Elbow Joint Stiffness Functional Scales Based on Hill’s Muscle Model and Genetic Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
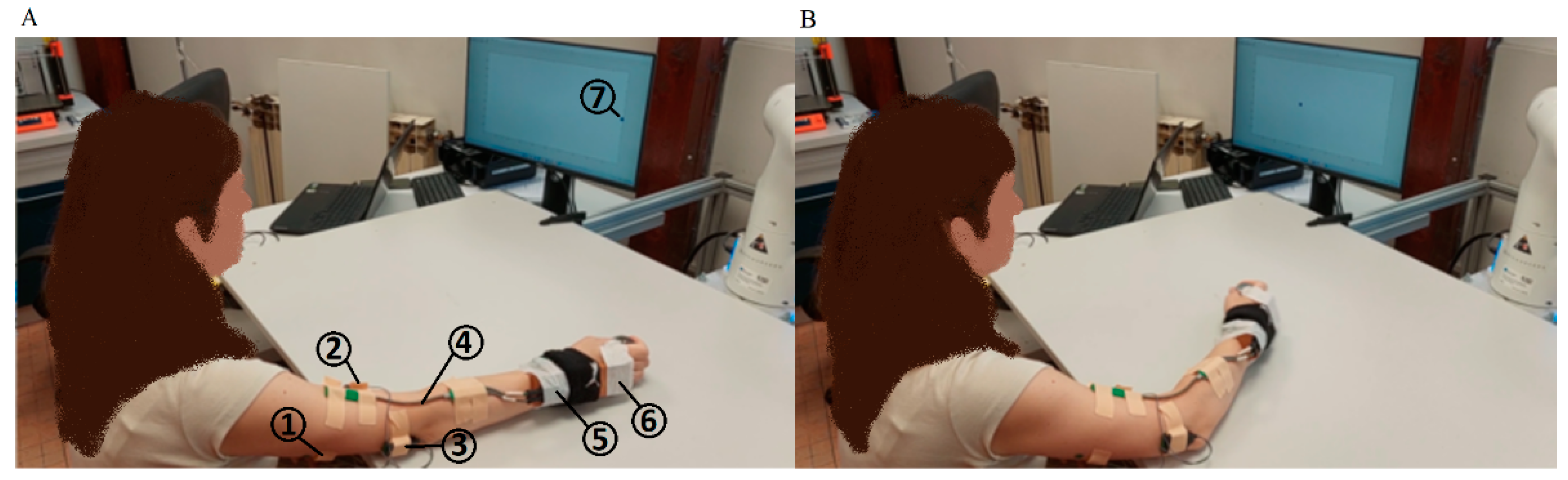
2.2. Experiment Description
- Maximum voluntary contraction (MVC) data acquisition for the triceps brachii long head and biceps brachii long head muscle against static resistance [33,34,35,36]. The recorded values of the MVC were used in all experiments for normalization of the recorded EMG signals (see Section 2.3).
- Training phase. In the beginning, the participant’s arm was in the initial position (Figure 1A). The participant repeated the WMFT8m rotation (while pulling the 0.5 kg weight) of the elbow joint following the tempo of the “moving dot” shown on the feedback display. The tempo of the “moving dot” was set to 15 beats per minute (bpm), 30 bpm, 45 bpm, and 60 bpm and it was changed for each 10 WMFT8m movement, respectively. This phase was used for estimating the parameters of Hill’s model (see Section 2.4.1) by GA, as described in Section 2.4.3.
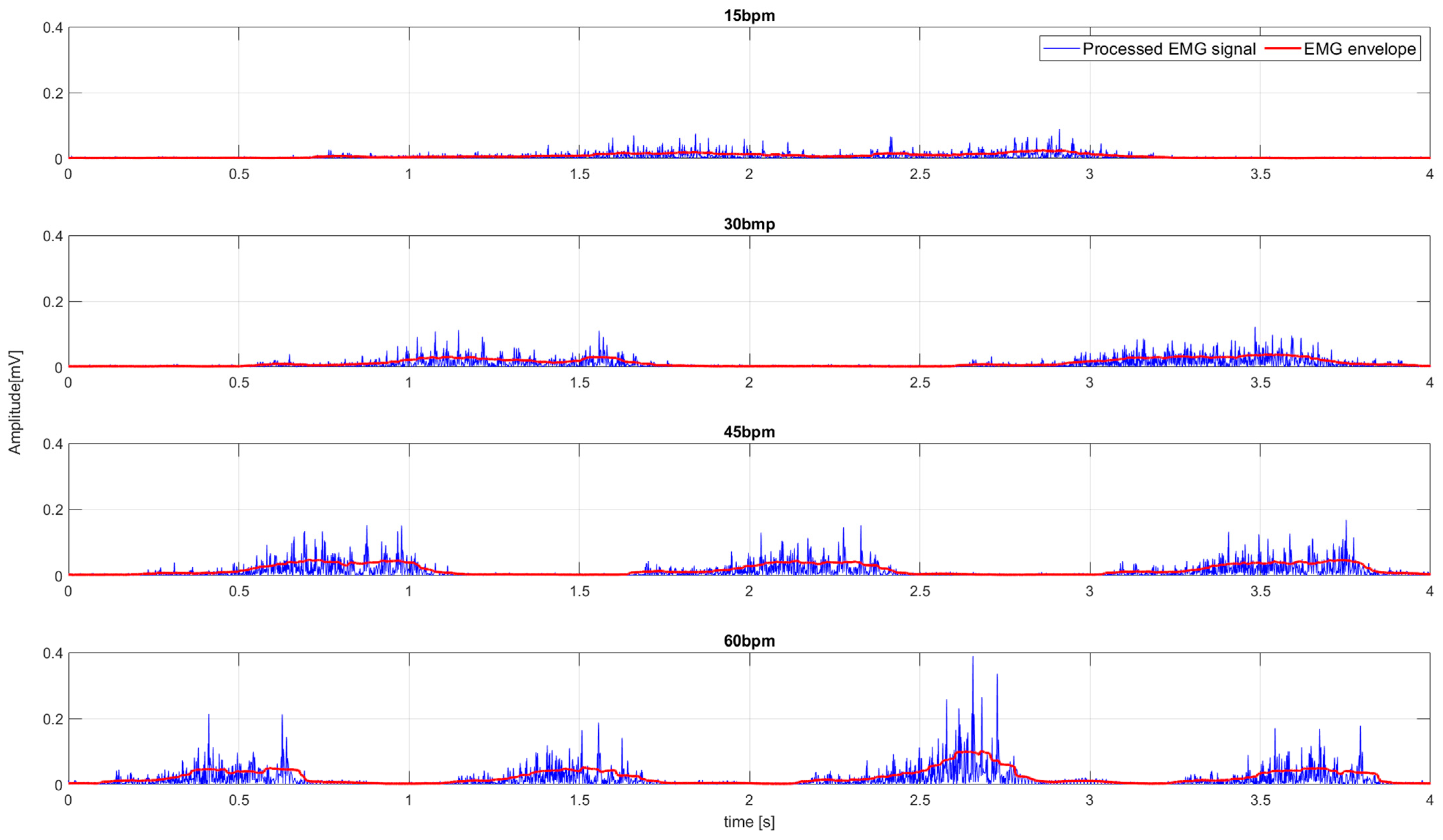
- Task frequency variation experiment (E1). In the beginning, the participant’s arm was in the initial position (Figure 1A). The participant repeated the WMFT8m rotation (while pulling the 0.5 kg weight) of the elbow joint following the tempo of the “moving dot” shown on the feedback display. The tempo of the “moving dot” was set to 15 bpm, 30 bmp, 45 bmp, and 60 bmp. The participant repeated the WMFT8m movement for each tempo 10 times in three series. The resting pause between series was 1 min. The resting pause between different tempos was 5 min.
