet 3D printed microfluidic device for growing seed root and stalk mechanical characterization. Sens. Actuators A Phys. 2019, 297, 111557. [Google Scholar] [CrossRef]
- Sathasivam, M.; Hosamani, R.; Swamy, B.K.; Kumaran G, S. Plant response to real and simulated microgravity. Life Sci. Space Res. 2021, 28, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Teoh, C.C.; Zulkifi, N.A.; Ong, K.K.; Norliza, A.B.; Rauf, U.F.A.; Wan Yunus, W.M.Z. Influence of clinorotation on total grain yield per plant of MR 219 rice seed. Mater. Proc. 2019, 19, 1446–1450. [Google Scholar] [CrossRef]
- Lüttge, U. Progress in Botany Volume 83; Springer: Berlin/Heidelberg, Germany, 2023; Volume 83, ISBN 978-3-031-12781-6. [Google Scholar]
- Prasad, B.; Richter, P.; Vadakedath, N.; Haag, F.; Strauch, S.; Mancinelli, R.; Lebert, M. How the space environment influences organisms: An astrobiological perspective and review. Int. J. Astrobiol. 2021, 20, 159–177. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Tallinen, S.; Kim, Y.J.; Kim, S.; Esterhuizen, M. Ageing affects microplastic toxicity over time: Effects of aged polycarbonate on germination, growth, and oxidative stress of Lepidium sativum. Sci. Total Environ. 2021, 790, 148166. [Google Scholar] [CrossRef] [PubMed]
- Kordyum, E.L. Plant cell gravisensitivity and adaptation to microgravity. Plant Biol. 2014, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Poghosyan, A.; Golkar, A. CubeSat evolution: Analyzing CubeSat capabilities for conducting science mission. Prog. Aerosp. Sci. 2017, 88, 59–83. [Google Scholar] [CrossRef]
- Cal Poly CubeSat Laboratory. CubeSat Design Specification (CDS) Revision 14; Cal Poly CubeSat Laboratory: San Luis Obispo, CA, USA, 2020. [Google Scholar]
- SpaceX. Falcon User’s Guide; SpaceX: Hawthorne, CA, USA, 2021. [Google Scholar]
- Mitchell, A.; Lafont, U.; Hołyńska, M.; Semprimoschnig, C. Additive manufacturing—A review of 4D printing and future applications. Addit. Manuf. 2018, 24, 606–626. [Google Scholar] [CrossRef]
- Walczak, R. Inkjet 3D printing—Towards new micromachining tool for MEMS fabrication. Bull. Pol. Acad. Sci. Tech. Sci. 2018, 66, 179–186. [Google Scholar]
- Walczak, R.; Adamski, K.; Kubicki, W. Inkjet 3D printed chip for gel electrophoresis. Sens. Actuators B Chem. 2018, 261, 474–480. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawa, B.; Śniadek, P.; Walczak, R.; Dziuban, J. Nanosatellite Payload for Research on Seed Germination in a 3D Printed Micropot. Sensors 2023, 23, 1974. https://doi.org/10.3390/s23041974
Kawa B, Śniadek P, Walczak R, Dziuban J. Nanosatellite Payload for Research on Seed Germination in a 3D Printed Micropot. Sensors. 2023; 23(4):1974. https://doi.org/10.3390/s23041974
Chicago/Turabian StyleKawa, Bartosz, Patrycja Śniadek, Rafał Walczak, and Jan Dziuban. 2023. "Nanosatellite Payload for Research on Seed Germination in a 3D Printed Micropot" Sensors 23, no. 4: 1974. https://doi.org/10.3390/s23041974
APA StyleKawa, B., Śniadek, P., Walczak, R., & Dziuban, J. (2023). Nanosatellite Payload for Research on Seed Germination in a 3D Printed Micropot. Sensors, 23(4), 1974. https://doi.org/10.3390/s23041974