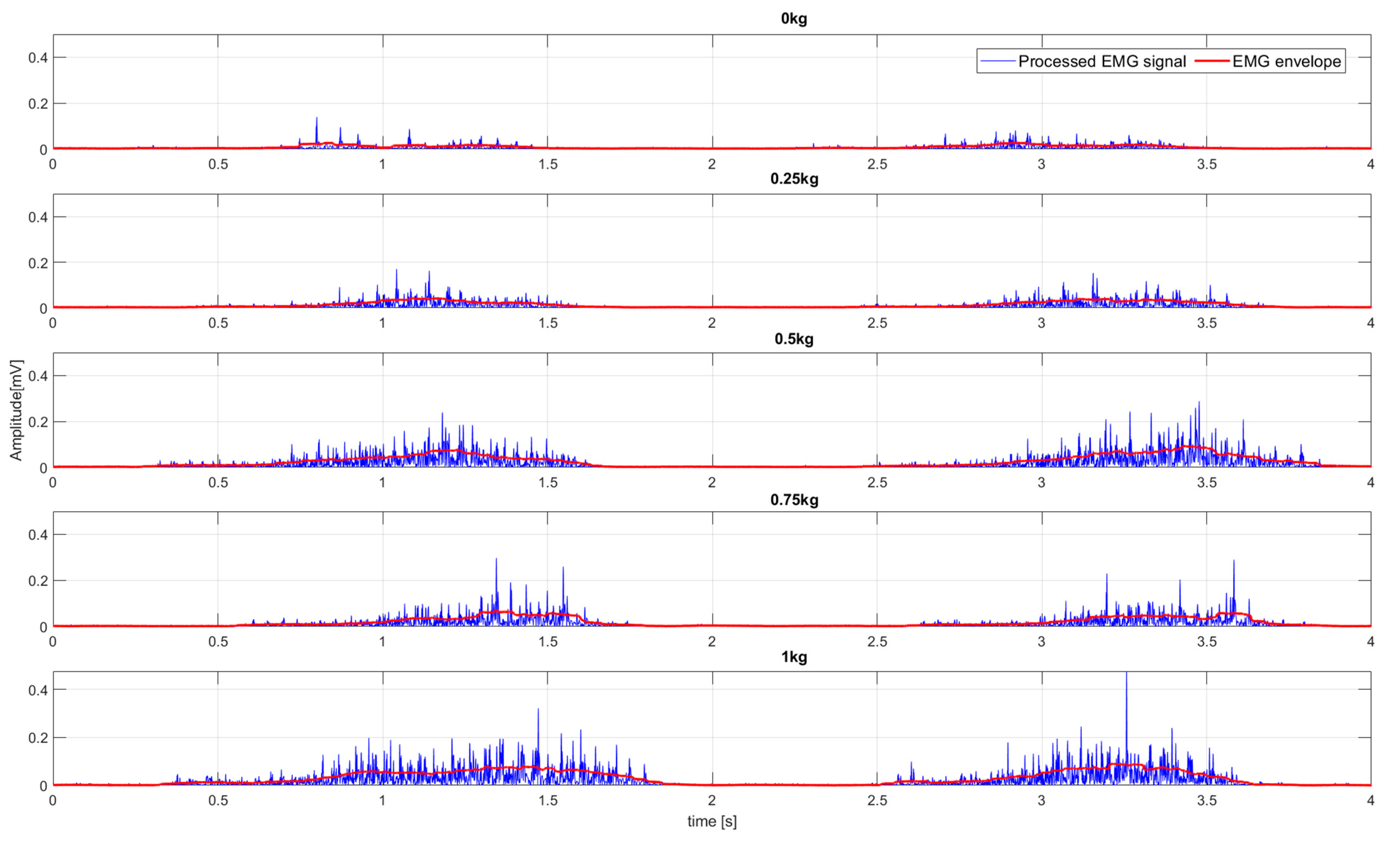
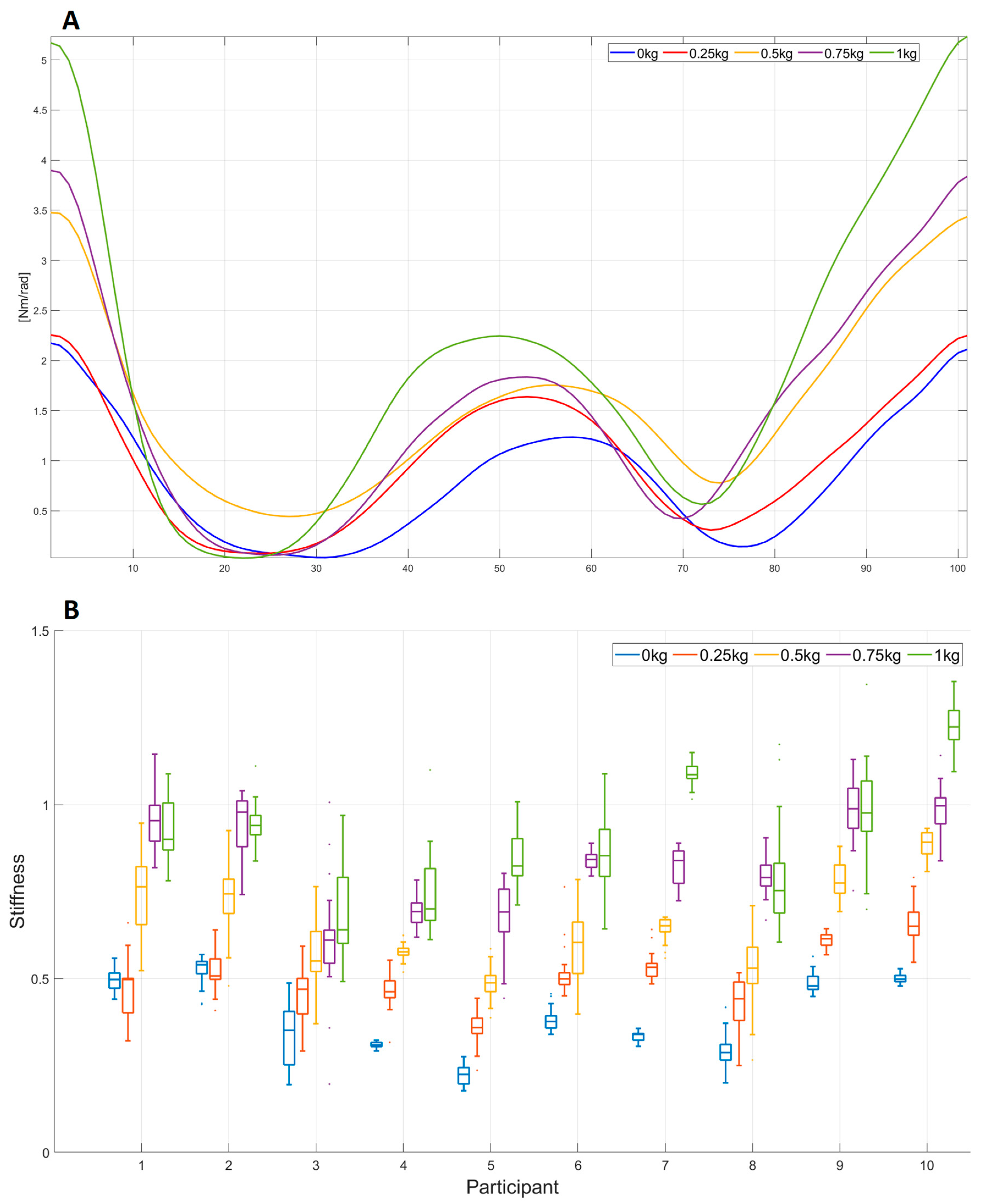
- Load variation experiment (E2). In the beginning, the participant’s arm was in the initial position (Figure 1A). The participant repeated the WMFT8m rotation of the elbow joint following the tempo of the “moving dot” shown on the feedback display. The tempo of the “moving dot” was set to 30 bmp. The participant repeated the same WMFT8m movement with different “pulling” weights: 0.25, 0.5, 0.75, and 1 kg. The participant repeated the WMFT8m movement for each weight 10 times in three series. The resting pause between series was 1 min. The resting pause between different weights was 5 min.
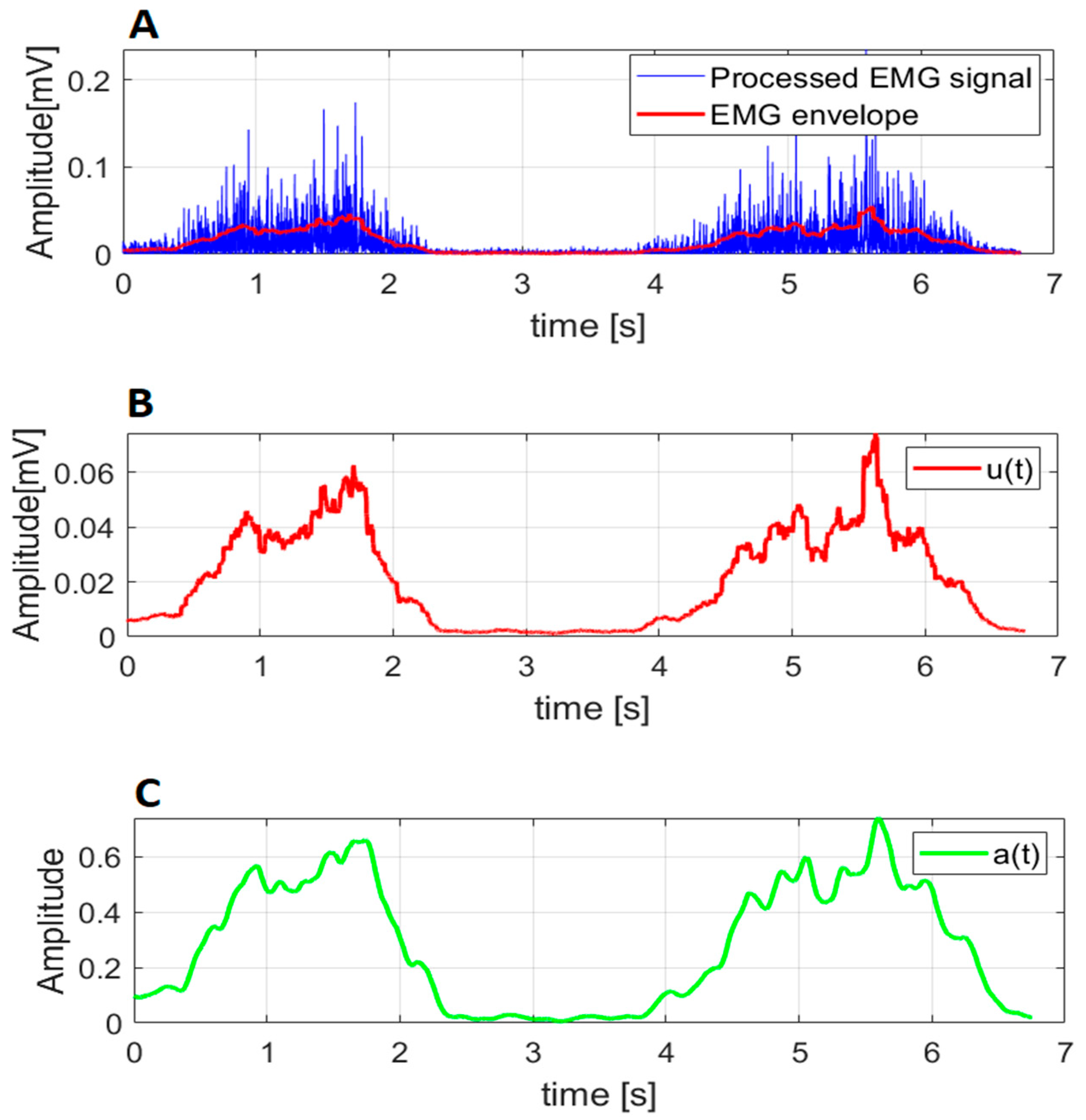
2.3. EMG Processing and Muscle Activation Dynamics
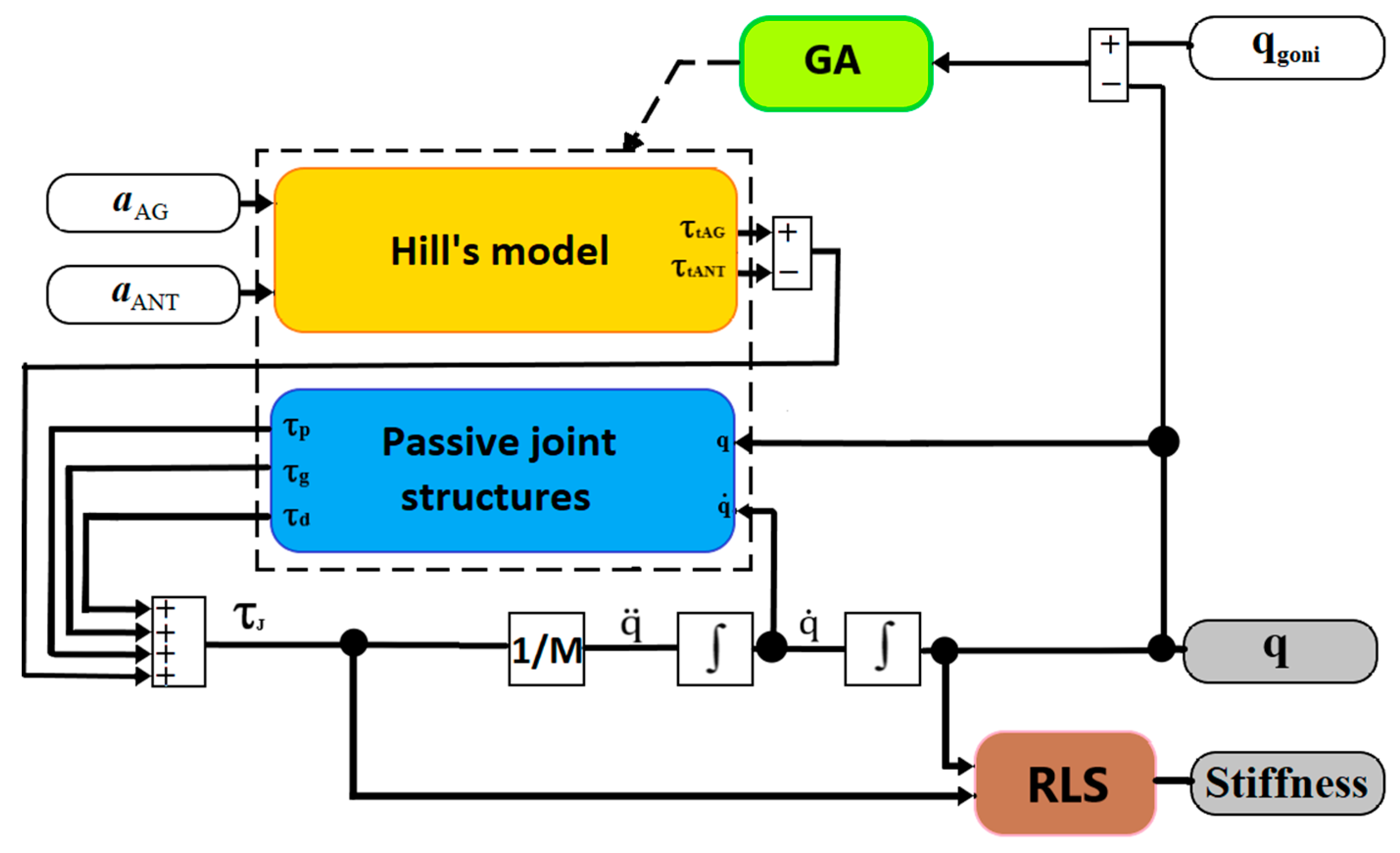
2.4. Algorithm for Elbow Joint Stiffness Estimation
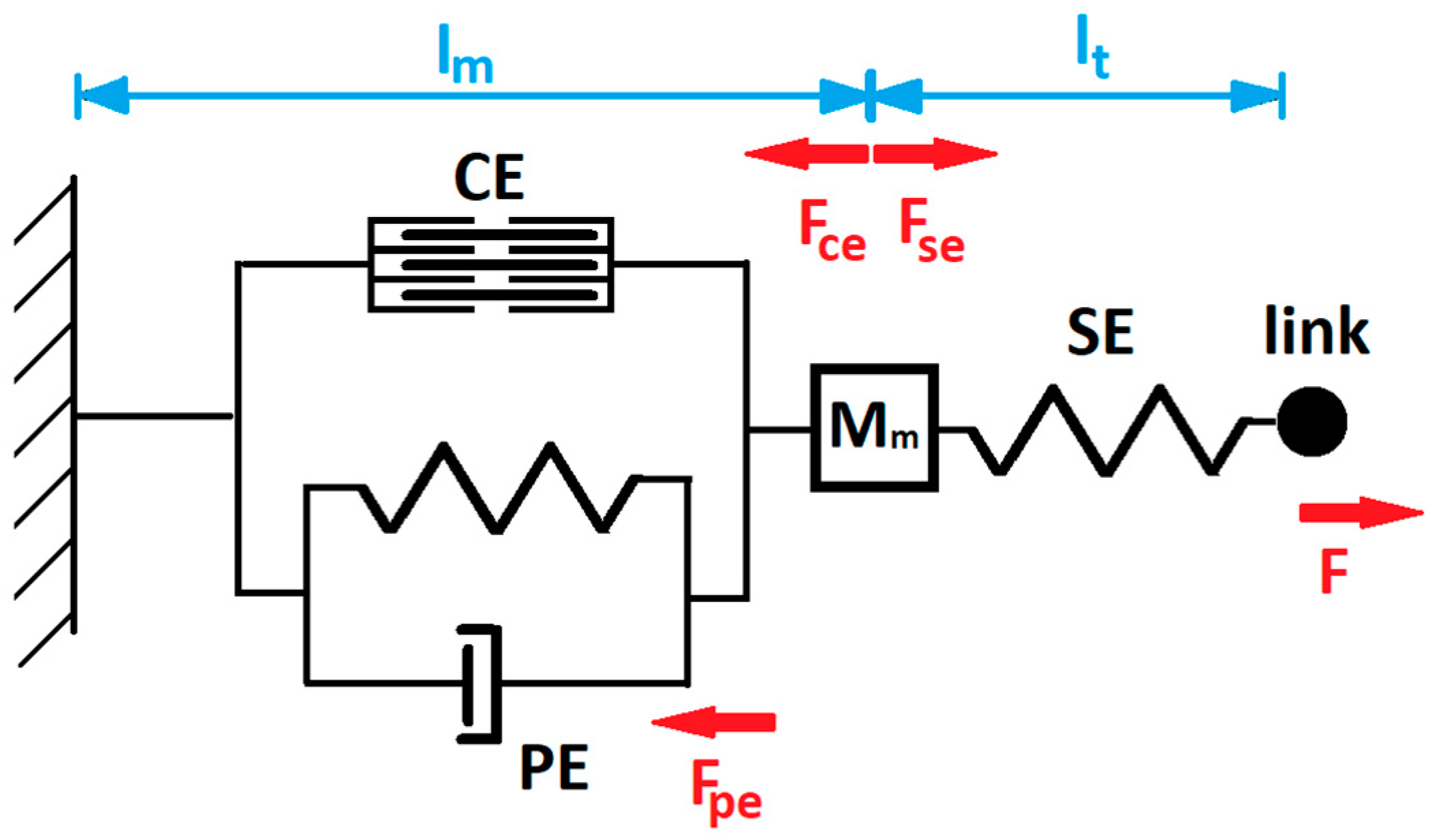
2.4.1. Hill’s Muscle Model
2.4.2. Passive Joint Structures
2.4.3. GA for Adjustment of Parameters for Hill’s Muscle Model and Passive Joint Structure Model
2.4.4. Verification of the Training GA Outputs
2.4.5. Recursive Least Square Algorithm
2.4.6. Stiffness Functional Scale
3. Results
3.1. Identification of Hill’s and Passive Joint Structure Model Parameters
3.2. Verification of Hill’s Model and Passive Joint Structure Parameter Estimation
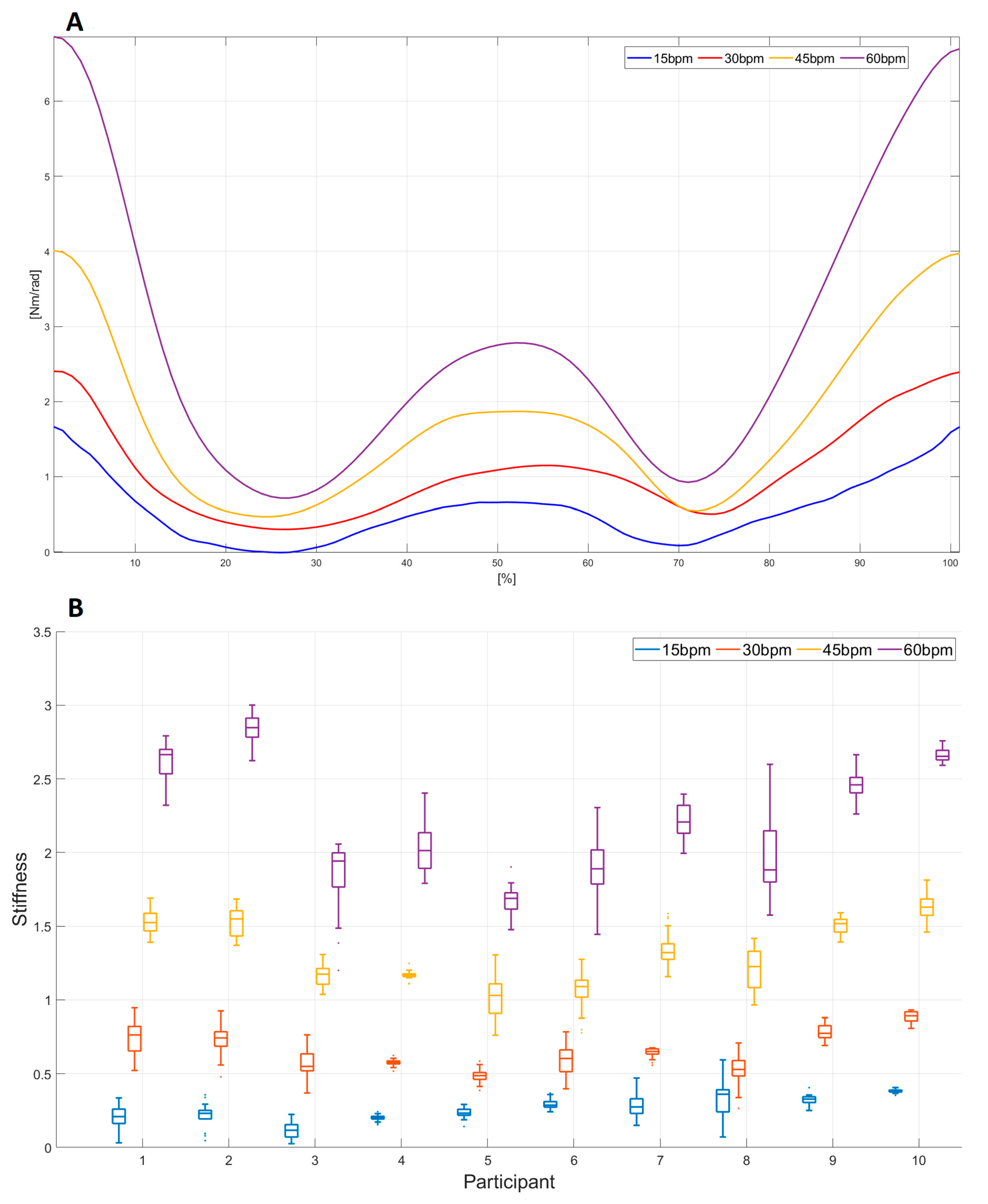
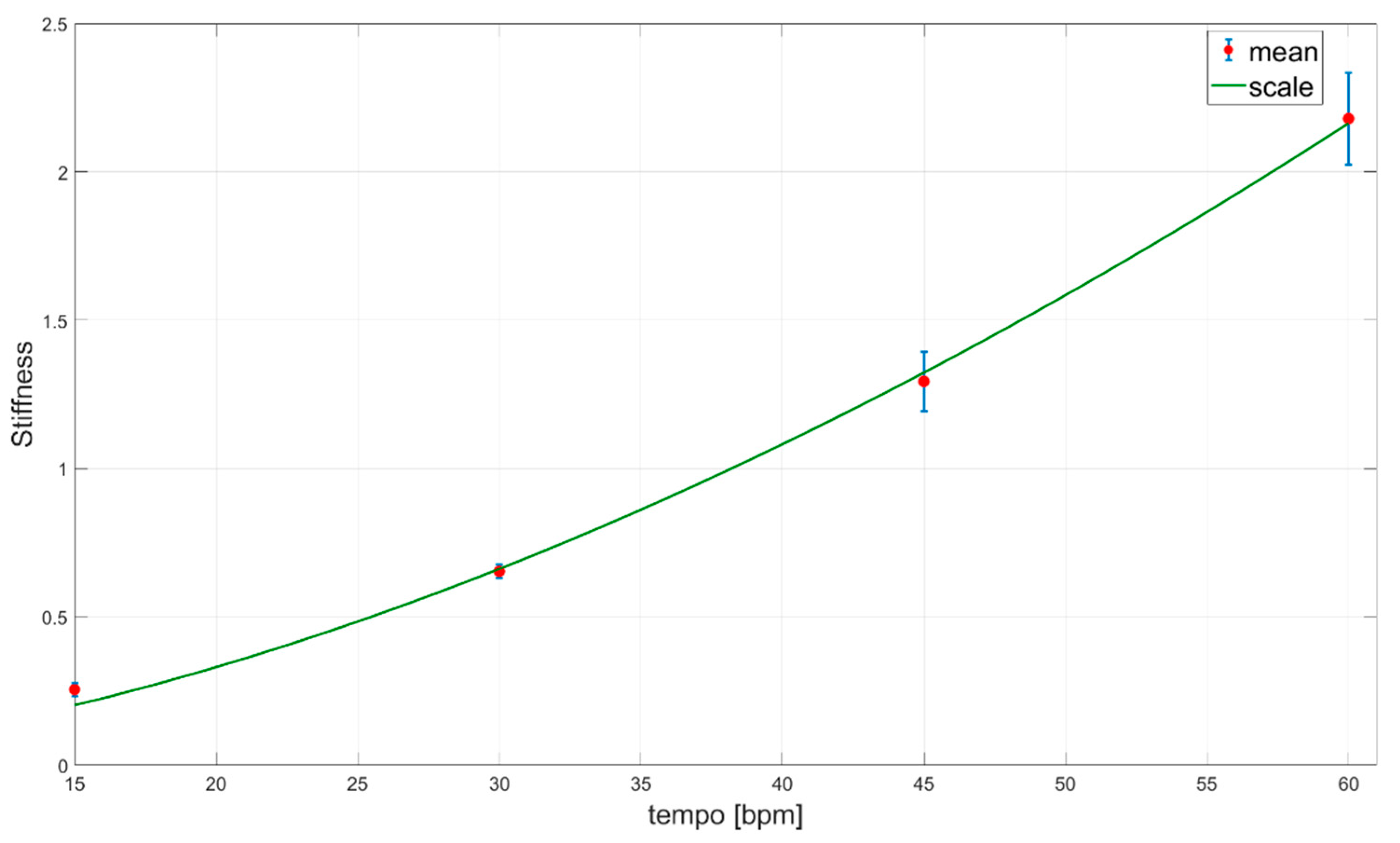
3.3. Elbow Joint Stiffness Estimation and Functional Scales
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tran, V.D.; Dario, P.; Mazzoleni, S. Kinematic Measures for Upper Limb Robot-Assisted Therapy Following Stroke and Correlations with Clinical Outcome Measures: A review. Med. Eng. Phys. 2018, 53, 13–31. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery After Stroke: A Critical Review of its Measurement Properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and A Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Bernaldo de Quirós, M.; Douma, E.H.; van den Akker-Scheek, I.; Lamoth, C.J.C.; Maurits, N.M. Quantification of Movement in Stroke Patients under Free Living Conditions Using Wearable Sensors: A Systematic Review. Sensors 2022, 22, 1050. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Lannin, N.A.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, J.W.; Winstein, C.; van Wegen, E.E.; Wolf, S.L.; et al. Standardized Measurement of Sensorimotor Recovery in Stroke Trials: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 31, 784–792. [Google Scholar] [CrossRef]
- Borzelli, D.; Pastorelli, S.; d’Avella, A.; Gastaldi, L. Virtual Stiffness: A Novel Biomechanical Approach to Estimate Limb Stiffness of a Multi-Muscle and Multi-Joint System. Sensors 2023, 23, 673. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, J.; Wang, L.; Zhang, M.; Li, J.; Bao, S. A Review of The Key Technologies for sEMG-Based Human-Robot Interaction Systems. Biomed. Signal Process. Control 2020, 62, 102074. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Zhang, J.; Zhang, M.; Hou, Z. Continuous Motion and Time-Varying Stiffness Estimation of the Human Elbow Joint Based on SEMG. J. Mech. Med. Biol. 2019, 19, 1950040. [Google Scholar] [CrossRef]
- He, B.; Huang, H.; Thomas, G.C.; Sentis, L. A Complex Stiffness Human Impedance Model with Customizable Exoskeleton Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2468–2477. [Google Scholar] [CrossRef]
- Perreault, E.J.; Kirsch, R.F.; Crago, P.E. Voluntary Control of Static Endpoint Stiffness During Force Regulation Tasks. J. Neurophysiol. 2002, 87, 2808–2816. [Google Scholar] [CrossRef]
- Perreault, E.; Kirsch, R.; Crago, P. Multijoint Dynamics and Postural Stability of the Human Arm. Exp. Brain Res. 2004, 157, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ajoudani, A.; Fang, C.; Tsagarakis, N.G.; Bicchi, A.A. A reduced-Complexity Description of Arm Endpoint Stiffness with Applications to Teleimpedance Control. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2015), Hamburg, Germany, 28 September–2 October 2015. [Google Scholar] [CrossRef]
- Ajoudani, A.; Fang, C.; Tsagarakis, N.; Bicchi, A. Reduced-Complexity Representation of the Human Arm Active Endpoint Stiffness for Supervisory Control of Remote Manipulation. Int. J. Robot. Res. 2017, 37, 155–167. [Google Scholar] [CrossRef]
- Fang, C.; Ajoudani, A.; Bicchi, A.; Tsagarakis, N.G. Online Model Based Estimation of Complete Joint Stiffness of Human Arm. IEEE Robot. Autom. Lett. 2018, 3, 84–91. [Google Scholar] [CrossRef]
- Stanev, D.; Moustakas, K. Stiffness Modulation of Redundant Musculoskeletal Systems. J. Biomech. 2019, 85, 101–107. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, F.; Kim, W.; Ajoudani, A. An Intuitive Formulation of the Human Arm Active Endpoint Stiffness. Sensors 2020, 20, 5357. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Y.; Xu, D.; Kang, S.H.; Zhang, L.Q. EMG-Based Real-Time Linear-Nonlinear Cascade Regression Decoding of Shoulder, Elbow, and Wrist Movements in Able-Bodied Persons and Stroke Survivors. IEEE Trans. Biomed. Eng. 2019, 67, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, K.; Vranić, J.; Miljković, N. Hill's and Huxley's Muscle Models: Tools for Simulations in Biomechanics. Serbian J. Electr. Eng. 2015, 12, 53–67. [Google Scholar] [CrossRef]
- Robleto, R. An Analysis of the Musculotendon Dynamics of Hill-Based Models. Master’s thesis, Texas Tech University Libraries, 2500 Broadway Lubbock, TX, USA, 1997. [Google Scholar]
- Cavallaro, E.E.; Rosen, J.; Perry, J.C.; Burns, S. Real-Time Myoprocessors for a Neural Controlled Powered Exoskeleton Arm. IEEE Trans. Bio-Med. Eng. 2006, 53, 2387–2396. [Google Scholar] [CrossRef]
- Garner, B.A.; Pandy, M.G. Musculoskeletal Model of the Upper Limb Based on the Visible Human Male Dataset. Comput. Methods Biomech. Biomed. Eng. 2001, 4, 93–126. [Google Scholar] [CrossRef]
- Winters, J.M.; Stark, L. Estimated Mechanical Properties of Synergistic Muscles Involved in Movements of a Variety of Human Joints. J. Biomech. 1988, 21, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Desplenter, T.; Trejos, A. Evaluating Muscle Activation Models for Elbow Motion Estimation. Sensors 2018, 18, 1004. [Google Scholar] [CrossRef] [PubMed]
- Holzbaur, K.R.S.; Murray, W.M.; Delp, S.L. A Model of the Upper Extremity for Simulating Musculoskeletal Surgery and Analyzing Neuromuscular Control. Ann. Biomed. Eng. 2005, 33, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Murray, W.M.; Buchanan, T.S.; Delp, S.L. The Isometric Functional Capacity of Muscles that Cross the Elbow. J. Biomech. 2000, 33, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.F.; Wilson, A.J. A Passive Movement Method for Parameter Estimation of a Musculoskeletal Arm Model Incorporating a Modified Hill Muscle Model. Comput. Methods Progr. Biomed. 2014, 114, e46–e59. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Lan, N.; Loeb, G.E.; Gordon, J. Model-Based Sensorimotor Integration for Multi-Joint Control: Development of a Virtual Arm Model. Ann. Biomed. Eng. 2008, 36, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Bingshan, H.; Haoran, T.; Hongrun, L.; Xiangxiang, Z.; Jiantao, Y.; Hongliu, Y. An Improved EMG-Driven Neuromusculoskeletal Model for Elbow Joint Muscle Torque Estimation. Appl. Bionics Biomech. 2021, 2021, 1985741. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, K.; Zhang, X.; Wang, K.; Ota, J. Motion Estimation of Elbow Joint From sEMG using Continuous Wavelet Transform and Back Propagation Neural Networks. Biomed. Signal Process. Control 2021, 68, 102657. [Google Scholar] [CrossRef]
- Lemerle, S.; Grioli, G.; Bicchi, A.; Catalano, M.G. A Variable Stiffness Elbow Joint for Upper Limb Prosthesis. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2019), Macau, China, 4–8 November 2019; pp. 7327–7334. [Google Scholar]
- Pearsall, D.J.; Reid, G. The Study of Human Body Segment Parameters in Biomechanics. Sports Med. 1994, 18, 126–140. [Google Scholar] [CrossRef]
- Wolf, S.L.; Lecraw, D.E.; Barton, L.A.; Jann, B.B. Forced Use of Hemiplegic Upper Extremities to Reverse the Effect of Learned Nonuse Among Chronic Stroke and Head-Injured Patients. Exp. Neurol. 1989, 104, 125–132. [Google Scholar] [CrossRef]
- Cestelein, B.; Cagnie, B.; Parlevliet; Danneels, T.L.; Cools, A. Optimal Normalization Tests for Muscle Activation of the Levator Scapulae, Pectoralis Minor, and Rhomboid Major: An Electromyography Study Using Maximum Voluntary Isometric Contractions. Arch. Phys. Med. Rehabil. 2015, 96, 1820–1827. [Google Scholar] [CrossRef]
- Nieminen, H.; Taklal, E.-P.; Viikari-Juntura, E. Normalization of Electromyogram in the Neck-Shoulder Region. Eur. Appl. J. Physiol. Occup. Physiol. 1993, 67, 199–207. [Google Scholar] [CrossRef]
- Ekstrom, R.A.; Soderberg, G.L.; Donatelli, R.A. Normalization Procedures Using Maximum Voluntary Isometric Contractions for the Serratus Anterior and Trapezius Muscles During Surface EMG Analysis. J. Electromyogr. Kinesiol. 2004, 15, 418–428. [Google Scholar] [CrossRef]
- Halaki, M.; Ginn, K. Normalization of EMG Signals: To Normalize or Not to Normalize and What to Normalize to? In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; InTech: Rijeka, Croatia, 2012; Chapter 7; ISBN 978-953-51-0805-4. [Google Scholar]
- Manal, K.; Buchanan, T.S. A One-Parameter Neural Activation to Muscle Activation Model: Estimating Isometric Joint Moments from Electromyograms. J. Biomech. 2003, 36, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V. The Heat of Shortening and the Dynamic Constants of Muscle. Proc. R. Soc. 1938, 126, 136–195. [Google Scholar] [CrossRef]
- He, J. A Feedback Control Analysis of the Neuro-Musculo-Skeletal System of a Cat Hindlimb. Ph.D. Dissertation, The University of Maryland, College Park, MD, USA, 1988. [Google Scholar]
- Audu, M.L.; Davy, D.T. The Influence of Muscle Model Complexity in Musculoskeletal Motion Modeling. J. Biomech. Eng. 1985, 107, 147–157. [Google Scholar] [CrossRef]
- Sakoe, H.; Seibi, C. Dynamic Programming Algorithm Optimization for Spoken Word Recognition. IEEE Trans. Acoust. Speech Signal Process. 1978, 26, 43–49. [Google Scholar] [CrossRef]
- Fagiolini, A.; Trumić, M.; Jovanović, K. An Input Observer-Based Stiffness Estimation Approach for Flexible Robot Joints. IEEE Robot. Autom. Lett. 2020, 5, 1843–1850. [Google Scholar] [CrossRef]
- Trumić, M.; Grioli, G.; Jovanović, K.; Fagiolini, A. Force/Torque-Sensorless Joint Stiffness Estimation in Articulated Soft Robots. IEEE Robot. Autom. Lett. 2022, 7, 7036–7043. [Google Scholar] [CrossRef]
- Ljung, L. System identification. In Signal Analysis and Prediction; Procházka, A., Uhlíř, J., Rayner, P.W.J., Kingsbury, N.G., Eds.; Springer, Birkhäuser: Boston, MA, USA, 1998; pp. 163–173. ISBN 978-1-4612-1768-8. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Li, Z.; Yang, Z.; Dehghani-Sanij, A.A.; Xie, S. An EMG-Driven Musculoskeletal Model for Estimating Continuous Wrist Motion. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.J.; Hollerbach, J.M.; Xu, J.; Hunter, I.W. Time-Varying Stiffness of Human Elbow Joint During Cyclic Voluntary Movement. Exp. Brain Res. 1992, 88, 433–442. [Google Scholar] [CrossRef]
- Shin, W.; Handdeut, C.; Kim, J. Human elbow motor learning skills of varying loads: Proof of internal model generation using joint stiffness estimation. J. Biomech. Sci. Eng. 2021, 16, 21–88. [Google Scholar] [CrossRef]
- Franklin, D.W.; Burdet, E.; Osu, R.; Kawato, M.; Milner, T.E. Functional Significance of Stiffness in Adaptation of Multijoint Arm Movements to Stable and Unstable Dynamics. Exp. Brain Res. 2003, 151, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shiller, D.M.; Gribble, P.L.; Ostry, D.J. Relationship Between Cocontraction, Movement Kinematics and Phasic Muscle Activity in Single-Joint Arm Movement. Exp. Brain Res. 2021, 140, 171–181. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Mambrito, B. Voluntary Control of Motor Units in Human Antagonist Muscles: Coactivation and Reciprocal Activation. J. Neurophysiol. 1987, 58, 525–542. [Google Scholar] [CrossRef]
- Schwarz, A.; Kanzler, C.M.; Lambercy, O.; Luft, A.R.; Veerbeek, J.M. Systematic Review on Kinematic Assessments of Upper Limb Movements After Stroke. Stroke 2019, 50, 718–727. [Google Scholar] [CrossRef]


| No | Parameter | No | Parameter |
|---|---|---|---|
| 1 | Length, Hand | 6 | Circumference, Forearm |
| 2 | Length, Wrist to Knuckle | 7 | Circumference, Elbow |
| 3 | Length, Forearm | 8 | Width, Hand |
| 4 | Circumference, Fist | 9 | Width, Wrist |
| 5 | Circumference, Wrist | 10 | Width, Elbow |
| Parameter | Mean Value ± Standard Deviation | Min Value | Max Value |
|---|---|---|---|
| k1 [Nm] | 7.16 ± 1.88 | 4.54 | 9.05 |
| k2 [] | 0.35 ± 0.05 | 0.25 | 0.43 |
| k4 [Nm] | 35.89 ± 13.24 | 15.00 | 60.5 |
| k5 [] | 0.026 ± 0.006 | 0.02 | 0.04 |
| q1 [] | 4.20 ± 0.77 | 3.11 | 5.49 |
| q2 [] | 5.25 ± 1.29 | 3.22 | 6.95 |
| Bp [Nms] | 3.32 ± 0.81 | 1.55 | 4.05 |
| Fmax [N] | 2289.76 ± 786.18 | 1401.59 | 3913.00 |
| w [n.u.] | 0.78 ± 0.14 | 0.64 | 0.96 |
| lmopt [m] | 0.19 ± 0.05 | 0.10 | 0.27 |
| af [n.u.] | 0.39 ± 0.19 | 0.25 | 0.75 |
| kte [] | 2611.95 ± 862.11 | 1511.02 | 4042.59 |
| ktl [N/m] | 27,230.04 ± 8846.59 | 15,570.31 | 43,133.01 |
| kt [N/m] | 538,599.51 ± 196,458.08 | 317,771.71 | 923,909.99 |
| ltc [m] | 0.34 ± 0.093 | 0.23 | 0.47 |
| lts [m] | 0.33 ± 0.070 | 0.24 | 0.46 |
| kml [N/m] | 408.21 ± 137.80 | 244.96 | 710.60 |
| kme [] | 75.84 ± 23.35 | 46.05 | 113.92 |
| km [N/m] | 4551.31 ± 1590.63 | 2500.06 | 7352.47 |
| lmc [m] | 0.14 ± 0.03 | 0.10 | 0.19 |
| lms [m] | 0.19 ± 0.05 | 0.10 | 0.27 |
| Bm [Ns/m] | 0.19 ± 0.05 | 130.97 | 342.17 |
| 241.77 ± 75.83 | 0.33 | 0.45 | |
| 0.39 ± 0.04 | 0.54 | 1.46 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Training phase | 8.47 | 4.46 | 8.02 | 1.95 | 8.67 | 9.16 | 4.89 | 2.56 | 2.77 | 1.83 | |
| E1 | 15 bpm | 2.78 | 5.21 | 8.43 | 4.37 | 5.42 | 5.48 | 6.67 | 1.63 | 3.55 | 5.80 |
| 30 bpm | 4.45 | 1.98 | 2.91 | 4.05 | 5.16 | 5.83 | 1.78 | 5.25 | 2.96 | 2.16 | |
| 45 bpm | 4.80 | 3.11 | 6.05 | 2.06 | 6.32 | 6.51 | 2.43 | 2.23 | 2.95 | 2.31 | |
| 60 bpm | 5.31 | 5.74 | 10.79 | 6.56 | 11.85 | 7.03 | 3.13 | 4.76 | 3.65 | 0.99 | |
| E2 | 0 kg | 4.55 | 7.80 | 8.47 | 2.52 | 4.35 | 12.16 | 11.43 | 5.10 | 4.75 | 6.94 |
| 0.25 kg | 4.76 | 6.55 | 6.64 | 4.91 | 5.33 | 3.53 | 10.15 | 6.29 | 2.23 | 3.02 | |
| 0.5 kg | 4.45 | 1.98 | 2.91 | 4.05 | 5.16 | 5.83 | 1.78 | 5.25 | 2.96 | 2.16 | |
| 0.75 kg | 3.70 | 7.22 | 11.22 | 3.20 | 3.93 | 8.65 | 5.49 | 1.69 | 2.93 | 11.14 | |
| 1 kg | 8.13 | 5.44 | 10.79 | 4.38 | 5.35 | 8.82 | 5.32 | 6.96 | 2.30 | 3.26 |
| Parameter | Percentage Variation | |||||
|---|---|---|---|---|---|---|
| −10% | +10% | −20% | 20% | −30% | 30% | |
| k1 | 0.0622 | 0.0429 | 0.1784 | 0.1370 | 0.2854 | 0.2916 |
| k2 | 0.1712 | 0.1341 | 0.3541 | 0.3405 | 0.4556 | 0.5504 |
| k4 | 0.0331 | 0.0353 | 0.1078 | 0.0916 | 0.2543 | 0.1592 |
| k5 | 0.0019 | 0.0019 | 0.0040 | 0.0038 | 0.0064 | 0.0059 |
| q1 | 0.0024 | 0.0024 | 0.0052 | 0.0050 | 0.0084 | 0.0077 |
| q2 | 0.1669 | 0.1934 | 0.3211 | 0.3747 | 0.4050 | 0.3827 |
| Bp | 0.0041 | 0.0040 | 0.0100 | 0.0094 | 0.0173 | 0.0155 |
| Fmax | 0.0239 | 0.0243 | 0.0538 | 0.0614 | 0.1028 | 0.1066 |
| w | 0.0142 | 0.0104 | 0.0446 | 0.0235 | 0.0994 | 0.0367 |
| lmopt | 0.0444 | 0.0287 | 0.0938 | 0.0548 | 0.1887 | 0.0899 |
| af | 0.0193 | 0.0159 | 0.0559 | 0.0377 | 0.0994 | 0.0609 |
| kte | 0.00035 | 0.00042 | 0.0005 | 0.00064 | 0.00057 | 0.00072 |
| ktl | 0.00028 | 0.00033 | 0.00037 | 0.00036 | 0.00047 | 0.00047 |
| kt | 0.00074 | 0.00071 | 0.0012 | 0.00087 | 0.0020 | 0.0011 |
| ltc | 5.0826 | 0.0038 | 7.1025 | 0.0038 | 5.8225 | 0.0038 |
| lts | 0.0038 | 4.9648 | 0.0038 | 6.9452 | 0.0038 | 6.2682 |
| kml | 0.00028 | 0.00031 | 0.00043 | 0.00044 | 0.0005 | 0.00051 |
| kme | 0.00032 | 0.00026 | 0.00046 | 0.00039 | 0.00056 | 0.00046 |
| km | 0.0074 | 0.0072 | 0.0186 | 0.0168 | 0.0322 | 0.0277 |
| lmc | 0.0082 | 0.0133 | 0.0110 | 0.0215 | 0.0114 | 0.0298 |
| lms | 0.0444 | 0.0287 | 0.0938 | 0.0548 | 0.1887 | 0.0899 |
| Bm | 0.00079 | 0.00079 | 0.0013 | 0.0012 | 0.0018 | 0.0018 |
| 0.00005 | 0.00009 | 0.00007 | 0.00014 | 0.00009 | 0.00017 | |
| 0.0254 | 0.0215 | 0.0733 | 0.0517 | 0.1440 | 0.0846 | |
| Reference | lmopt [m] | lts [m] | lms [m] | Fmax [N] | Mm [kg] | af [n.u.] | vmax [m/s] |
|---|---|---|---|---|---|---|---|
| Cavallaro et al. [20] | 0.15 | 0.19–0.23 | n.a. | 393–1000 | n.a. | n.a. | n.a. |
| Garner et al. [21] | 0.15 | 0.19–0.23 | n.a. | 462–629 | n.a. | n.a. | n.a. |
| Winters et al. [22] | 0.09–0.14 | 0.22 | 0.31–0.37 | n.a. | 0.06–0.13 | 0.35–0.45 | 0.49–0.76 |
| Desplenter et al. [23] | 0.11–0.17 | n.a. | 0.16–0.25 | 2875–2397 | n.a. | n.a. | n.a. |
| Holzbaur et al. [24] | 0.11–0.13 | 0.14–0.27 | n.a. | 624–799 | n.a. | n.a. | n.a. |
| Murray et al. [25] | 0.13 | 0.22–0.23 | 0.22–0.36 | n.a. | n.a. | n.a. | n.a. |
| Yu et al. [26] | 0.15 | 0.11–0.13 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Song et al. [27] | 0.16–0.17 | 0.21–0.23 | 0.39–0.41 | 630–764 | 0.34–0.45 | n.a. | n.a. |
| Bingshan et al. [28] | 0.12–0.13 | 0.14–0.27 | n.a. | 624–799 | n.a. | n.a. | 0.96–1.04 |
| This paper | 0.1–0.27 | 0.24–0.46 | 0.1–0.27 | 1400–3900 | 0.33–0.45 | 0.25–0.75 | 0.54–1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radmilović, M.; Urukalo, D.; Janković, M.M.; Dujović, S.D.; Tomić, T.J.D.; Trumić, M.; Jovanović, K. Elbow Joint Stiffness Functional Scales Based on Hill’s Muscle Model and Genetic Optimization. Sensors 2023, 23, 1709. https://doi.org/10.3390/s23031709
Radmilović M, Urukalo D, Janković MM, Dujović SD, Tomić TJD, Trumić M, Jovanović K. Elbow Joint Stiffness Functional Scales Based on Hill’s Muscle Model and Genetic Optimization. Sensors. 2023; 23(3):1709. https://doi.org/10.3390/s23031709
Chicago/Turabian StyleRadmilović, Marija, Djordje Urukalo, Milica M. Janković, Suzana Dedijer Dujović, Tijana J. Dimkić Tomić, Maja Trumić, and Kosta Jovanović. 2023. "Elbow Joint Stiffness Functional Scales Based on Hill’s Muscle Model and Genetic Optimization" Sensors 23, no. 3: 1709. https://doi.org/10.3390/s23031709
APA StyleRadmilović, M., Urukalo, D., Janković, M. M., Dujović, S. D., Tomić, T. J. D., Trumić, M., & Jovanović, K. (2023). Elbow Joint Stiffness Functional Scales Based on Hill’s Muscle Model and Genetic Optimization. Sensors, 23(3), 1709. https://doi.org/10.3390/s23031709





